Abstract
Background: FOLFIRINOX chemotherapy displays significant survival improvements in patients with pancreatic cancer. However, toxicities have hampered enthusiasm for the use of FOLFIRINOX in full dose. In order to increase the tolerability, many researchers focused on the modification of FOLFIRINOX. On the other hand, hyperthermia (HT) has been considered as an effective ancillary treatment for cancer therapy. Up to now, there is no report evaluating combining deep regional hyperthermia (DRHT) with modified-FOLFIRINOX for pancreatic cancer patients.
Methods: In this study, we conducted a retrospective review of pancreatic cancer patients treated with the combination of new form modified-FOLFIRINOX and DRHT (BSD2000). Patients underwent chemotherapy that included low-dose irinotecan (70–130 mg/m2), oxaliplatin (65–70 mg/m2) on day 1 and 5-FU (2400 mg/m2 as a 46 h continuous infusion, no bolus) or capecitabine (CAP) (1000 mg/m2 twice daily on days 1–10) or tegafur, gimeracil and oteracil potassium (TS-1) (80–120 mg/d twice daily on days 1–10), 2-week schedule. Generally, DRHT treatment was performed weekly, 45 min for each time during chemotherapy.
Results: The patients receiving mFOLFIRINOX as the first line chemotherapy combining with DRHT, obtained an improvement in OS and PFS, 17 months (95% CI 1.97–32.03 months) and 4 months (95% CI 0–8.29 months) respectively. Overall, this combination regimen was safe; 17.6% patients suffered from grade 3/4 toxicities.
Conclusions: In conclusion, we conducted a retrospective study combining mFOLFIRINOX and DRHT, which was well tolerated. The efficacy in the treatment of pancreatic cancer was encouraging, but further studies would be required to prove its merit, compared with conventional treatment.
Introduction
Pancreatic cancer remains the fourth leading cause of cancer-related death over the world in 2015 [Citation1], with a 5-year survival rate of 1–5% [Citation2]. In 2015, there are approximately 90,100 new pancreatic cancer patients in China, with an estimated 79,400 deaths [Citation3,Citation4]. Of all cancers, pancreatic cancer is ranked 10th in incidence and mortality is ranked 7th [Citation3]. Because of lateness in diagnosis, and the biology of pancreas cancer, only 10–20% pancreatic cancer patients present with resectable disease [Citation5]. For most pancreatic cancer patients, especially for those with locally advanced or metastatic pancreatic cancer, systemic chemotherapy is the main choice of treatment [Citation6,Citation7].
The standard treatment for pancreatic cancer has evolved over the last few decades, moving away from 5-fluorouracil (5-FU) or 5-FU combination chemotherapy [Citation8,Citation9] to gemcitabine (Gem) or Gem combination chemotherapy [Citation10]. 5-FU has been studied extensively by altering different doses and schedules. However, its response rate rarely exceeds 20% [Citation11]. 5-FU in combination with other drugs offers little improvement, in fact, more toxicities over single-agent 5-FU [Citation11–13]. In 1997, a randomize controlled trial (RCT) demonstrated that Gem was more effective than 5-FU in treating pancreatic cancer, with better median survival (5.6 vs. 4.4 months; p = .0025) [Citation11]. Subsequently, a series of studies focused on Gem combination treatment to prolong the survival time of pancreatic cancer patients [Citation14–25]. Gem and Gem combination regimens have been the standard treatment of locally advanced or metastatic pancreatic cancer for over a decade [Citation14–25]. Despite the improvements, overall survival (OS) time has not been satisfactory; median OS ranges from 5.0 to 10.1 months [Citation18,Citation25–27]. Recently, attention has been focused onto the more complex regimen, FOLFIRINOX. The synergism between irinotecan, oxaliplatin and 5-FU has formed the FOLFIRINOX regimen, which was initially prescribed in metastatic colorectal cancer, displaying significant improvements in OS (11.1 vs. 6.8 months), progression-free survival (PFS) (6.4 vs. 3.3 months) and response rate (31.6% vs. 9.4%) comparing to Gem [Citation28] in treating pancreatic cancer.
The survival gain came at a cost, however. Toxicities have hampered enthusiasm for the use of FOLFIRINOX in full dose. Many studies tried to reduce this regimen toxicity without compromising efficacy [Citation29–33]. Stein et al. reduced the dose of FOLFIRINOX with irinotecan (85%) and bolus 5-FU (85%). This attempt decreased the neutropenia, vomiting and fatigue to a great extent, meanwhile maintained the efficacy [Citation31]. Gunturu et al. also tried to decrease the dose of the FOLFIRINOX and indeed obtained a satisfactory result [Citation30]. Mahaseth et al. published his research that during modified-FOLFIRINOX (no 5-FU bolus) regimen, providing growth factors to all patients could improve the safety and guarantee the efficacy [Citation29]. There are few reports about modified-FOLFIRINOX treating Chinese patients. One institution in China evaluated modified-FOLFIRINOX (75% irinotecan, 85% oxaliplatin and no 5-FU bolus) in Chinese metastatic pancreatic cancer patients [Citation4], which indeed provided a better tolerance with similar efficacy to FOLFIRINOX.
