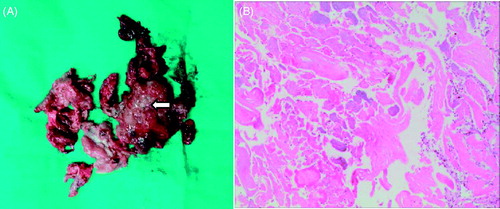
Abstract
Objective: To evaluate the safety and feasibility of combined procedures: HIFU combined with systemic MTX followed by ultrasound-guided curettage or hysteroscopic resection while treating placenta accreta (PA).
Method: This study included 21 patients diagnosed with retained PA with marked vascularity after abortion or delivery from July 2015 to December 2017. Patients with high serum β-hCG level (≥100 mIU/mL) received systemic MTX + HIFU treatment for 3 days and the ones with low β-hCG level (<100 mIU/mL) only received USgHIFU treatment for 3 days before ultrasound-guided curettage or hysteroscopic resection. All patients had completed follow-up data. The safety and feasibility of the treatment were evaluated retrospectively.
Result: Sixteen patients received systemic 100 mg MTX without myelosuppression. All patients received three days of HIFU ablation therapy; the median of HIFU treatment time was 60 minutes. Ultrasound-guided curettage and ovum forceps were used to extract planted placental tissue in 5 patients with one week after birth or after abortion. Sixteen patients received a hysteroscopic operation after the HIFU treatment. The median of intraoperative blood loss was 30 ml. Twenty patients had recovered normal menstruation on average 32 days (range 14–60) after the operation.
Conclusion: Based on the results of this study, with a relatively small number of patients, it seems that three-days’ therapy of HIFU ± systemic MTX followed by ultrasound-guided curettage or hysteroscopic resection, is a safe and feasible treatment for retained PA with marked vascularity after abortion or delivery.
Introduction
Placenta accreta (PA) is a general term used to describe the clinical condition when part or all placenta invades and becomes inseparable from the uterine wall after abortion or delivery [Citation1]. As a result, a part of the placenta cannot separate after delivery, which may trigger life-threatening complications such as a catastrophic hemorrhage, hemorrhagic shock, uterine rupture, infection and coagulation disorders when the implanted tissue is massive and deep, with a rich blood supply. The increasing number of cesarean sections has increased the incidence of PA [Citation2]. The incidence of PA has increased ten-fold over the past 30 years in China [Citation3]. The incidence of conservative treatments such as uterine artery embolization (UAE) or methotrexate has increased gradually, replacing hysterectomy. However, the disadvantage of conservative treatment still exist, including the risk of bleeding or infection and the risk of requiring a secondary hysterectomy [Citation3,Citation4]. PA is an important obstetric problem in medical practice and the preservation of the uterus remains a clinical challenge in the management of retained PA with marked vascularity. Over the past decade, ultrasound-guided high-intensity focused ultrasound (USgHIFU) has been used to treat PA [Citation5–8], however, such combined procedures: HIFU combined with systemic MTX followed by ultrasound-guided curettage or hysteroscopic resection, have never been systemically studied.
In the present report, safety and feasibility of ultrasound-guided high-intensity focused ultrasound were evaluated as key preoperative pretreatment of curettage or hysteroscopic resection of retained PA with marked vascularity after abortion or delivery.
Materials and methods
This study was approved by the ethics committee at the First Hospital of Jinan University. Informed consent was obtained from every patient before HIFU treatment.
Patients
This single-center retrospective study described the clinical backgrounds, therapeutic modalities, and treatment outcomes of 21 patients with retained PA or increta with marked vascularity after abortion or delivery from July 2015 to December 2017. The detailed process was meticulously recorded. Inclusion criteria for patients: (1) stable vital signs, no active vaginal bleeding or infection, normal liver and renal function; hemoglobin >70 g/l; (2) ultrasound and MRI indication PA (); the maximum diameter of residual placenta larger than 3 cm; (3) absence of serious delivery complications. Patients with retained placenta without vascularization or with acute postpartum hemorrhage were excluded from the present study. In this study, 21 patients diagnosed with PA with marked vascularity were treated with HIFU ± systemic MTX followed by curettage or hysteroscopic resection guided by ultrasound.
