Abstract
Background: Studies suggest volatile anesthetics and opioids may enhance the malignant potential of cancer cells. The objective of this single institution retrospective study was to evaluate the survival impact of a multimodal opioid-sparing nonvolatile anesthetic technique (MA) in a group of patients who had undergone cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) for appendiceal carcinomatosis.
Methods: Propensity score matching (PSM) and Cox proportional hazard models were used to compare the survivals of patients who received MA (MA group), to those who received volatile-opioid anesthesia (volatile-opioid group).
Results: Of the 373 patients, 110 (29%) were in the MA group and 263 (71%) in the volatile-opioid group. The MA group was older (mean ± standard deviation (SD): 55 ± 11 versus 53 ± 10 years, p = .035) and had more patients with ASA scores 3 or 4 (90% versus 81%, p = .032), and those with high grade tumors (18% versus 12%, p = .009). Intraoperative opioid consumption was lower in the MA group (mean morphine equivalents ± SD: 13 ± 10 versus 194 ± 789, p < .0001). After PSM, 107 patients remained in each group. In the adjusted Cox proportional hazards model after PSM, MA was not associated with improved progression free survival (PFS) (HR 1.45, 95% CI [0.94–2.22], p = .093) or overall survival (OS) (HR 1.66, 95% CI [0.86–3.20], p = .128), when compared to volatile-opioid anesthesia.
Conclusions: In this retrospective study, a multimodal opioid-sparing nonvolatile anesthetic approach was not associated with improved survival.
Precis’ statement: In this study of patients undergoing major cancer surgery, the use of multimodal anesthetic and analgesic agents, while avoiding volatile anesthetics and minimizing opioid use was not associated with improved survival.
Introduction
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is an extensive surgical procedure that has significantly improved the survival of patients with carcinomatosis of appendiceal origin [Citation1–4]. Several factors including age, tumor burden, histology and the completeness of cytoreduction have been shown to influence the survival of patients undergoing CRS-HIPEC for appendiceal carcinomatosis [Citation5].
It has been suggested that the type of anesthetic used during major oncologic surgery might affect cancer recurrence [Citation6,Citation7]. This is based on the results of in vitro studies which indicate that volatile anesthetics and opioids may enhance the malignant potential of tumors [Citation7,Citation8]. Proposed mechanisms include the promotion of cancer cell proliferation and invasion, and the induction of immunosuppression, inflammation and angiogenesis [Citation7,Citation8]. To the contrary, other anesthetics and analgesics such as propofol, lidocaine, ketamine and celecoxib have been shown to possess antitumor properties [Citation6,Citation9–11]. Combinations of these agents are widely used as part of multimodal anesthetic and analgesic regimens, and have been associated with improved pain control, reduced opioid consumption, shorter hospital stay and lower rates of complications [Citation12,Citation13].
Furthermore, in a large cohort study of more than 10,000 cancer patients, the mortality was 46% higher with volatile-opioid anesthesia, than with propofol-based total intravenous anesthesia (TIVA) [Citation14]. The anti-tumor properties of propofol and several of the other adjuncts used in multimodal anesthesia, makes it biologically plausible that using combinations of these agents while avoiding volatile anesthetics might contribute to improved survival in cancer patients. To the best of our knowledge, this hypothesis has not been evaluated in patients undergoing cancer surgery. In this retrospective study, our objective was to compare the survivals of patients who received opioid-sparing multimodal anesthesia without volatile agents, to that of those who received volatile-opioid anesthesia while undergoing CRS-HIPEC for appendiceal carcinomatosis. Our hypothesis was that patients who received multimodal opioid-sparing anesthesia without volatile agents would have better progression free survival (PFS) or overall survival (OS) compared to those who received volatile-opioid anesthesia.