On the other hand, regional therapeutic hyperthermia (HT) has been considered as an effective ancillary treatment for cancer therapy, especially in the treatment of head and neck tumors, cervix cancer, breast cancer, melanoma and glioblastoma [Citation34–39]. HT uses high-frequency electromagnetic waves to heat tumor cells to 41–45 °C, which can alter the metabolism and the surrounding microenvironment of the tumor cells. Besides that, HT can facilitate the delivery and diffusion of chemotherapeutics by improving blood flow and at the same time strengthen the drug cytotoxicity to tumor cells [Citation40,Citation41]. Thus, HT combining with chemotherapy would enhance the efficacy of chemotherapy.
In this study, we conducted a retrospective review of pancreatic cancer patients treated with the combination of new form modified-FOLFIRINOX (mFOLFIRINOX) (irinotecan, oxaliplatin, 5-FU/5-FU analog) and deep regional hyperthermia (DRHT) in the Second Affiliated Hospital of Dalian Medical University, in order to assess the efficacy and the toxicity of this combination treatment. As far as we know, this is the first report evaluating combining DRHT with new form modified-FOLFIRINOX for pancreatic cancer patients [Citation42]. We hope this report can benefit more pancreatic cancer patients.
Materials and methods
Patients
This report is a retrospective study involving locally advanced, metastatic and postoperative pancreatic cancer patients receiving modified-FOLFIRINOX (irinotecan, oxaliplatin and 5-FU/5-FU analog) and DRHT from January 2014 to February 2018 at the Department of Oncology, the Second Affiliated Hospital, Dalian Medical University. Patients who were histologically or cytologically diagnosed with pancreatic cancer prescribing modified-FOLFIRINOX (mFOLFIRINOX), with or without prior treatments, were eligible for inclusion. Written informed consent was obtained from each patient’s family for the publication of this study. Before each cycle of mFOLIRINOX regimen, patients were evaluated regularly to make sure they were able to tolerate the treatment (Eastern Cooperative Oncology Group Performance Status Scale ≤1, granulocyte count ≥1500 per cubic millimeter, hemoglobin count ≥80 g/L, platelet count ≥80,000 per cubic millimeter, bilirubin ≤1.5 times the upper limit of the normal range, and creatinine ≤the upper limit of the normal range).
Chemotherapy
Full dose FOLFIRINOX consists of oxaliplatin (85 mg/m2), followed by irinotecan (180 mg/m2) and leucovorin (400 mg/m2), followed by 5-FU (400 mg/m2) as a bolus and a 46 h continuous infusion (2400 mg/m2), 2-week schedule. In view of racial differences, Western people’s experience may be not suitable for Chinese patients. The FOLFIRINOX regimen needs to be modified to get a better outcome while attracting more Chinese pancreatic cancer patients to receive this therapy. In our study, the FOLFIRINOX regimen was modified in all patients starting with the first cycle. Dose modifications were made at the treating physician’s discretion, mainly based on patients’ performance status. During the treatment process, we modulated the dose according to the status of patients as well. Patients underwent chemotherapy that included low-dose irinotecan (70–130 mg/m2), oxaliplatin (65–70 mg/m2) on day 1 and 5-FU (2400 mg/m2 as a 46 h continuous infusion, no bolus) or capecitabine (CAP) (1000 mg/m2 twice daily on days 1–10) or tegafur, gimeracil and oteracil potassium (TS-1) (80–120 mg/d twice daily on days 1–10), 2-week schedule. The treatment period could be prolonged depending on patient tolerance.
Patients routinely received palonosetron and dexamethasone for antiemetic prophylaxis. Before initiation of chemotherapy, patients were provided with antidiarrheal medications and were asked to administrate these medications at the first sign of diarrhea. When patients suffered from jaundice, biliary stenting or drainage procedures were performed. Chemotherapy would continue until disease progression, unacceptable toxicity or patients’ refusal.
Deep regional hyperthermia (DRHT)
DRHT integrated in multimodal approaches is recommended for patients with unresectable deep-seated tumors. DRHT was carried out according to European Society for Hyperthermic Oncology (ESHO) quality and safety assurance guidelines [Citation43,Citation44] using the annular phased-array system BSD-2000 (BSD Medical Corporation, Pyrexar Medical, Salt Lake City, UT) [Citation45]. The target area of HT was focused on the pancreas or metastatic lesion. The frequency and average output power were 75–120 MHz and 450–550 W, respectively. Based on ESHO protocols quality assurance guidelines, in terms of electromagnetic heating techniques, CT and/or MRI were applied to locate the target area. The amplitude and phase of each channel were adjusted to form a thermal field suitable for the specific tumor shape, which can enable a positioning of the tumor with an accuracy of 1 cm. This approach can reduce the damage to the surrounding normal tissue [Citation46].