The patients were 19–44 years old, and the mean age was 29.61 ± 4.59 years. These patients had a median of two pregnancies (range 1–6) and one delivery (range 0–3). Fifteen patients induced abortion (the gestational week at abortion was 10–27 weeks), four patients underwent vaginal delivery and the other two patients underwent cesarean section. Average vaginal bleeding period before the treatment was 26.00 ± 14.56 days (range 7–120) ().
Table 1. Demographic characteristics of the patients with placenta accreta.
Diagnostic imaging and serum β-hCG measurement
A routine physical examination, laboratory tests including serum β-hCG measurement, transvaginal color Doppler ultrasound and MRI were performed before determining the treatment procedure (). Serum β-hCG level showed less than 100 mIU/mL in 5 patients and more than 100 mIU/mL in 16 patients. When an increased vascular flow was detected in retained uteroplacental tissue by transvaginal color Doppler ultrasonography, further evaluations were made by MRI to evaluate the depth of the placental invasion into the myometrium as well as the shape of the retained placental. If the placental invasion was attached to the myometrium, rather than being restricted within the decidua basalis, PA was diagnosed (), but if the placental tissue invaded into the myometrium, it was diagnosed as placenta increta () [Citation9,Citation10]. Ten patients were PA and 11 were placenta increta. The median residual placental volume (calculated using the formula of the ellipsoid volume: V = 0.52 × length × anteroposterior diameter × transverse diameter, measured by MRI) was 61.11 cm3 (range 6.01–338.95). The minimum distance from the implantation site to the uterine serosa was 4.04 mm (range 0–20.29). According to the Adler semi-quantitative method [Citation11], five lesions were classified as level I, ten lesions as level II and six lesions as level III ().
Figure 1. Therapeutic algorithm for management of retained placenta accreta or increta with marked vascularity after abortion or delivery. After identifying vascular lesion in retained placenta tissue by transvaginal color Doppler ultrasound and MRI and serum β-hCG measurement, cases were divided into low (≤100 mIU/mL) and high (>100 mIU/mL) serum β-hCG groups. Cases with low serum β-hCG were managed by HIFU + ultrasound-guided curettage or hysteroscopic resection, while cases with high serum β-hCG were managed by HIFU + systemic MTX + ultrasound-guided curettage or ultrasound-guided hysteroscopic resection.
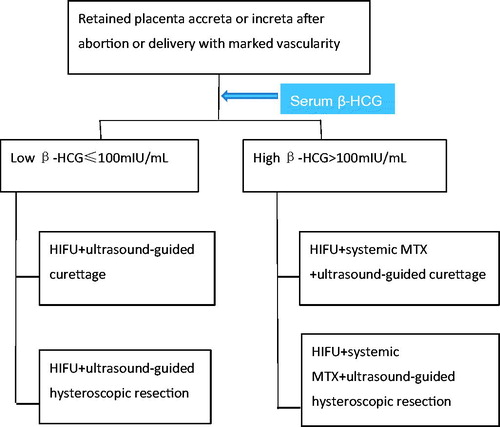
Figure 2. Retained placenta accreta marked vascularity after abortion. (A) MRI showed the retained placenta accreta as irregular low signal intensity; (B) Ultrasound before operation: 32 mm × 20 mm × 19 mm hyperechogenic shadow in the endometrial cavity with patchy blood flow signal, and unclear boundary with the muscular layer of the uterus was shown; (C) The lesion was not seen at 1 month after operation.
