Abstract
Purpose: Mouse double-stranded DNA-dependent protein kinase (DNA-PK) activity is heat sensitive. Recovery of heat-inactivated DNA repair activity is a problem after combination therapy with radiation and heat. We investigated the mechanism of recovery of heat-inactivated DNA-PK activity.
Methods: Hybrid cells containing a fragment of human chromosome 8 in scid cells (RD13B2) were used. DNA-PK activity was measured by an in vitro assay. Immunoprecipitation of the nuclear extract was performed with an anti-Ku80 antibody. Proteins co-precipitated with Ku80 were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and detected by Western blotting using anti-heat shock protein (HSP)72 and anti-heat shock cognate protein (HSC)73 antibodies. HSC73 was overexpressed with the pcDNA3.1 vector. Short hairpin (sh)RNA was used to downregulate HSC73 and HSP72.
Results: The activity of heat-inactivated DNA-PK recovered to about 50% of control during an additional incubation at 37 °C after heat treatment at 44 °C for 15 min in the presence of cycloheximide (which inhibits de novo protein synthesis). Maximal recovery was observed within 3 h of incubation at 37 °C after heat treatment. Constitutively expressed HSC73, which folds newly synthesized proteins, reached maximal levels 3 h after heat treatment using a co-immunoprecipitation assay with the Ku80 protein. Inhibiting HSC73, but not HSP72, expression with shRNA decreased the recovery of DNA-PK activity after heat treatment.
Conclusions: These results suggest that de novo protein synthesis is unnecessary for recovery of some heat-inactivated DNA-PK. Rather, it might be reactivated by the molecular chaperone activity of HSC73, but not HSP72.
Introduction
Heat treatment enhances the lethal effect of ionizing radiation to cells. This phenomenon is called thermal radiosensitization. The lethal lesions induced by ionizing radiation are mainly double-strand breaks (DSBs) in DNA molecules. The aim of combining radiation with hyperthermia is to depress DNA repair activity for the DSBs induced by ionizing radiation, thereby enhancing the effectiveness of radiation-therapy against cancer [Citation1,Citation2]. However, a major problem with this combination therapy is the recovery of heat-inactivated DNA repair activity by heat shock proteins (HSPs) with molecular chaperone activity [Citation3]. HSPs are proteins universally conserved throughout evolution. Their amino acid sequences show at least 50% homology between eukaryotes and prokaryotes [Citation4]. The function of these proteins is to protect other proteins from heat-inactivation and to help the refolding of heat-inactivated proteins [Citation5,Citation6]. Among the HSPs, HSP72 (inducible HSP70) and heat shock cognate protein 73 (HSC73; constitutive HSP70) are well characterized [Citation7–9].
There are two main repair processes for DSBs, homologous recombinational repair and non-homologous end-joining (NHEJ) repair. Double-stranded DNA-dependent protein kinase (DNA-PK) plays an important role in the NHEJ repair process [Citation10]. Recently, we reported that the DNA-PK activities of NHEJ repair contribute in ‘fast repair processes’; i.e., within early periods after damage [Citation11]. DNA-PK activity was detected only when the Ku70/80 heterodimer, the DNA end-binding component, was bound to the end of the DSB [Citation12]. Mutant cells lacking DNA-PK activity are hypersensitive to ionizing radiation [Citation13,Citation14]. This suggests that DNA-PK activity plays the most important role of repairing ionizing radiation-induced damage.
In our previous study, we found that mouse DNA-PK activity was heat sensitive [Citation11]. Further results indicated that heat inactivation of DNA-PK activity was the result of heat-induced inactivation of Ku protein(s) [Citation15]. Burgman et al. were the first to report that heat treatment inactivated Ku80 and Ku70 [Citation16]. In this report, we describe that some heat-inactivated DNA-PK activity (provably heat-inactivated Ku70 and/or Ku80) was recovered by an additional incubation at 37 °C after a 44 °C heat treatment by a mechanism involving HSC73 chaperone activity.
Methods
Cell culture
Hybrid cells containing a fragment of human chromosome 8 in scid mouse cells (RD13B2) [Citation17] were cultured in Dulbecco’s modified Eagle’s minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Equitech-Bio, Kerrville, TX). For studying the inhibition of de novo protein synthesis, cycloheximide (20 µg/mL) was added to the culture medium.
Heat treatment
Hybrid cells (5 × 106 cells/plate) were inoculated into 100-mm cell culture dishes and grown at 37 °C for 24 h. The cells were then treated at 44 °C for 15 min by immersing the dishes, sealed by parafilm, into a water bath. Then, the cells were incubated at 37 °C for the indicated hours.
Assay of DNA-PK activity
DNA-PK activity was determined as described previously [Citation15]. In brief, reaction mixtures consisted of the crude cell extract (10 µg protein), substrate buffer (with the p53 oligopeptide as the substrate), double-stranded DNA, and [γ-32P] ATP (111 TBq/mmol). The reaction mixtures were incubated at 30 °C for 30 min, then transferred to phosphocellulose disks. The disks were washed with 1% acetic acid and distilled water. DNA-PK activity was calculated from the radioactivity bound to the disks. DNA-PK activity in the control hybrid cells without heat treatment was set to 100%.