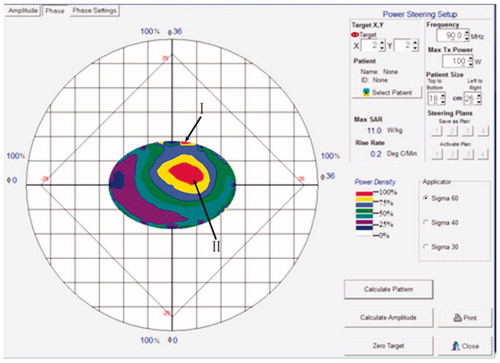
The temperature control is a key element influencing the efficacy and security of HT. As the interior temperature of tumor cannot be obtained directly and noninvasively, BSD2000 establishes a simulation system, giving the thermal mapping according to the parameters of physical characteristic, location and size of tumor, heating frequency, and output power. A typical thermal mapping is shown in . The energy densities of different areas are indicated by colors, which can reflect the temperature change of different zones under HT treatment. With the help of unique technique and based on great amount of experiments, BSD2000 achieves that the temperature changes on the projection point (I) and in the tumor area (II) are approximately the same. On the other hand, the temperature of point (I) is measured directly by a noninvasive thermometry subsystem through a probe attached on the skin. Through this measured temperature, we could get the temperature change in area II. The temperature feedback is collected by a computer and can be modulated by the output power so that the tumor temperature is kept at 41–43 °C.
A water bag is used to protect the skin from overheating. Generally, the HT treatment was performed weekly, on the second and ninth day of the chemotherapy regimen, 45 min for each time during the treatment of mFOLFIRINOX regimen. The temperature profile for each HT treatment was collected, based on which an average maximum temperature and the temperatures achieved in 20, 50 and 90% (T20, T50 and T90, respectively) time of all treatments were calculated. Patients were carefully instructed to report any discomfort during treatment. HT treatment was stopped when patients suffered from unacceptable adverse events, or patients refused to receive any more treatment.
Assessment
Imaging studies (CT and/or MRI) were checked at baseline and every 8 weeks to assess the tumor burden variation. All scans were systematically reviewed by the investigators for response by RECIST (complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD)). Two physicians reviewed the results of imaging studies independently and compared the results with official radiology reports. Images were included in response analysis if patients had received at least two cycles of mFOLFIRINOX regimen prior to image acquisition. Adverse events were graded using National Cancer Institute Common Toxicity Criteria version 4.0 (Rockville, MD). PFS was defined from the start of therapy until the date of first documented progression. OS was calculated from initial time of therapy until date of death or loss to follow-up. Data collection was stopped in February 2018.
Statistical analysis
Survival was calculated by the Kaplan–Meier method. All calculations and survival displays were conducted using the SPSS version 21.0 statistical software package (SPSS, Chicago, IL) [Citation29].
Results
Patient characteristics
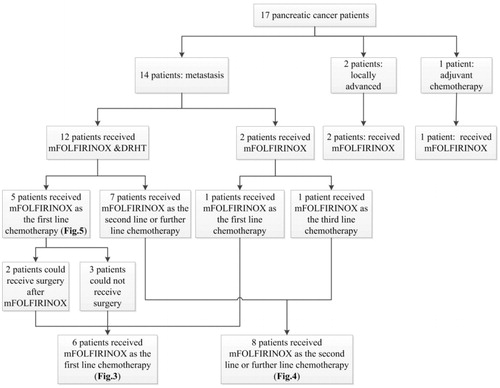
From January 2014 to February 2018, 17 patients, with locally advanced, metastatic and postoperative pancreatic cancer were treated with at least one period of mFOLFIRINOX. The baseline patient characteristics are shown in . The range of age was 46–74, with a mean age of 56. All the patients had an Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1. The primary tumor of pancreatic cancer located in the head, body, and tail of the pancreas in 7 (41.1%), 5 (29.4%) and 5 (29.4%) patients, respectively. The past treatments that these patients had are shown in CONSORT diagram (. Twelve patients received mFOLFIRINOX combined with DRHT, while the other five patients received only mFOLFIRINOX. Fourteen patients (82.3%) had metastatic disease and two patients had locally advanced pancreatic cancer at the beginning of mFOLFIRINOX regimen. Only one patient received mFOLFIRINOX as adjuvant chemotherapy. Of the 14 metastatic pancreatic cancer patients, 6 (42.8%) were first line of chemotherapy, 8 (57.1%) had two more further lines of chemotherapy. Seven (41.1%) patients received CAP and six (35.2%) patients prescribed TS-1, while, four (23.5%) patients received 5-FU during the treatment. Two patients received the percutaneous transhepatic cholangial drainage (PTCD) because of obstructive jaundice. The baseline laboratory testing outcomes, such as tumor biomarkers, hematological index and hepatorenal function are also listed in . Elevated CA19-9 levels were found in 15 patients (88.2%) before chemotherapy. Average maximum temperature was 41.8 °C (95% CI 40–43.2 °C). T20, T50 and T90 were 40.8 °C (95% CI 39–43 °C), 40.3 °C (95% CI 38.5–42.5 °C) and 39.2 °C (95% CI 37.5–40 °C) respectively.