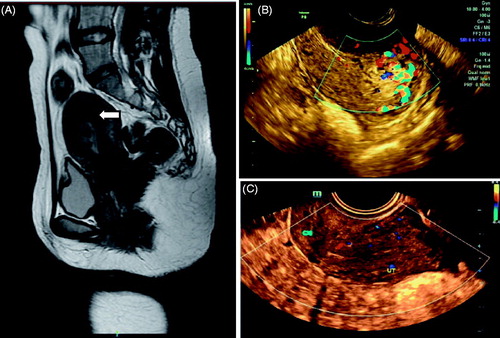
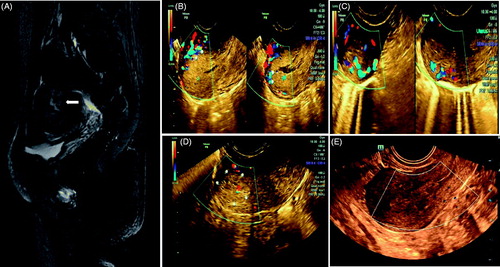
Figure 3. Retained placenta increta marked vascularity after delivery. (A) MRI showed the retained placenta increta as high signal intensity (arrow); (B) Ultrasound imaging before operation: 65 mm × 38 mm × 43 mm hyperechogenic shadow in the endometrial cavity with marked vascularity and deep myometrial invasion reached to uterine serosa was shown; (C) 25 mm × 20 mm × 23 mm hyperechogenic shadow at one month after operation; (D) 16 mm × 14 mm × 18 mm hyperechogenic shadow at three months after operation; (E) The lesion was not seen at six months after operation.
Systemic MTX
The patient with high serum β-hCG level (≥100 mIU/mL) received systemic MTX, which MTX 20 mg/d was administered intramuscularly for 5 days. From the third day of systemic MTX, HIFU treatment was performed for 3 days simultaneously. Patients with low serum β-hCG (<100 mIU/mL) only received USgHIFU treatment for 3 days without systemic MTX.
USgHIFU treatment
HIFU was performed using the ‘PRO2008’ high intensity focused ultrasound system (SHENZHEN PROMETHE MEDICAL SCI-TECH CO., LTD., Shenzhen, China). Before starting the HIFU treatment, all patients were required to bladder filling. During the treatment, the patients were placed in the supine position, and the treatment area of the abdominal wall is uniformly applied with a coupling agent to ensure no bubble generation. Ultrasonic external probe positioning, laser light positioning, and accurate positioning of the built-in ultrasonic probe, the degassing water capsule membrane of the C-arm in the treatment system are in close contact with the skin of the treatment area coated with the coupling agent, and the treatment is started. The patients were treated in an awake state and communicate with the doctor at any time. Treatment parameters: emission frequency (1.2 ± 0.18) MHz, acoustic emission power 5000 W/cm2; focal length 12.6 cm adjustable; adjustable focal length size adjustable. The transmission time was adjustable from 0.01 to 0.50 s, and the interval was adjustable from 0.01 to 0.50 s. The number of launches was adjustable from 0 to 50 times, the number of transducers was one, and the power was 60%–100%. HIFU phased arrays give a highly heating at 3 mm-interval, which covers the area of the placental tissue rich in blood vessels rather than the implanted myometrium, keeping a certain distance from the uterine serosa (average thickness of the muscle layer or 20 mm) ().
Figure 4. HIFU PRO2008 ablation therapy on placenta accreta with marked vascularity. (A,B,C) showed HIFU ablation arrays, (A) 31st slice, (B) 32nd slice, (C) 33rd slice. (D) Doppler ultrasound before HIFU, (E) Doppler ultrasound after HIFU treatment at 31st slice showed blood flow reduction.
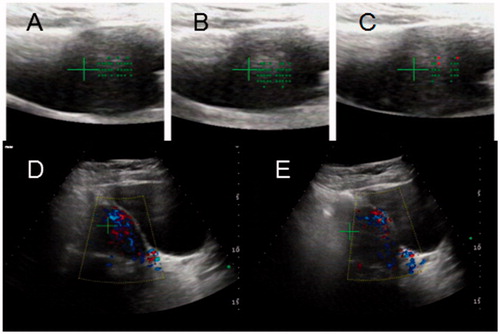
During the whole course of HIFU treatment, color Doppler ultrasound was used to determine the location of the target area and to monitor the response to HIFU. The sagittal view of the ultrasound scanning mode was selected, and the treatment plan was made by dividing the placenta into different slices with a thickness of 3 mm each. The ablation procedure began from the innermost slice. During surgery, we compared the ultrasound image with the target image, the grey-scale values with each other and identified any coagulation necrosis by observing the lesions (). HIFU ablation was terminated when any sign of placenta tissue blood flow disappeared or a grey-scale change in the target tissue could be observed on the color Doppler ultrasound.
