Abstract
Benign thyroid nodules are a common clinical occurrence and usually do not require treatment unless symptomatic. During the last years, ultrasound-guided minimally invasive treatments (MIT) gained an increasing role in the management of nodules causing local symptoms. In February 2018, the Italian MIT Thyroid Group was founded to create a permanent cooperation between Italian and international physicians dedicated to clinical research and assistance on MIT for thyroid nodules. The group drafted this list of statements based on literature review and consensus opinion of interdisciplinary experts to facilitate the diffusion and the appropriate use of MIT of thyroid nodules in clinical practice. (#1) Predominantly cystic/cystic symptomatic nodules should first undergo US-guided aspiration; ethanol injection should be performed if relapsing (level of evidence [LoE]: ethanol is superior to simple aspiration = 2); (#2) In symptomatic cystic nodules, thermal ablation is an option when symptoms persist after ethanol ablation (LoE = 4); (#3) Double cytological benignity confirmation is needed before thermal ablation (LoE = 2); (#4) Single cytological sample is adequate in ultrasound low risk (EU-TIRADS ≤3) and in autonomously functioning nodules (LoE = 2); (#5) Thermal ablation may be proposed as first-line treatment for solid, symptomatic, nonfunctioning, benign nodules (LoE = 2); (#6) Thermal ablation may be used for dominant lesions in nonfunctioning multinodular goiter in patients refusing/not eligible for surgery (LoE = 5); (#7) Clinical and ultrasound follow-up is appropriate after thermal ablation (LoE = 2); (#8) Nodule re-treatment can be considered when symptoms relapse or partially resolve (LoE = 2); (#9) In case of nodule regrowth, a new cytological assessment is suggested before second ablation (LoE = 5); (#10) Thermal ablation is an option for autonomously functioning nodules in patients refusing/not eligible for radioiodine or surgery (LoE = 2); (#11) Small autonomously functioning nodules can be treated with thermal ablation when thyroid tissue sparing is a priority and ≥80% nodule volume ablation is expected (LoE = 3).
Introduction
Benign thyroid nodules are a common occurrence in the general population and usually do not require treatment unless symptomatic [Citation1]. In this case, patients generally report local compressive symptoms or esthetic concerns, due to the enlarging size of the nodule. Treatment options currently include open or minimally invasive surgery, aspiration with or without ethanol injection for cystic nodules, and thermal ablations. In autonomously functioning thyroid nodules (AFTNs), thyroid function is also affected and in these cases, pharmacological therapy and/or radioiodine ablation are also traditionally indicated [Citation2].
Over the last years, ultrasound (US)-guided minimally invasive treatment (MIT) (including chemical ablation and energy-based ablations) gained an increasing role in the management of benign thyroid nodules [Citation3]. They have proven efficacy and safety for the management of compressive symptoms and the improvement of esthetic concerns [Citation4–9] and are also demonstrating a promising role in the treatment of selected AFTNs [Citation10–14].
In February 2018, a meeting involving different specialists with specific expertise in the use of MIT for thyroid nodules was held in Milan, Italy [Citation15]. It included dedicated radiologists, endocrinologists, nuclear medicine physicians, pathologists, and surgeons who founded the Italian MIT Thyroid (MITT) Group. This interdisciplinary group had the purpose of creating a network between Italian and international physicians who are dedicated to clinical research and care in the field of MIT of thyroid nodules [Citation15].
The purpose of this document drafted by the Italian MITT Group was to provide a series of statements based on literature review and consensus opinion of interdisciplinary experts to facilitate both the diffusion and the appropriate use of MIT for the management of thyroid nodules in clinical practice.
Materials and methods
Expert selection
For this consensus, an interdisciplinary board of 29 physicians with specific expertise in the management of thyroid nodules was appointed by the Italian MITT Group for their proven experience in clinical practice and research on the topic. The panel included radiologists, endocrinologists, nuclear medicine physicians, pathologists, and surgeons.