Table 1. Patient characteristics at baseline.
Toxicity
Modified-FOLFIRINOX
The adverse events reported in this study are shown in . We observed three patients (17.6%) with grade 3/4 toxicities. Two patients who received combination treatment of chemotherapy and regional HT developed grade 3 neutropenia. One patient treated with chemotherapy alone developed grade 4 diarrhea. Three patients (17.6%) treated with the combination treatment stopped treatment after the first cycle when suffering grade 2/3 adverse events. For hematologic toxicities, 11 patients (8 with combination treatment and 3 with chemotherapy) required granulocyte-colony stimulating factor (G-CSF) treatment during chemotherapy. Moreover, 8 patients (6 with combination treatment and 2 with chemotherapy alone) received interleukin 11 therapy. No patients were treated with provided with hemopoietin. No other unexpected or severe toxicities, such as neutropenia or thromboembolism were observed in our study. Overall mFOLFIRINOX was safe.
Table 2. Adverse events.
Hyperthermia (HT)
Overall, grade 1/2 (mild) position-related pain during HT treatment was the main side effect. No patient gave up HT as a result of adverse events and there was no treatment-related death.
Efficacy
The median number of mFOLFIRINOX treatment cycles was 5 (range, 1–12 cycles). Fourteen patients received mFOLFIRINOX for at least two cycles, among which, 10 patients had SD, 3 patients got PD, and one was lost to follow up. The disease control rate (CR + PR + SD) was 76.9%.
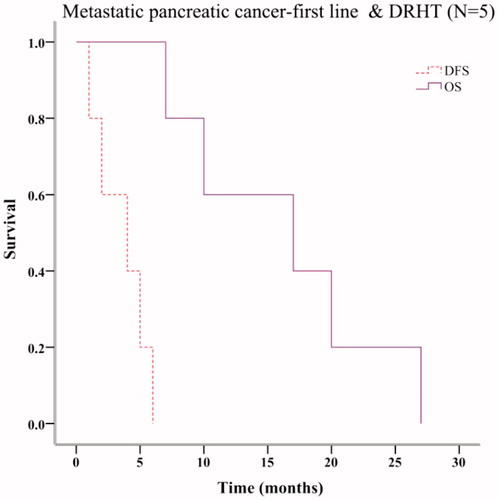
Seventeen patients, with locally advanced, metastatic and postoperative pancreatic cancer were treated with at least one cycle of mFOLFIRINOX. The past treatments that these patients received are shown in CONSORT diagram (. The corresponding relationship between these patients and the OS & PFS results (Kaplan–Meier plots in ) is also indicated in the CONSORT diagram (. Fourteen patients had metastatic pancreatic cancer. Six patients received mFOLFIRINOX as first line chemotherapy. Their median OS and PFS were 10 months (95% CI 0.00–22.00 months) and 2 months (95% CI 0.0–5.60 months), respectively (. Eight patients underwent mFOLFIRINOX as the second line or further line chemotherapy. Their median OS and PFS were 6 months (95% CI 1.08–10.92 months) and 1 month (95% CI not estimable), respectively (. Twelve metastatic patients underwent mFOLFIRINOX combined with DRHT. Five received mFOLFIRINOX as first line chemotherapy. Their OS and PFS were 17 months (95% CI 1.97–32.03 months) and 4 months (95% CI 0–8.29 months), respectively (. Two of the five patients were able to undergo resection after mFOLFIRINOX regimen. Actually, only one of the two patients chose to receive the surgery and indeed benefit from the excision.
Figure 3. Survival analysis of metastatic pancreatic cancer patients receiving mFOLFIRINOX as the first line chemotherapy.
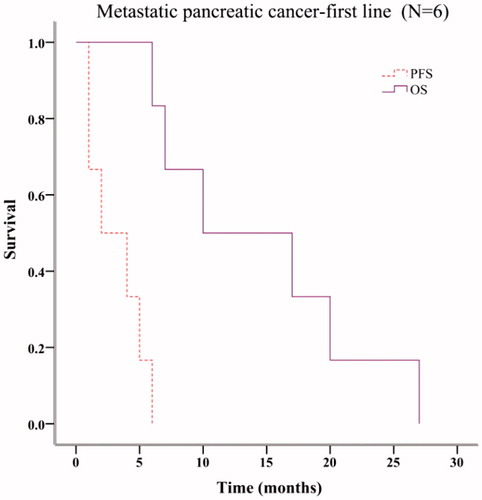
Figure 4. Survival analysis of metastatic pancreatic cancer patients receiving mFOLFIRINOX as the second or further line chemotherapy.