Ultrasound-guided hysteroscopy resection or curettage
Hysteroscopic resection was performed under spinal anesthesia. The patients were placed in a dorsal lithotomy position. After disinfecting the operating field, the depth of the uterus was measured before the procedure. Cervix dilators were used to dilate the cervix to 9.5 mm. Suction curettage and ovum forceps were used to extract planted placental tissue under transabdominal ultrasound guided. Next, a resectoscope (Olympus 9.0 mm) was used to remove any parts of the placenta that were difficult to separate from the uterine wall; any implanted parts of the placenta were left in situ. If bleeding on the wound surface still occurred after placental tissues were removed, a 10-IU oxytocin solution was injected into the cervical stroma, and electrocoagulation used. Foley catheter balloon was used in some patients to reduced uterine bleeding and prevention of intrauterine adhesions. Foley catheter balloon was removed at the 5th day after the operation. All the removed placenta tissues were sent to pathological examination.
Follow-up observations
After treatment, patient’s temperature and any sign of vaginal bleeding were observed in the hospital. If vital signs were stable, all patients were discharged from the hospital 3–5 days after the operation. According to the approved protocol, the serum β-HCG level was monitored weekly until it returned to normal. Routine outpatient follow-up at 1, 3, 6 months after the operation. The duration of vaginal bleeding, abdominal pain and the first return of the menstrual cycle should be recorded. Postoperative uterine cavity was assessed by transvaginal ultrasound at the first follow-up and sequential treatment of estrogen and progesterone for 3 cycles were given in patients with heterogeneous echo light clusters.
Statistical methods
All data are presented as mean standard deviation (SD) or median (range). SPSS 15.0 software (SPSS, Inc., Chicago, IL) was used for statistical analysis.
Results
Systemic MTX and USgHIFU treatment
Sixteen patients received systemic 100 mg MTX without myelosuppression. All patients received three days’ HIFU ablation therapy. The median HIFU treatment time was 60 min (range 30–100) and the median sonication time was 700 s (range 227–2500). It was shown that all significant grey-scale changed. Color Doppler ultrasound showed the blood perfusion among all the lesion was reduced (). The HIFU procedure went well in all patients and they all completed the whole process. During treatment, all patients complained about mild lower abdominal and sacrococcygeal pain. Nineteen of them scored the pain between 1 and 2 points, whereas the other 2 reported pain scores of 3 points. No serious complications occurred during or after these procedures ().
Table 2. HIFU treatment results of placenta accreta.
Ultrasound-guided curettage and hysteroscopic resection
Ultrasound-guided curettage and ovum forceps were used to extract planted placental tissue in 5 patients within one week after birth or after abortion, which was difficult to finish hysteroscopic resection due to enlarging large uterine cavity. The other patients received a hysteroscopic operation after the HIFU treatment. The aim of the operation was to remove the non-implanted part of the placenta, the deeply implanted part of the placenta was left in situ, to be absorbed slowly after the HIFU ablation. The median volume of any intraoperative blood loss was 30 ml (range 5–200). All patients preserved their uterus and were able to tolerate the hysteroscopic operation well. No severe complications, such as water intoxication or gas embolism, uterus perforation occurred during the operation.