Literature search and evaluation
After a thorough review of current literature, two authors (GM and LMS) drafted a list of statements addressing the most relevant questions on the use of MIT for thyroid nodules. For each statement, literature evidence was assessed, with a search on major online databases (MEDLINE, Web of Science, EMBASE, and Google, with search terms (thyroid ablation) OR (thyroid radiofrequency) OR (thyroid ethanol) OR (thyroid laser) OR (thyroid microwave) OR (thyroid high-intensity focused ultrasound) OR (thyroid radioiodine) OR (thyroid fine-needle aspiration and their expansions), and levels of evidence were assigned according to the criteria proposed by the Oxford Center of Evidence-Based Medicine in 2011 [Citation16].
Consensus process
The Delphi method was used for the consensus process on the clinical use of MITT. This system consists of a sequence of structured group rounds designed to survey the expert opinion on potentially controversial topics and to reach a final shared agreement [Citation17,Citation18]. Thus, in this process, each participant was asked to agree, disagree, or abstain with the proposed statements and the contributors had also the opportunity to provide comments on each topic to appropriately improve the statements. Finally, the AGREE II instrument (a specific tool for assessing the quality and reporting of practice guidelines) was used to verify the minimum quality requirements of the produced document [Citation19,Citation20]. An electronic questionnaire was built on the Google Forms platform (Google LLC, CA) and was disseminated to all the participants.
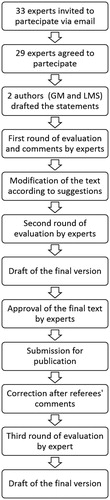
Two Delphi rounds were initially performed and a final document was prepared to be submitted for publication. After the manuscript was sent back for revisions, the referees’ comments were evaluated, the text was modified accordingly, and a third Delphi round was performed. The detailed consensus process is represented in .
Statistical analysis
For each statement, the consensus opinion was established at the end of the Delphi process. The consensus was considered as strong when >95% participants agreed, while it was considered as broad when >80% but less than 95% agreed on each statement [Citation21].
The paper was then prepared based on the results of the Delphi process and circulated via email among all participants for its final approval.
Statements
Statement #1. Predominantly cystic (51–90% of fluid component) or cystic (>90% of fluid component) thyroid nodules that become symptomatic should first undergo US-guided percutaneous aspiration for both diagnostic and therapeutic purposes, and image-guided ethanol ablation in case of relapse
Summary of evidence. Predominantly cystic or purely cystic thyroid nodules are a common clinical finding that does not require treatment unless symptomatic. In case of compressive symptoms or cosmetic concerns, the use of MIT can provide a clinically significant benefit. Simple aspiration, US-guided ethanol ablation, and thermal ablation have been proposed for the management of cystic nodules. Current evidence demonstrates that the outcomes of ethanol ablation are superior to both conservative treatment [Citation22] and simple aspiration [Citation4,Citation23–25], while are nearly equivalent to thermal ablation [Citation26,Citation27], a procedure that is more complicated and expensive. Thus, US-guided ethanol ablation is the most appropriate procedure for relapsing cysts in terms of efficacy, invasiveness, rapidity, and costs [Citation28].
Level of evidence: 2
Level of agreement: strong (agree, n = 28; abstain, n = 1; disagree, n = 0)
Statement #2. In symptomatic predominantly cystic thyroid nodules, image-guided thermal ablation should be considered as an option when local symptoms persist after ethanol ablation.
Summary of evidence. A similar efficacy of ethanol ablation and thermal ablation has been reported in the treatment of predominantly cystic thyroid nodules [Citation26,Citation27]. However, thermal ablation should be considered as a further therapeutic option in cystic lesions that relapse after ethanol ablation or that would remain symptomatic due to the residual solid component [Citation29–31]. Thus, the use of thermal ablation as a second line of treatment seems to be appropriate in selected patients with symptoms persistence after ethanol ablation.
Level of evidence: 4
Level of agreement: strong (agree, n = 28; abstain, n = 1; disagree, n = 0)
Statement #3. A double cytological confirmation of benignity should be obtained prior to image-guided thermal ablation of thyroid nodules.
Summary of evidence. Generally, a double cytological confirmation should be obtained in thyroid nodules prior to thermal ablation to rule out the risk of missing malignancy [Citation28,Citation32]. A low rate (<3%) of false negative results has been reported after a single fine needle aspiration, so generally, a repeated aspiration after benign report is suggested, unless US appearance of low risk of malignancy. However, as concerns remain regarding a possible higher rate of false negative results, particularly in large nodules, double aspiration is suggested before image-guided thermal ablation of a thyroid nodule [Citation33–35].