Methods
After identifying a study population and defining the study objectives, statistical plan and outcomes of interest, a retrospective protocol was submitted to the intuitional review board (IRB) of the MD Anderson Cancer Center. Upon obtaining IRB approval (MD Anderson Cancer Center, PA 14–1087), demographic, perioperative and survival data of adults (>18 years old) who had undergone CRS-HIPEC for appendiceal carcinomatosis between January 2006 and January 2017 were extracted from the medical records. Patients who had undergone a repeat CRS-HIPEC were excluded.
Comparisons were made between patients who received multimodal opioid-sparing anesthesia without volatile agents and those who received volatile-opioid anesthesia. The primary outcomes of interest were PFS and (OS. PFS was defined as the length of time from the date of surgery to the date of first evidence of new peritoneal disease or an increase in the size of any residual peritoneal lesions, or the date of death (whichever occurred first). OS was defined as the length of time from the date of surgery to the date of death or last follow-up date.
Anesthetic management
Several aspects of care including the choice of anesthetic agents and pain management were not standardized. However, typical anesthetic management involved the placement of an epidural catheter or transversus abdominus plane block (with liposomal bupivacaine), followed by a general anesthesia with either volatile-opioid anesthesia (VO group) or multimodal opioid-sparing TIVA (MA group). The VO group commonly received desflurane in a mixture of air and oxygen, and intravenous (IV) infusions of an opioid (sufentanil or fentanyl) and muscle relaxant (rocuronium or cisatracurium). Occasionally, IV infusions of lidocaine, ketamine, dexmedetomidine or low dose propofol were used in combination with volatile agents. Patients in the MA group received the preoperative administration of pregabalin (75 mg), celecoxib (200–400 mg) and tramadol (300 mg), followed by continuous infusions of propofol (50–150 mcg/kg/min), dexmedetomidine (0.075–0.3 mcg/kg/h), ketamine (2.5–10 mg/h), IV lidocaine (0.5 mg/min), and a muscle relaxant (rocuronium or cisatracurium). Intermittent boluses of opioids (sufentanil or fentanyl) were administered according to clinical judgment. In both groups, a continuous epidural infusion of bupivacaine (0.075%) with hydromorphone (5–10 mcg/ml) was used in patients with epidural catheters.
The majority of the patients was extubated in the operating room and recovered on a monitored ward for the initial 24–48 h. Pain management over the immediate 3–5 postoperative days consisted of epidural analgesia (bupivacaine 0.075% with hydromorphone 5–10 mcg/ml) when available, and supplemental IV opioids, IV acetaminophen and ketorolac. Oral opioids, gabapentinoids and celecoxib were introduced upon transition to oral feeding.
Statistical methods
Descriptive statistics including mean, standard deviation, median and range were calculated for continuous variables such as age, body mass index (BMI), and intraoperative opioid consumption (in morphine dose equivalents). Frequency counts and percentages were calculated for categorical variables such as gender, American Society of Anesthesiologists physical status score (ASA), and general anesthesia technique (MA or VO). The Chi-square test was used to evaluate the association between two categorical variables. Wilcoxon rank sum test was used to evaluate the difference in a continuous variable between patient groups.
The Kaplan–Meier method was used for time-to-event analysis including PFS and OS. Median time to event in months with 95% confidence interval (CI) was calculated. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. Univariate Cox proportional hazards models were fitted to evaluate the effects of continuous variables on time-to-event out comes. Multicovariable Cox proportional hazard models were used for multivariate analysis to include important and significant covariates. Statistical software SAS version 9.3 (SAS, Cary, NC) and S-Plus version 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for all the analyses. A p value < .05 was considered statistically significant.
To adjust for selection bias in the observational study, a propensity score matching (PSM) analysis was conducted. The following prognostic covariates were included in the multicovariate logistic model to estimate the propensity scores: age, gender, BMI, ASA, tumor grade, extra-abdominal disease, neoadjuvant chemotherapy, preoperative PLR and estimated blood loss (EBL). The Greedy 5 → 1 digit match algorithm was used to match the baseline covariates, so that the two study groups (MA or VO) would have similar propensity scores [Citation15].