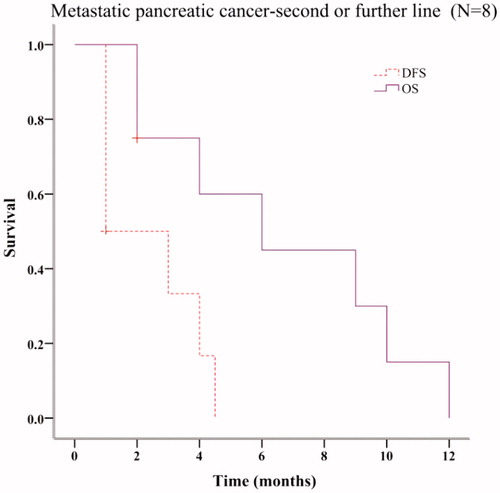
Figure 5. Survival analysis of metastatic pancreatic cancer patients receiving mFOLFIRINOX as the first line chemotherapy combining with deep regional hyperthermia.
For the two locally advanced pancreatic cancer patients, they were both alive in February 2018. Their survivals were 9 and 3 months, respectively. For the one patient receiving mFOLFIRINOX as adjuvant chemotherapy, tumor recurrence had not happened. As of the time of writing of this article, the survival of this patient was >4 years.
Discussion
Pancreatic cancer’s prognosis is extremely dismal. Therefore, multi-disciplinary therapy and selection of optimal treatment means are crucial for achieving prolonged survival. FOLFIRINOX chemotherapy can provide dramatically efficacy in locally advanced and metastatic pancreatic cancer patients with observably increased survival, compared to Gem monotherapy or combined treatment [Citation4,Citation25,Citation26].
The different mechanisms of the three drugs (irinotecan, oxaliplatin and 5-FU) and their nonoverlapping toxicities provided the rationale for regimen of FOLFIRINOX [Citation47], which has initially been used in several gastrointestinal malignancies. Irinotecan, a camptothecin analog, has been shown to have a higher growth inhibitory effect than cisplatin, mitomycin and 5-FU inpancreatic adenocarcinoma cells in vitro [Citation48]. SN-38, the main active metabolite of irinotecan, and oxaliplatin showed synergistic activity in vitro, delaying the reversion of oxaliplatin inducing DNA interstrand cross-links [Citation49]. Preclinical studies have indicated SN-38 sequentially diminishes DNA synthesis, inhibiting dUMP synthesis and enhancing efficacy of 5-FU when irinotecan precedes 5-FU/leucovorin [Citation50–52]. On the basis of these encouraging results, FOLFIRINOX has been used in pancreatic cancer treatment with promising results. However, many physicians hesitate to prescribe FOLFIRINOX for pancreatic cancer patients because of its considerable toxicity. The association of three drugs is reported with a higher rate of grade 3/4 adverse toxicities, compared with Gem, including hematologic toxicity, sensory neuropathy and digestive system toxicity, which may limit its applicability [Citation26]. Many investigators have tried to improve patients’ tolerance to this chemotherapy through decreasing the dose of the regimen and they indeed get some satisfactory outcomes [Citation29–33].
In this article, we applied a novel combination regimen, which united mFOLFIRINOX with DRHT. The dose of irinotecan was as low as 70–130 mg/m2, meanwhile, CAP or TS-1 were applied as alternatives of 5-FU. The pancreatic cancer patients receiving this regimen indeed benefited in both tolerability and efficacy. The metastatic pancreatic cancer patients who received mFOLFIRINOX as the first line chemotherapy obtained an OS of 17 months, which was longer than that reported by Corney’s (11.1 months) [Citation26]. Eight patients underwent mFOLFIRINOX as the second line or further line chemotherapy with median OS of 6 months. It was quite a remarkable result which can be compared with other studies’ outcomes of second line FOLFIRINOX chemotherapy [Citation53,Citation54].
Overall our modified regimen was well tolerated. Only 17.6% patients suffered grade 3/4 neutropenia and diarrhea. What is more, we observed no grade 3/4 fatigue, sensory neuropathy or vomiting. Compared with former studies [Citation29–33], our modification suggested a good tolerability. Combinations of DRHT with mFOLFIRINOX did not yield any additional toxicities over those yielded by FOLFIRINOX or mFOLFIRINOX regimen.
Low dose irinotecan and replacing 5-FU with oral drugs can significantly decrease adverse events. It is a recommended way to reduce the adverse events by decreasing the dose of regimen. By reducing the dose of irinotecan, diarrhea has been reduced, which was caused by SN-38, active form of irinotecan, accumulating on the intestinal epithelium. What is more, hematologic toxicities, hepatotoxicity and many other kinds of toxicities were also decreased, compared with low dose irinotecan. As a result, in our study, we observed good tolerability.