Level of evidence: 2
Level of agreement: strong (agree, n = 29; abstain, n = 0; disagree, n = 0)
Statement # 4. A single cytological sample can be considered adequate to confirm nodule benignity if the US appearance is at low risk of malignancy (EU-TIRADS ≤3) and in AFTN.
Summary of evidence. Risk stratification at US is a crucial predictive factor for thyroid nodule management. In this setting, the EU-TIRADS system has been proven to be a valuable tool. Thyroid nodules classified as EU-TIRADS 3 have been proven to have a low risk of malignancy, which ranges between 2% and 4% [Citation36,Citation37]. In nodules that demonstrate both cytological and US low-risk features, the risk of a false negative result at cytology is definitely low, probably ranging from 1% to 3%. Similarly, the risk of malignancy in AFTNs is low in the adult population [Citation38]. Therefore, in these low-risk nodules, a single negative cytological examination may be sufficient to candidate patients for thermal ablation [Citation39–42]. Conversely, MIT management of AFTNs with TIR 3 A cytology is still unsettled.
Level of evidence: 2
Level of agreement: strong (agree, n = 29; abstain, n = 0; disagree, n = 0)
Statement #5. Thermal ablation may be proposed as a first-line treatment for solid nonfunctioning thyroid nodules that are benign at cytology when they become symptomatic.
Summary of evidence. Traditionally, open thyroidectomy is the standard of care for benign thyroid nodules presenting with cosmetic and/or compressive issues, even though benign at cytology [Citation43]. Limitations of surgery are the frequent need for replacement therapy, the risk of complications, and the elevated costs [Citation28]. Thermal ablation is an effective outpatient procedure, with high technical feasibility [Citation6], elevated clinical success rate [Citation7,Citation8], low frequency of minor (0.5–4%) [Citation7,Citation9] and major complications (less than 3.9%) [Citation6], and outcomes sustained up to five years [Citation7,Citation44–47]. A few comparative studies between thermal ablation and open thyroidectomy showed that the former resulted in shorter operation time, no need of hospitalization, and absence of post-treatment scar [Citation48], less complications and side effects, no functional changes, higher patients’ satisfaction [Citation48–53] and, as a whole, lower direct and long-term costs.
Level of evidence: 2
Level of agreement: strong (agree, n = 28; abstain, n = 0; disagree, n = 1)
Statement #6. Thermal ablation may be proposed as a treatment for nonfunctioning benign multinodular goiter only in patients who refuse or who cannot undergo surgery.
Summary of evidence. Currently, a single paper reported about the use of thermal ablation in multinodular goiter, showing that sequential treatment in the same session is effective and safe even if with longer procedure time and less tolerable pain in the day after treatment [Citation54]. Due to the limited evidence and the burdensome modality of treatment, thermal ablation should be considered as a possible therapeutic option only in patients with well-defined dominant nodules who refuse or cannot undergo surgery [Citation4].
Level of evidence: 5
Level of agreement: strong (agree, n = 28; abstain, n = 0; disagree, n = 1)
Statement #7. Thyroid nodules treated with thermal ablation should be routinely followed-up with clinical and US examination.
Summary of evidence. US is the most reliable method to evaluate thyroid nodules, both in the pretreatment setting and after thermal ablation [Citation7,Citation9,Citation45,Citation55,Citation56]. Contrast-enhanced US provides a clear representation of parenchymal vascularization immediately after thermal ablation, when the presence of gas bubbles generated by ablation may impair the assessment of the treated area [Citation57,Citation58]. During follow-up, simple B-mode US nodule evaluation is generally not adequate to distinguish between the still vital part of the nodule and the area of ablated tissue. So, the use of other modalities such as color-Doppler or contrast-enhanced US may be considered for the detection of the still vital part of the treated nodule [Citation4,Citation52,Citation59].
Level of evidence: 2
Level of agreement: strong (agree, n = 27; abstain, n = 1; disagree, n = 1)
Statement #8. In case of incomplete symptom resolution, symptom relapse, or nodule regrowth, a re-treatment with thermal ablation may be considered.