Among the 373 patients in the entire study population, the propensity score was calculated for 358 patients. Of those, 109 patients were in the MA group and 249 in the VO group. One hundred and seven patients in the MA group with non-missing values for the selected covariates were matched on a 1:1 ratio to the patients in the VO group with non-missing values for the same covariates. The standardized differences for all covariates were ≤7.81% in the post-matching cohort, suggesting substantial reduction of bias between the two groups ().
Table 1. Baseline and perioperative characteristics of 373 patients with appendiceal carcinomatosis grouped according to the type of anesthetic management (multimodal anesthesia versus volatile-opioid).
Results
From January 2006 to January 2017, 389 adult patients underwent CRS-HIPEC for appendiceal carcinomatosis. After excluding 16 patients who had undergone repeat CRS-HIPEC, 373 patients were included in the study. The average age (±standard deviation) of the study population was 53 (±10) years. Two hundred and twenty-five (60%) were female and 148 (40%) were male. Of these, 110 (29%) were in the MA group and 263 (71%) in the VO group. The MA group was older (median age [interquartile (IQR)] 55 years (50, 63) versus 53 years (45, 61), p = .035) and had a higher proportion of patients who received neoadjuvant chemotherapy (44% versus 31%, p = .0175). The MA group also had a higher proportion of patients with higher ASA scores (90% versus 81%, p = .032), as well as those with high-grade tumors (18% versus 12%, p = .009). Other baseline and perioperative characteristics of the study population were not significantly different (). Over the intraoperative period, the MA group had a significantly lower opioid consumption (median morphine dose equivalents [IQR]; 12 (8, 15) versus 114 (67, 170), p < .0001), and a lower proportion of patients with epidural analgesia (81% versus 90%, p = .010). The platelet:lymphocyte ratio (PLR) on postoperative day one and length of stay were significantly lower in the MA group (mean, 271 versus 318, p = .002 and median, 11 versus 16, p < .0001, respectively), suggesting an anti-inflammatory effect of the MA technique.
Progression free survival
The median PFS (95% CI) for the entire study population was 39 months (32, 79). Compared to the VO group, the median PFS (95% CI) was significantly shorter in the MA group (21 months [17, 49], versus 46 months [37, 93], p = .005). In the unadjusted Cox proportional hazards model before PSM, the following covariates were significantly associated with worse PFS; tumor burden (hazard ratio [HR] 1.04, 95% CI [1.02, 1.06], p < .001), preoperative neutrophil:lymphocyte ratio (NLR) (HR 1.07, 95% CI [1.00, 1.16], p = .048), preoperative PLR (HR 1.00, 95% CI [1.00, 1.00], p = .026), EBL (HR 1.00, 95% CI [1.00, 1.00], p < .001), and MA (HR 1.64, 95% CI [1.16, 2.33], p = .006). After PSM, the association between MA and PFS was statistically significant in the unadjusted model (HR 1.64, 95% CI [1.07–2.52], p = .022) (), but was not significant after adjusting for tumor grade, extra-abdominal disease, neoadjuvant chemotherapy, preoperative PLR and EBL (HR 1.45, 95% CI [0.94–2.22], p = .093). The following covariates remained statistically significant in the adjusted model after PSM: tumor grade (HR 2.27, 95% CI [1.19–4.34], p = .013), neoadjuvant chemotherapy (HR 2.85, 95% CI [1.66–4.88], p < .001), and EBL (HR 1.03, 95% CI [1.01–1.05], p = .014) ().
Figure 1. Kaplan–Meier curves showing the progression free survival of patients who received volatile-opioid anesthesia and those who received multimodal anesthesia (MA).
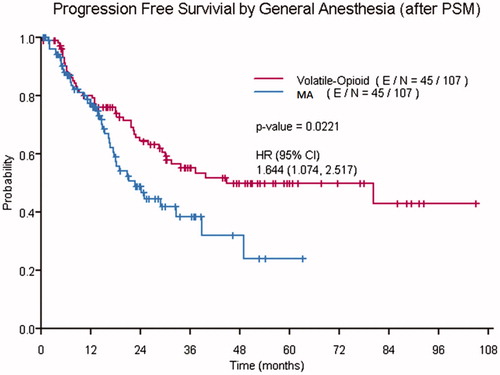
Table 2. Multivariate Cox Proportional Hazards Model for progression free survival.