CAP and TS-1 are orally-administered chemotherapeutic agents [Citation55,Citation56], which can be well absorbed after oral ingestion and gradually converted to 5-FU in the body. Thus, they have widely been applied in multiple kinds of tumors treatment as alternatives of 5-FU. Meanwhile, the convenience of oral administration makes CAP and TS-1 attractive treatment options in various kinds of cancers [Citation18,Citation57]. Experiments have demonstrated that CAP and TS-1 can provide improved tolerability and similar efficacy of 5-FU in the treatment of pancreatic cancer [Citation17,Citation18,Citation27,Citation58–60]. Thus in this study, we replaced 5-FU with CAP or TS-1 in the regimen of FOLFIRINOX, increasing the convenience and tolerance to the chemotherapy, at the same time, maintaining efficacy.
Combining DRHT with mFOLFIRINOX can help to kill tumor cells and at the same time enhance the efficacy of chemotherapeutics. The regimen of mFOLFIRINOX consists of three different kinds of chemotherapy agents, oxaliplatin, irinotecan and 5-FU. Rietbroek et al. [Citation61] demonstrated that the efficacy of oxaliplatin was increased by 180% at 43 °C, in vitro. This occurred because of enhancement of platinum-DNA adduct formation in human lung cancer cells. Urano et al. [Citation62] showed that the thermal enhancement ratio of oxaliplatin increased with the rise in the temperature in mouse fibrosarcoma cells, in vitro. Kondo et al. [Citation63] showed that HT increased irinotecan induced DNA strand breaks in mouse mammary carcinoma FM3A cells. Katschinski et al. [Citation64] confirmed that HT can enhance the cytotoxicity of SN-38, the main active metabolite of irinotecan, through increased Topo I activity in human lung cancer cells.
A number of investigators have indicating that exposure time of 5-FU before the administration of HT is critical determinant of its cytotoxicity. Urano et al. [Citation65] and Monge [Citation66] demonstrated a weak relationship between 5-FU and HT when graded doses of 5-FU were given within 5 or 15 min before HT in mouse tumors. Takemoto et al. [Citation67] also reported that 5-FU has the smallest thermal enhancement ratio (1.1) in mice mammary carcinoma when the agents were given by intraperitoneal injection immediately before HT. Oppositely, when 5-FU was administered before HT for over 2 h, some positive results were observed. In vitro, Kido et al. [Citation68] showed that 48 h exposure to 5-FU caused Chinese hamster lung fibroblasts to accumulate in S-phase. Cells in this part of the cell cycle were shown to be more sensitive to HT. Mini et al. [Citation69] showed that cytotoxic effects would be enhanced when a longer exposure (4 and 8 h) to 5-FU followed heat (42 °C for 1 and 2 h). Heat exposure (42 °C for 1 and 2 h) induced a rapid decrease in the synthesis of DNA of human leukemia cells. In our study, 5-FU was either orally taken or intravenously injected over 2 h before HT. According to the above references, we presumed that HT could help to increase the cytotoxicity of 5-FU.
The above research results basically indicated the enhanced cytotoxicities of oxaliplatin, irinotecan and 5-FU by HT. This positive effect could be explained by two aspects. On one hand, HT can alter the pathophysiology of tumor cells by many modalities, such as DNA adduct formation [Citation61], DNA strand breaking [Citation63], Topo I activity [Citation64], synthesis of DNA [Citation69] and cell cycle [Citation68].
On the other hand, HT is also believed to be able to affect the microenvironmental factors of tumor cells, such as perfusion and oxygenation, improving its sensitivity to chemotherapeutics [Citation70–73]. Song et al. [Citation74] summarized in a review that an improvement in tumor oxygenation caused by HT resulted from a heat-induced increase in blood perfusion in human breast, head and neck cancers. They confirmed that 39–42 °C was the temperature range which can improve oxygenation for up to 1–2 days. Jones et al. [Citation75] also found that HT was able to improve the tumor oxygenation which leaded to consequent treatment response in locally advanced human breast cancer. In normal conditions, pancreatic cancer is quite resistant to conventional therapies. This resistance can be explained by abundant and compact accumulation of nontumor cells and extracellular matrix, known as the stroma, which can hamper vascularization and the delivery of chemotherapeutics, at the same time, obstruct the transportation of oxygen, causing hypoxia [Citation76]. Sensitivity to chemotherapeutics could be decreased by hypoxic microenvironment, leading to negative prognosis [Citation77,Citation78]. Although no direct experimental evidence has revealed that HT would lead to improved perfusion/oxygenation in pancreatic cancer. Considering its effects on breast, head and neck cancers listed above, we suppose there may also exist an enhancement effect in blood flow in pancreatic cancer. Interstitial pressure can be reduced and vessel permeability can be promoted slightly by HT in the region of interest. Thus hypoxia is decreased and PH value around tumor cells can be modulated. All these effects may facilitate a better inflow of the chemotherapy drugs into the region of tumor [Citation79–81], in addition to increasing tumor sensitivity to chemotherapy [Citation82].