Summary of evidence. The clinical purpose of thermal ablation is the improvement of local symptoms and signs due to thyroid nodule pressure. If symptoms persist after initial therapy [Citation60–62], an additional treatment may be performed, which usually results in symptom improvement. Re-treatment after thermal ablation is reported as required in a minority of cases [Citation60,Citation63,Citation64]. The proper timing of re-treatment is presently unsettled.
Level of evidence: 2
Level of agreement: strong (agree, n = 29; abstain, n = 0; disagree, n = 0)
Statement # 9. In case of nodule regrowth, a new cytological assessment is suggested before the second ablation
Summary of evidence. No papers specifically addressed the risk of malignancy in nodules regrowing after image-guided thermal ablation. However, as nodule regrowth (defined as ≥50% nodule volume increase compared to the minimum recorded volume during whatever previous follow-up time point [Citation3]), maybe a potential sign of malignancy a further cytological assessment is suggested before re-treatment.
Level of evidence: 5
Level of agreement: strong (agree, n = 29; abstain, n = 0; disagree, n = 0)
Statement #10. Thermal ablation may be proposed as a treatment option for AFTN in patients who refuse or cannot undergo traditional treatments with radioiodine or surgery.
Summary of evidence: Thermal ablation treatment of AFTN has been reported to result in volume reduction, symptom amelioration, and improvement or normalization of hyperthyroidism [Citation10–12,Citation47]. However, the success rate of thermal ablation in AFTN appears variable and is influenced by the baseline volume and severity of hyperfunction [Citation13]. Thus, thermal ablation can be proposed as an alternative treatment only in patients with large AFTN or overt hyperthyroidism who refuse or who cannot undergo standard treatment (i.e., radioiodine or surgery) [Citation11,Citation14,Citation28].
Level of evidence: 2
Level of agreement: strong (agree, n = 27; abstain, n = 1; disagree, n = 1)
Statement #11. Small size AFTN can be treated with thermal ablation when the preservation of normal thyroid tissue function is a priority and it is reasonable to expect at least 80% nodule volume ablation.
Summary of evidence. In case of AFTN, the higher the ablated volume, the better the functional outcome. Patients with AFTN are reported to have a higher probability of symptom resolution and normalization of thyroid function after thermal ablation if the treatment results in the ablation of at least 80% of nodule volume [Citation12,Citation14,Citation65–67]. Thus, a favorable outcome may generally be expected in small AFTN [Citation66], while in larger nodules a combined treatment with thermal ablation and radioiodine may be planned, as it is reported resulting in a more rapid volume reduction and in a lower irradiation than with radioiodine alone [Citation68]. Due to the lack of changes in the function of the surrounding parenchyma and the absence of irradiation, thermal ablation is specifically appealing in young patients or patients with subclinical hyperthyroidism and incomplete suppression of normal thyroid tissue [Citation69].
Level of evidence: 3
Level of agreement: strong (agree, n = 27; abstain, n = 1; disagree, n = 1)
Discussion
This paper represents the first consensus statement of the Italian MITT Group regarding the MIT of benign thyroid nodules. For all statements, strong agreement among the members of the Group was achieved after the third round of Delphi process.
Both a robust literature evidence and an ample consensus among the experts indicated ethanol ablation as the first treatment modality in case of relapse of cystic nodules after the aspiration, due to its low-cost, rapidity, and safety. So, thermal ablation should be reserved only to prevalently cystic lesions that are not controlled by a prior treatment with ethanol ablation.
Strong agreement was found among the participants about the need of two consistently benign cytological samples prior to undergo MIT, as a general rule. Similarly to what reported in the Korean guideline [Citation38], we evaluated the opportunity of considering as sufficient a single benign cytological report. This issue was extensively discussed and the initial statement was modified, as most experts recommended a second cytological sampling regardless the US appearance of nodules. Thus, even though at the end of the process a satisfactory agreement was achieved, we point out the necessity of controlled studies to better clarify this relevant issue due to the weakness of the available evidence.
Regarding the proposal of MIT as the first treatment option for benign thyroid nodules, a robust evidence demonstrates clinically significant and sustained results in most cases with a very low complication rate. Even though well conducted comparative studies on the direct and indirect costs of the two therapeutic approaches are limited, thermal ablation has the advantage of an outpatient procedure and lower expenses in comparison with surgery when a single application is needed. Thus, there is a strong consensus that MIT should be considered as a first-line treatment for symptomatic solid nonfunctioning benign thyroid nodules that become symptomatic. So, at variance with former recommendations, MIT should not be reserved only for patients refusing or who are not suitable for surgery. According to all experts, MIT represents only a second-line therapeutic option in multi-nodular goiter and should be reserved only to patients who are not suitable for surgery.