Overall survival
The 3 and 5 year (95% CI) OS rates of the MA group (72% [6, 85] and 57% [41, 78], respectively) were lower than those of the VO group (85% [81, 90], 80% [74, 85], respectively). The unadjusted Cox proportional hazards model before PSM demonstrated a statistically significant association between OS and BMI (HR 0.96, 95% CI [0.92, 0.99], p = .031), preoperative NLR (HR 1.13, 95% CI [1.04, 1.24], p = .006), preoperative PLR (HR 1.02, 95% CI [1.00, 1.04], p = .018), EBL (HR 1.02, 95% CI [1.00, 1.03], p = .043), and MA (HR 2.29, 95% CI [1.35, 3.90], p = .002). After PSM, the unadjusted Cox proportional hazards model demonstrated a statistically significant association between MA and OS (HR 1.958, 95% CI [1.03–3.72], p = .040) (). After adjusting for tumor grade, extra-abdominal disease, and preoperative PLR, this association between MA and OS was not statistically significant (HR 1.66, 95% CI [0.86–3.20], p = .128). As shown in , the following covariates remained statistically significant in the adjusted model after PSM: tumor grade (HR 12.42, 95% CI [4.99–30.92], p < .001), extra-abdominal disease (HR 13.41, 95% CI [3.64–49.33], p < .001), and preoperative PLR (HR 1.05, 95% CI [1.01–1.09], p = .014).
Figure 2. Kaplan–Meier curves showing the overall survival of patients who received volatile-opioid anesthesia and those who received multimodal anesthesia (MA).
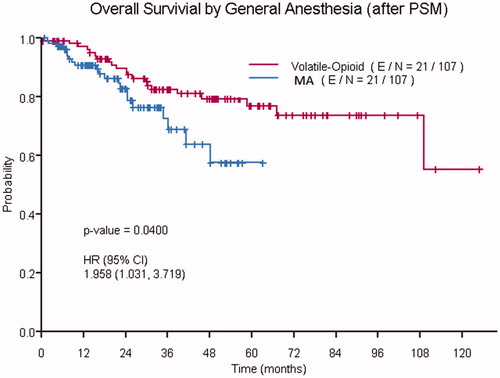
Table 3. Multivariate Cox Proportional Hazard Models for overall survival.
Discussion
In this retrospective study of patients who had undergone CRS-HIPEC, compared to volatile-opioid anesthesia, there was no demonstrable survival benefit in patients who received MA despite a substantial reduction in intraoperative opioid use and the avoidance of volatile anesthetics. Furthermore, although the MA technique was associated with a significant reduction in postoperative inflammatory markers, our results do not support our hypothesis that combining several anesthetics/analgesics with anti-tumor properties would be associated with improved survival. Our findings are also not consistent with those of other studies which have demonstrated a favorable association between opioid-sparing TIVA and cancer progression [Citation14,Citation16]. Among several other factors, the differences in our findings may be explained by the components of MA in our study, the aggressive nature of appendiceal carcinomatosis, or the slightly older and sicker demographic of our MA group.