In this combination regimen, low dose irinotecan can significantly decrease the adverse events. Replacing 5-FU with oral drugs can provide patients more flexibility and convenience. What is more, HT is an appropriate method to enhance the effect of chemotherapy and help killing tumor cells. These three aspects produce synergy effect which enable this combination treatment to achieve a satisfactory outcome, reducing the adverse events and maintaining the efficacy. This combination regimen provides a promising choice for pancreatic cancer treatment.
Conclusion
In conclusion, we conducted a retrospective study about combination mFOLFIRNOX and DRHT, which demonstrated well tolerability and efficacy in pancreatic cancer patients. Limitation of this study is small sample size. Based on the results of this study, a randomized trial focusing on first line or further line treatment of locally advanced or metastatic pancreatic cancer will be necessary in the near future to further clarify the factors and mechanism influencing the efficacy of this combination therapy.
Acknowledgments
We are grateful to all of the staff of Second Affiliated Hospital of Dalian Medical University for their work on this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
- He Y, Zheng R, Li D, et al. Pancreatic cancer incidence and mortality patterns in China, 2011. Chin J Cancer Res. 2015;27:29–37.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Li X, Ma T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: a prospective study in Chinese population. Cancer Lett. 2017;406:22–26.
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057.
- Sohal DP, Walsh RM, Ramanathan RK, et al. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. 2014;106:dju011.
- Suker M, Beumer BR, Sadot E, et al. A patient-level meta-analysis of FOLFIRINOX for locally advanced pancreatic cancer. Lancet Oncol. 2016;17:801–810.
- Carter SK, Comis RL. The integration of chemotherapy into a combined modality approach for cancer treatment. Cancer Treat Rev. 1975;2:193–214.
- Oster MW, Robert G, Lawrence P, et al. Chemotherapy for advanced pancreatic cancer. A comparison of 5-fluorouracil, adriamycin, and mitomycin (FAM) with 5-fluorouracil, streptozotocin, and mitomycin (FSM). Cancer. 1986;57:29–33.
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–620.
- Burris HA3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413.
- DeCaprio JA, Mayer RJ, Gonin R, et al. Fluorouracil and high-dose leucovorin in previously untreated patients with advanced adenocarcinoma of the pancreas: results of a phase II trial. J Clin Oncol. 1991;9:2128–2133.
- Crown J, Casper ES, Botet J, et al. Lack of efficacy of high-dose leucovorin and fluorouracil in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 1991;9:1682–1686.
- Huguet F, Girard N, Guerche CS-E, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277.
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–2615.
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: eastern cooperative oncology group trial E2297. J Clin Oncol. 2002;20:3270–3275.
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82.
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217.
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516.
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952.
- Lima CMR, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783.
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the cancer and leukemia group B (CALGB 80303). J Clin Oncol. 2010;28:3617–3622.
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: southwest oncology group–directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825.
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648.
- Conroy T, Paillot B, Francois E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer–a groupe tumeurs digestives of the federation nationale des centres de lutte contre le cancer study. J Clin Oncol. 2005;23:1228–1236.
- Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311–1315.
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. 2012;30:361.
- Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:809.
- Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the Princess Margaret Cancer Centre experience. Br J Cancer. 2016;115:649.
- Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer. 2014;13:232–238.
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1–18.
- Senior K. Hyperthermia and hypoxia for cancer-cell destruction. Lancet Oncol. 2001;2:524.
- Hiraoka M, Jo S, Akuta K, et al. Radiofrequency capacitive hyperthermia for deep-seated tumors. II. Effects of thermoradiotherapy. Cancer. 1987;60:128–135.
- Hornback NB. Historical aspects of hyperthermia in cancer therapy. Radiol Clin North Am. 1989;27:481–488.
- Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg. 1998;227:380–389.
- Qing Yu F, Bao An M, Xiu Chun Q, et al. Preliminary report on treatment of bone tumors with microwave‐induced hyperthermia. Bioelectromagnetics. 1996;17:218–222.
- Fan YF, Qin Y, Li DG, et al. Retrospective clinical study of advanced pancreatic cancer treated with chemotherapy and abdominal hyperthermia. J Glob Oncol. 2018;4:1–4.
- Mohamed F, Marchettini P, Stuart OA, et al. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–468.
- Ishikawa T, Kokura S, Oyamada H, et al. Effects of a sequential combination of hyperthermia and gemcitabine in the treatment of advanced unresectable pancreatic cancer: a retrospective study. Thermal Med. 2008;24:131–139.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570.
- Lagendijk JJW, Van Rhoon GC, Hornsleth SN, et al. Esho quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14:125–133.
- Tschoep-Lechner KE, Milani V, Berger F, et al. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8–16.