Follow-up results
The average hospital stay was 8.5 ± 1.6 days (range 6–11). During the follow-up period, patients had a small amount of vaginal bleeding with a median duration of 14 days (range 7–60). Twenty patients recovered normal menstruation an average of 32 days (range 14–60) after the operation, and the other one patient complained of less menstruation was verified intrauterine adhesion by hysteroscopy. During the follow-up period, no hemorrhage, infection or other complications occurred (). Ultrasound showed no lesions in the uterus at one-month after operation in 12 patients. Eight patients restore normal uterine cavity at 3-month and 1 patient at 6-month after the operation. Three patients had next pregnancy within postoperative 6 months.
Table 3. Follow-up results of placenta accreta.
Discussion
As a noninvasive treatment technique, the application of HIFU on the management of PA has been reported in several preliminary studies in recent years [Citation5–7]. During HIFU ablation, the ultrasound beams generated from the transducer can penetrate through abdominal skin, subcutaneous tissue and the bladder, then focused at the targeted PA lesion. When the temperature at the target increases to over 60 °C, coagulate necrosis occurs [Citation12–14]. The cavitation effect of HIFU may also help increase the temperature of the targeted tissue and loosen the adhesion of the placenta tissue to the myometrium [Citation3]. HIFU has unique advantages in the treatment of PA with marked vascularity. A previous study has also shown that HIFU can destroy small vessels with a diameter of less than 2 mm [Citation15–18], which could reduce the chances of hemorrhage related to PA. Although there are several clinical studies that have shown the effectiveness and safety of HIFU in treating PA [Citation5–8]. Ye et al. [Citation5] reported that, two patients had uterine perforation during the first operation of the 25 patients and one converted into laparoscopic operation which was found whole layers of the uterus that underwent HIFU therapy, became thin and brittle, and the lesion in the uterus was resected and sutured. The volume of blood loss in 2 cases, were more than 400 ml and nine patients underwent a second session of curettage guided by hysteroscopy.
The present study showed that 3-day scheme of HIFU combined with systemic MTX followed by ultrasound-guided curettage or hysteroscopic resection was safety and feasibility in treating PA. HIFU was performed using the ‘PRO2008’ high intensity focused ultrasound system. The patient was placed in the supine position and had only slight lower abdominal and sacrococcygeal pain, which no anesthesia was needed during the HIFU treatment. All placental tissues in the uterine cavity were successfully removed for one session of hysteroscopy or curettage with intraoperative blood loss less than 200 ml. Ultrasound-guided hysteroscopic resection is also safer and more effective, as the procedure is visible. The aim of the hysteroscopic electrotomy was to remove the non-implanted part of the placenta. The deeply implanted part of the placenta was left in situ, to be absorbed slowly after the HIFU ablation, which may be also partly attributed to the killing effect of MTX on villous trophoblasts avoiding second sessions of hysteroscopic operation. It was verified in the present study that all patients showed no lesions in the uterine cavity by ultrasound latest 6 months. Sequential treatment of estrogen and progesterone can also promote the recovery of endometrium.
Three days’ therapy of HIFU may be better than one day’s therapy to prevent over-ablation. It was found in our practice that some blood vessels of target lesions can be recanalized after one-day HIFU therapy. If increase the dose of HIFU ablation or prolong the ablation time at one-day therapy, the risk of over-ablation will increase at the same time. The success rate of the vascular occlusion, ultrasound-guided HIFU, which was quoted in published literature, varies from 63–100% [Citation18–21]. Especially 91% in vivo placental vascular occlusion of the sheep [Citation18]. After some pre-experiments, we chose a three days’ therapy resulting in good effect and avoiding over-ablation. On the second day and the third-day therapy, some supplements ablation was applied to the lesion according to the blood flow of Doppler ultrasound. Herein, blood perfusion of implant placental tissues significantly dropped, or even disappeared after MTX and HIFU treatment. It can also avoid heavy bleeding and uterus perforation during the following hysteroscopic resection. The scope of HIFU ablation treatment covers the area of the placental tissue rich in blood vessels, rather than the implanted myometrium, avoiding damage to the myometrium and uterine horn, in order to the preservation of the patient's fertility. Compared with some complication of UAE such as ovarian insufficiency and uterine rupture [Citation22], MTX and HIFU treatment were safer and cheaper. This study is limited because it is a retrospective descriptive study and the non-randomization case control may cause result bias. Another limitation of this study is that the number of subjects is small and the follow-up time was short. Therefore, RCT with larger recorded samples are required in future studies.