In a large retrospective study by Wigmore et al. [Citation14], patients who received volatile anesthesia while undergoing oncologic surgery had a 46% increased risk of death compared to those who received propofol-based TIVA. In a smaller group of patients undergoing colon cancer surgery, Wu et al. [Citation16] reported a 64% reduction in the risk of death of patients who received propofol-based TIVA, compared to those who received desflurane. Unlike our study population where patients received propofol in combination with other anesthetics including dexmedetomidine, ketamine, lidocaine, celecoxib, pregabalin and tramadol, patients in the studies by Wigmore et al., and Wu et al. received a predominantly propofol-based TIVA without any of the oral and IV adjuncts administered in our MA group. Unlike propofol which has mainly been associated with properties which may reduce the survival of tumors [Citation17–19], some of the components of MA in our study namely, dexmedetomidine and ketamine, have been associated with some pro-tumoral effects [Citation20–23]. For example, dexmedetomidine has been shown to increase tumor cell retention and promote metastasis in rodent models of breast, lung and colon cancers [Citation21]. Furthermore, in a retrospective study of patients undergoing lung cancer surgery, the intraoperative administration of dexmedetomidine was associated with a 28% increase in the risk of death [Citation20]. The underlying mechanisms for this association may be related to the modulation of NK cell cytotoxicity and increased capillary permeability to tumor cells [Citation21].
The results of studies investigating the effects of ketamine on cancer cells have been conflicting [Citation10,Citation22,Citation23]. In an in vitro study by Zhou et al. [Citation10], ketamine was shown to induce apoptosis of lung adenocarcinoma cell lines in a concentration-dependent manner. However, in another study on a rat model of pulmonary metastasis, ketamine was shown to reduce NK cell activity and increase lung tumor retention [Citation22]. The effect of ketamine on NK cell activity and the metastatic activity of mammary adenocarcinoma cell lines has also been shown to vary over time, and to be dependent upon the age of the animals used in the study [Citation23]. The reasons behind these conflicting results are unclear. However, based on the above, we are led to speculate that despite providing a significant anti-inflammatory effect, the proposed oncological benefits of propofol, lidocaine and celecoxib could be offset when used in combination with other anesthetics.
Appendiceal carcinomatosis encompasses a variety of tumor types some of which are characterized by high-grade aggressive disease. In the most recent American Joint Committee on Cancer staging system, all appendiceal tumors with peritoneal dissemination are considered stage IV disease [Citation24]. In other studies where a survival benefit of opioid-sparing TIVA has been demonstrated, there has been a trend toward a lesser benefit in patients with metastatic disease and higher ASA scores [Citation14,Citation16]. For example in the study by Wigmore et al. [Citation14], the risk associated with volatile anesthetic exposure to patients with metastatic disease was less than that of patients with no metastases. A similar trend was observed with ASA scores, with the hazard ratios of ASA III patients being lowest. It is may be possible that beneficial effects of our MA technique are non-significant in patients with such aggressive and widespread disease as appendiceal carcinomatosis.
In this study, patients in the MA group were significantly older, had higher ASA scores, and a higher proportion of patients with high-grade tumors. With age, higher ASA scores and tumor-grade being relevant to survival outcomes, there is a possibility that we may not have adequately controlled for the combination or interaction of these factors, PSM notwithstanding. This may have been evidenced by the significantly higher risk of progression and death in the MA group in unadjusted models, which remained higher (although insignificant) in the adjusted models; perhaps an effect of our small sample size. That said, a somewhat similar observation could be made about the studies by Wigmore et al. [Citation14] and Wu et al. [Citation16]. In both studies, patients who received volatile-opioid anesthesia had higher ASA scores and more advanced disease, and demonstrated worse survival after PSM. Although robust statistical methods are employed by both studies, it is impossible to completely rule out the effect residual confounding of unmeasured variables, particularly in the sicker patients. Similar to the findings of previous studies, higher-grade tumors, higher EBl, higher preoperative PLR and the presence of extra-abdominal disease were associated with reduced survival in our study [Citation25–27].
This study has several limitations. First, its retrospective and non-randomized nature means that unaccounted confounding variables and missing data may have played a role in our findings. Second, while the PSM addressed some of the selection bias, some essential components could not be addressed. For example, biases related to the year of the procedure (given an 11 year time period with changes in practice), and the reasoning behind the anesthetic choices (volatile versus MA) could not be addressed. Third, a type 2 statistical error might have existed because of the low number of patients included in each group after PSM. A post priori sample size analysis assuming a HR of 1.59, overall probability of event of 0.65, an expected reduction of 30% in recurrence-free survival rate at 5 years and a power (1-β) of 0.9 indicated that we would have needed a total of 232 patients to demonstrate a significant difference in survival between both groups of treatment. Finally, we did not conduct a sample size analysis to determine the number of patients needed in the study. Our decision was based on lack of literature or any other information to determine an adequate effect size.