Regarding follow-up after thermal ablation, there was strong agreement on the crucial role of ultrasound, which should be considered as the imaging method of choice. Other modalities such as color Doppler or contrast-enhanced ultrasound offer complementary information for better differentiation of the ablated area from the still vital part of the nodule tissue, but the evidence is still not robust enough to recommend their routine use in clinical practice.
Strong agreement was achieved on the opportunity of a repeated treatment for benign thyroid nodules in case of incomplete symptoms resolution, or if symptoms relapse after thermal ablation. Presently, there is a lack of studies specifically addressing the issue of the risk of malignancy in nodules that re-growth over time after thermal ablation. In these cases, however, a strong agreement was achieved on the opportunity of performing a further cytological assessment before a second thermal ablation to rule out the possibility of an underlying malignancy.
The appropriateness of the use of MIT for the management of ATFN has been one of the most debated topics during the Delphi consensus. Although evidence on the topic is fair, the clinical role of MITT in clinical practice is still argued. Specifically, the potential advantages and disadvantages of MITT versus radioiodine ablation are still not well established and further controlled studies on this topic are warranted to better define the appropriate role of MIT in AFTN. Thus, at present, MIT should be proposed as a treatment option only in patients who refuse or cannot undergo traditional treatments (i.e., radioiodine ablation or surgery). A strong agreement, however, was reached on the use of thermal ablation for small AFTN in which the majority of nodule volume (at least 80%) may be expected to be ablated, as the available data demonstrate satisfactory results in small size lesions. Notably, a complete consensus was reached about the preferential use of thermal ablation in young patients or patients with subclinical hyperthyroidism and incomplete suppression of normal thyroid tissue in order to spare the function of surrounding normal thyroid tissue.
In conclusion, this consensus document is based on the assessment of the best available evidence and on the combined opinion of an interdisciplinary group of specialists with specific interest in thyroid nodules and their minimally-invasive management. After this first proposal for the appropriate use of MIT in clinical practice, future updates are warranted on the basis of the improvement of the available evidence.
Disclosure statement
No participant declared competing or conflicting interests regarding the development of the present statements.
References
- Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707–735.
- Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. Forthcoming 2019.
- Papini E, Gugliemi R, Pacella CM. Laser, radiofrequency, and ethanol ablation for the management of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2016;23:400–406.
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015;18:423–430.
- Pacella CM. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:347–349.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167.
- Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol. 2005;152:341–345.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Spiezia S, Vitale G, Di Somma C, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003;13:941–947.
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25:112–117.
- Bernardi S, Stacul F, Michelli A, et al. 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine. 2017;57:402–408.
- Døssing H, Bennedbaek FN, Bonnema SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157:95–100.
- Pacella CM, Mauri G. Is there a role for minimally invasive thermal ablations in the treatment of autonomously functioning thyroid nodules? Int J Hyperth. 2018;34:636–638.
- Mauri G, Pacella CM, Papini E, et al. Proceedings of the first Italian conference on thyroid minimally invasive treatments and foundation of the Italian research group for thyroid minimally invasive procedures. Int J Hyperth. 2018;34:603–605.
- Oxford Center of Evidence Based Medicine. OCEBM Levels of Evidence 2016 v. 2.1. 2016 [cited 2019 Feb 4]. https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf.
- Steurer J. The Delphi method: an efficient procedure to generate knowledge. Skeletal Radiol. 2011;40:959–961.
- Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;28:5338–5351.
- Messina C, Bignotti B, Tagliafico A, et al. A critical appraisal of the quality of adult musculoskeletal ultrasound guidelines using the AGREE II tool: an EuroAIM initiative. Insights Imaging. 2017;8:491–497.
- Brouwers M, Kho M, Browman G, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2001;182:839–842.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44:14–36.
- Ferreira MC, Piaia C, Cadore AC. Percutaneous ethanol injection versus conservative treatment for benign cystic and mixed thyroid nodules. Arch Endocrinol Metab. 2016;60:211–216.
- Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004;10:269–275.
- Verde G, Paplni E, Pacella CM, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clin Endocrinol. 1994;41:719–724.
- Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777.
- Baek JH, Ha EJ, Choi YJ, et al. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015;16:1332.
- Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269:293–300.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules–2016 Update. Endocr Pr. 2016;22:622–639.
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81:905–910.
- Lee JH, Kim YS, Lee D, et al. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010;34:1488–1493.
- Døssing H, Bennedbaek FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules - a prospective randomized trial. J Clin Endocrinol Metab. 2013;98:E1213–E1217.
- Pacini F, Basolo F, Bellantone R, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. 2018;41:849–876.
- Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27:1341–1346.
- Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141:1089–1095.
- Shi H, Bobanga I, McHenry CR. Are large thyroid nodules classified as benign on fine needle aspiration more likely to harbor cancer? Am J Surg. 2017;213:464–466.
- Russ G, Bonnema SJ, Erdogan MF, et al. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–237.
- Persichetti A, Di Stasio E, Guglielmi R, et al. Predictive value of malignancy of thyroid nodule ultrasound classification systems: a prospective study. J Clin Endocrinol Metab. 2018;103:1359–1368.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19:632–655.
- Kim JY, Jung SL, Kim MK, et al. Differentiation of benign and malignant thyroid nodules based on the proportion of sponge-like areas on ultrasonography: imaging-pathologic correlation. Ultrasonography. 2015;34:304–311.
- Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207–213.
- Moon W-J, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation-multicenter retrospective study. Radiology. 2008;247:762–770.
- Hong MJ, Na DG, Baek JH, et al. Cytology-ultrasonography risk-stratification scoring system based on fine-needle aspiration cytology and the Korean-thyroid imaging reporting and data system. Thyroid. 2017;27:953–959.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20:1253–1261.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99:3653–3659.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100:460–466.
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34:784–791.
- Zhi X, Zhao N, Liu Y, et al. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34:644–652.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:1–10.
- Bernardi S, Dobrinja C, Carere A, et al. Patient satisfaction after thyroid RFA versus surgery for benign thyroid nodules: a telephone survey. Int J Hyperth. 2018;35:150–158.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Cervelli R, Mazzeo S, De Napoli L, et al. Radiofrequency ablation in the treatment of benign thyroid nodules: an efficient and safe alternative to surgery. J Vasc Interv Radiol. 2017;28:1400–1408.
- Yan J, Qiu T, Lu J, et al. Microwave ablation induces a lower systemic stress response in patients than open surgery for treatment of benign thyroid nodules. Int J Hyperthermia. 2018;34:606–610.
- Lang BHH, Woo YC, Chiu KWH. Sequential high intensity focused ultrasound (HIFU) ablation in the treatment of benign multinodular goitre: an observational retrospective study. Eur Radiol. 2018;28:3237–3244.
- Mauri G, Sconfienza LM. Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperth. 2017;33:301–302.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperth. 2017;33:295–299.
- Papini E, Pacella CM, Misischi I, et al. The advent of ultrasound-guided ablation techniques in nodular thyroid disease: towards a patient-tailored approach. Best Pract Res Clin Endocrinol Metab. 2014;28:601–618.
- Ma S, Zhou P, Wu X, et al. Detection of the single-session complete ablation rate by contrast-enhanced ultrasound during ultrasound-guided laser ablation for benign thyroid nodules: a prospective study. Biomed Res Int. 2016;2016:956536.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session—prospective randomized study. Radiology. 2012;263:909–916.
- Zhao CK, Xu HX, Lu F, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. Clin Hemorheol Microcirc. 2017;65:393–405.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperth. 2017;33:905–910.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Døssing H, Bennedbaek FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165:123–128.
- Amabile G, Rotondi M, Pirali B, et al. Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med. 2011;43:797–803.
- Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperth. 2018;34:617–623.
- Gambelunghe G, Stefanetti E, Colella R, et al. A single session of laser ablation for toxic thyroid nodules: three-year follow-up results. Int J Hyperthermia. 2018;34:631–635.
- Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab. 2014;99:E1283–E1286.
- Døssing H, Bennedbaek FN, Hegedüs L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid. 2003;13:885–888.