Conclusions
Based on the results of this study with a relatively small number of patients, it seems that three days’ therapy of HIFU ± systemic MTX followed by curettage or hysteroscopic resection guided by ultrasound, is a safety and effective treatment for retained PA with marked vascularity after abortion or delivery.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- ACOG Committee on Obstetric Practice. ACOG Committee opinion. Number 266, January 2002: placenta accreta. Obstet Gynecol. 2002;99:169–170.
- Committee on Obstetric Practice. Committee opinion no. 529: placenta accreta. Obstet Gynecol. 2012;120:207–211.
- Legendre G, Zoulovits FJ, Kinn J, et al. Conservative management of placenta accreta: hysteroscopic resection of retained tissues. J Minim Invasive Gynecol. 2014;21:910–913.
- Verspyck E, Resch B, Sergent F, et al. Surgical uterine devascularization for placenta accreta: immediate and long-term follow-up. Acta Obstet Gynecol Scand. 2005;84:444–447.
- Ye M, Yin Z, Xue M, et al. High-intensity focused ultrasound combined with hysteroscopic resection for the treatment of placenta accreta. BJOG: Int J Obstet Gy. 2017;124:71–77.
- Lee JS, Hong GY, Park BJ, et al. High-intensity focused ultrasound combined with hysteroscopic resection to treat retained placenta accreta. Obstet Gynecol Sci. 2016;59:421–425.
- Bai Y, Luo X, Li Q, et al. High-intensity focused ultrasound treatment of placenta accreta after vaginal delivery: a preliminary study. Ultrasound Obstet Gynecol. 2016;47:492–498.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterinediseases. Int J Hyperthermia. 2018;24:1–6.
- Takeda A, Koike W. Conservative endovascular management of retained placenta accreta with marked vascularity after abortion or delivery. Arch Gynecol Obstet. 2017;296:1189–1198.
- Cramer SF, Heller DS. Placenta accreta and placenta increta: an approach to pathogenesis based on the trophoblastic differentiation pathway. Pediatr Dev Pathol. 2016;19:320–333.
- Adler DD, Carson PL, Rubin JM, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. 1990;16:553–559.
- Zhang Y, Zhang C, He J, et al. The impact of gestational sac size on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment for caesarean scar pregnancy. Int J Hyperthermia. 2019;35:291–297.
- Jiang J, Xue M. The treatment of cervical pregnancy with high-intensity focusedultrasound followed by suction curettage: report of three cases. Int J Hyperthermia. 2019;24:1–4.
- Copelan A, Hartman J, Chehab M, et al. High-Intensity focused ultrasound: current status for image-guided therapy. Semin Intervent Radiol. 2015;32:398–415.
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76:590–599.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73:396–403.
- Wu F, Chen WZ, Bai J, et al. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28:535–542.
- Shaw CJ, Rivens I, Civale J, et al. Trans-abdominal in vivo placental vessel occlusion using high intensity focused ultrasound. Sci Rep. 2018;8:13631.
- Martin RW, Vaezy S, Kaczkowski P, et al. Hemostasis of punctured vessels using Doppler-guided high-intensity ultrasound. Ultrasound Med Biol. 1999;25:985–990.
- Vaezy S, Martin R, Kaczkowski P, et al. Use of high-intensity focused ultrasound to control bleeding. J Vasc Surg. 1999;29:533–542.
- Ichihara M, Sasaki K, Umemura S, et al. Blood flow occlusion via ultrasound image-guided high-intensity focused ultrasound and its effect on tissue perfusion. Ultrasound Med Biol. 2007;33:452–459.
- Ando M, Goto M, Matsuoka S, et al. Case of uterine rupture after multiple intrauterine operations and uterine artery embolization. J Obstet Gynaecol Res. 2018. [Epub ahead of print]