Conclusion
In this single-center retrospective study of adults who had undergone CRS-HIPEC for appendiceal carcinomatosis, multimodal opioid-sparing TIVA was not associated with a survival benefit, when compared to volatile-opioid anesthesia. Due to the unique nature of CRS-HIPEC, broad conclusions cannot be based on our findings, and further studies are required.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sugarbaker PH. Management of peritoneal metastases - basic concepts. J Buon. 2015;20(1):S2–S11.
- Ung L, Chua TC, Morris DL. Cure for peritoneal metastases? An evidence-based review. ANZ J Surg. 2013;83:821–826.
- Grotz TE, Royal RE, Mansfield PF, et al. Stratification of outcomes for mucinous appendiceal adenocarcinoma with peritoneal metastasis by histological grade. World J Gastrointest Oncol. 2017;9:354–362.
- Ronnett BM, Yan H, Kurman RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456.
- Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16:8.
- Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anesth. 2016;63:184–192.
- Connolly C, Buggy DJ. Opioids and tumour metastasis: does the choice of the anesthetic-analgesic technique influence outcome after cancer surgery? Curr Opin Anaesthesiol. 2016;29:468–474.
- Onizuka S, Tamura R, Yonaha T, et al. Clinical dose of lidocaine destroys the cell membrane and induces both necrosis and apoptosis in an identified Lymnaea neuron. J Anesth. 2012;26:54–61.
- Zhou X, Zhang P, Luo W, et al. Ketamine induces apoptosis in lung adenocarcinoma cells by regulating the expression of CD69. Cancer Med. 2018;7:788–795.
- Wang D, Fu L, Sun H, et al. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–1895.e4.
- Helander EM, Webb MP, Bias M, et al. A comparison of multimodal analgesic approaches in institutional enhanced recovery after surgery protocols for colorectal surgery: pharmacological agents. J Laparoendosc Adv Surg Tech A. 2017;27:903–908.
- Memtsoudis SG, Poeran J, Zubizarreta N, et al. Association of multimodal pain management strategies with perioperative outcomes and resource utilization: a population-based study. Anesthesiology. 2018;128:891–902.
- Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79.
- Austin PC. A comparison of 12 algorithms for matching on the propensity score. Statist Med. 2014;33:1057–1069.
- Wu ZF, Lee MS, Wong CS, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129:932–941.
- Yang N, Liang Y, Yang P, et al. Propofol suppresses LPS-induced nuclear accumulation of HIF-1alpha and tumor aggressiveness in non-small cell lung cancer. Oncol Rep. 2017;37:2611–2619.
- Chen X, Wu Q, You L, et al. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur J Pharmacol. 2017;795:150–159.
- Liu S, Gu X, Zhu L, et al. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine (Baltimore). 2016;95:e5479.
- Cata JP, Singh V, Lee BM, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33:317–323.
- Lavon H, Matzner P, Benbenishty A, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120:188–196.
- Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–1339.
- Forget P, Collet V, Lavandʼhomme P, et al. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur J Anaesthesiol. 2010;27:233–240.
- Wagner PL, Austin F, Zenati M, et al. Oncologic risk stratification following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendiceal carcinomatosis. Ann Surg Oncol. 2016;23:1587–1593.
- Owusu-Agyemang P, Zavala AM, Williams UU, et al. Assessing the impact of perioperative blood transfusions on the survival of adults undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal carcinomatosis. Vox Sang. 2017;112:567–577.
- Bong TSH, Tan GHC, Chia C, et al. Preoperative platelet-lymphocyte ratio is an independent prognostic marker and superior to carcinoembryonic antigen in colorectal peritoneal carcinomatosis patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Clin Oncol. 2017;22:511–518.
- Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14:1807–1817.