- He L, Wang J, Chen H, et al. Hyperthermia as an adjuvant therapy to chemotherapy for the treatment of advanced ovarian cancer complicated by ascites. Biomed Res. 2017;28:8115–8120.
- Ychou M, Conroy T, Seitz JF, et al. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2003;14:481–489.
- Matsuoka H, Yano K, Seo Y, et al. Cytotoxicity of CPT-11 for gastrointestinal cancer cells cultured on fixed-contact-sensitive plates. Anti-Cancer Drugs. 1995;6:413–418.
- Zeghari-Squalli N, Raymond E, Cvitkovic E, et al. Cellular pharmacology of the combination of the DNA topoisomerase I inhibitor SN-38 and the diaminocyclohexane platinum derivative oxaliplatin. Clin Cancer Res. 1999;5:1189–1196.
- Mullany S, Svingen PA, Kaufmann SH, et al. Effect of adding the topoisomerase I poison 7-ethyl-10-hydroxycamptothecin (SN-38) to 5-fluorouracil and folinic acid in HCT-8 cells: elevated dTTP pools and enhanced cytotoxicity. Cancer Chemother Pharmacol. 1998;42:391–399.
- Pavillard V, Formento P, Rostagno P, et al. Combination of irinotecan (CPT11) and 5-fluorouracil with an analysis of cellular determinants of drug activity. Biochem Pharmacol. 1998;56:1315–1322.
- Mans DRA, Grivicich I, Peters GJ, et al. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999;35:1851–1861.
- Assaf E, Verlinde-Carvalho M, Delbaldo C, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301–306.
- Lee MG, Lee SH, Lee SJ, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013;59:273–279.
- Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–1097.
- Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281.
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23–44.
- Ozaka M, Matsumura Y, Ishii H, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol. 2012;69:1197–1204.
- Saif MW, Syrigos KN, Katirtzoglou NA. S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs. 2009;18:335–348.
- Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol. 2009;39:2–15.
- Rietbroek R, Van De Vaart P, Haveman J, et al. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol. 1997;123:6–12.
- Urano M, Ling CC. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int J Hyperthermia. 2002;18:307–315.
- Kondo T, Ueda K, Kano E. Combined effects of hyperthermia and CPT-11 on DNA strand breaks in mouse mammary carcinoma FM3A cells. Anticancer Res. 1995;15:83–86.
- Katschinski DM, Robins HI. Hyperthermic modulation of SN-38-induced topoisomerase I DNA cross-linking and SN-38 cytotoxicity through altered topoisomerase I activity. Int J Cancer. 1999;80:104–109.
- Urano M, Kahn J, Reynolds R. The effect of 5-fluorouracil at elevated temperatures on a spontaneous mouse tumour: arrhenius analysis and tumour response. Int J Radiat Biol. 1991;59:239–249.
- Monge OR. Rofstad EKKaalhus O thermochemotherapy in vivo of a C3H mouse mammary carcinoma: single fraction heat and drug treatment. Eur J Cancer Clin Oncol. 1988;24:1661–1669.
- Takemoto M, Kuroda M, Urano M, et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int J Hyperthermia. 2003;19:193–203.
- Kido Y, Kuwano H, Maehara Y, et al. Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol. 1991;28:251–254.
- Mini E, Dombrowski J, Moroson BA, et al. Cytotoxic effects of hyperthermia, 5-fluorouracil and their combination on a human leukemia T-Lymphoblast cell line, CCRF-CEM. Eur J Cancer Clin Oncol. 1986;22:927–934.
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497.
- Olch AJ, Kaiser LR, Silberman AW, et al. Blood flow in human tumors during hyperthermia therapy: demonstration of vasoregulation and an applicable physiological model. J Surg Oncol. 1983;23:125–132.
- Song CW, Shakil A, Osborn JL, et al. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia. 1996;12:367–373.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465.
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res. 2001;155:515–528.
- Jones EL, Prosnitz LR, Dewhirst MW, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004;10:4287–4293.
- Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249–4256.
- Oei AL, Ahire VR, van Leeuwen CM, et al. Enhancing radiosensitisation of BRCA2-proficient and BRCA2-deficient cell lines with hyperthermia and PARP1-i. Int J Hyperthermia. 2018;34:39–48.
- Ino Y, Yamazaki-Itoh R, Oguro S, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146.
- Hu Y, Li Z, Mi DH, et al. Chemoradiation combined with regional hyperthermia for advanced oesophageal cancer: a systematic review and meta-analysis. J Clin Pharm Ther. 2017;42:155–164.
- Roesch M, Mueller-Huebenthal B. Review: the role of hyperthermia in treating pancreatic tumors. Indian J Surg Oncol. 2015;6:75–81.
- Pestieau SR, Stuart OA, Chang D, et al. Pharmacokinetics of intraperitoneal gemcitabine in a rat model. Tumori. 1998;84:706–711.
- Oei AL, Vriend LE, Krawczyk PM, et al. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;33:419–427.