Preparation of nuclear extracts
Nuclear extracts were prepared as described previously [Citation18]. In brief, cells (1 × 108) were suspended and disrupted in buffer A (10 mM HEPES-KOH (pH 7.8), 10 mM KCl, 0.1 mM EDTA (pH 8.0), 1 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride) with a Dounce homogenizer (Wheaton Industries Inc., Millville, NJ). After centrifugation (15,000 g), nuclear extracts were prepared by treating nuclei with buffer C (50 mM HEPES-KOH (pH 7.8), 420 mM KCl, 0.1 mM EDTA (pH 8.0), 5 mM MgCl2, 20% glycerol, 1 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride). After centrifugation (15,000 g), supernatants were stored as nuclear extracts.
Immunoprecipitation and Western blotting
Immunoprecipitation was performed as described by Suwa et al. [Citation19]. A goat anti-mouse Ku80 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was incubated with Protein G-Sepharose CL-4B beads (GE Healthcare, Buckinghamshire, UK). After washing with wash buffer (10 mM Tris–HCl (pH 8.0), 0.15 M NaCl), nuclear extract was added and incubated at 4 °C for 2 h. After again washing with wash buffer, the beads were treated with sodium dodecyl sulfate (SDS) sample buffer (0.125 M Tris–HCl (pH 6.8), 4% SDS, 20% glycerol, 0.01% bromophenol blue and 10% β-mercaptoethanol) and heated at 100 °C for 3 min. Proteins were analyzed by Western blotting. Monoclonal rat anti-HSC73 (1B5) [Citation20] and mouse anti-HSP72 (C92F3A-5: SPA-810) (StressGen Biotechnologies, Victoria, Canada) antibodies were used for the detection of HSC73 and HSP72, respectively. A monoclonal mouse anti-β-actin (2F3) (Fujifilm Wako Pure Chemical, Osaka, Japan) antibody was used for the detection of β-actin as the loading control. After treatment with horseradish peroxidase-conjugated goat anti-rat or anti-mouse IgG, proteins were visualized by an enhanced chemiluminescence system (GE Healthcare, Chicago, IL). Band intensities were measured by densitometry (Photometrics, Tucson, AZ). An intensity of one indicates the band intensity of hybrid cells not treated with heat.
Construction of HSC73 overexpressing hybrid cells
The mouse HSC73 gene was cloned into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA). The resultant plasmid was introduced into hybrid cells by using Lipofectin (Invitrogen, Carlsbad, CA).
Short hairpin (sh)RNAs of HSP72 and HSC73
Inhibition of HSP72 and HSC73 expression was achieved using the pSilencer version 2.1 Hygro vector (Invitrogen, Carlsbad, CA). Target sequences were: HSP72, GGCCAACAAGATCACCATC; HSC73, GATTCTTGACAAGTGCAAT. Plasmids were introduced into cells with Lipofectin.
Results
Recovery of heat-inactivated DNA-PK activity with or without cycloheximide
The time course of the recovery of DNA-PK activity after heat treatment at 44 °C for 15 min is shown in . The recovery of heat-inactivated DNA-PK activity was rapid, with 40% of the activity being recovered within 3 h of incubation at 37 °C. DNA-PK activity then increased gradually to a maximum of about 50% after a 10 h incubation at 37 °C following heat treatment.
Figure 1. (A) Recovery of DNA-dependent protein kinase (DNA-PK) activity after heat treatment of hybrid cells at 44 °C for 15 min with (■) or without (●) cycloheximide. Non-treated cell DNA-PK activity was set to 100%. Data are the average of two independent experiments. Error bars show the range. (B) Typical Western blot result of HSP72 and β-actin after heat treatment of hybrid cells with and without cycloheximide (CHX).
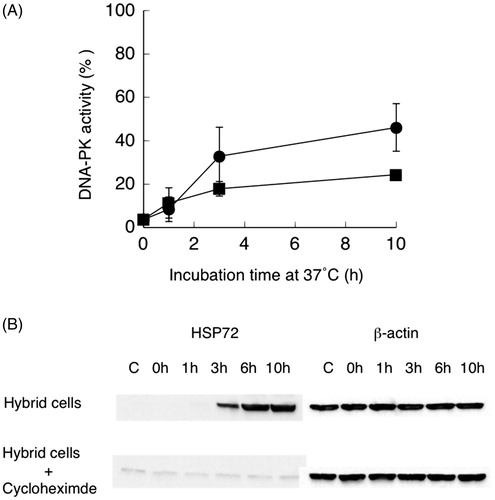
The effect of the protein synthesis inhibitor cycloheximide (20 µg/mL) on the recovery of heat-inactivated DNA-PK activity was studied. About half of the activity of non-treated cells (about 20% of control activity) was recovered in cycloheximide-treated cells within 3 h of incubation. This result indicates that at least half of the recovered DNA-PK activity was not dependent on de novo protein synthesis. Induction of HSP72 in the presence of cycloheximide was not observed even after incubation for 10 h after heat treatment (). This indicates that HSP72 did not participate in the recovery of heat-inactivated DNA-PK activity in the presence of cycloheximide.
Detection of proteins co-precipitated with Ku80
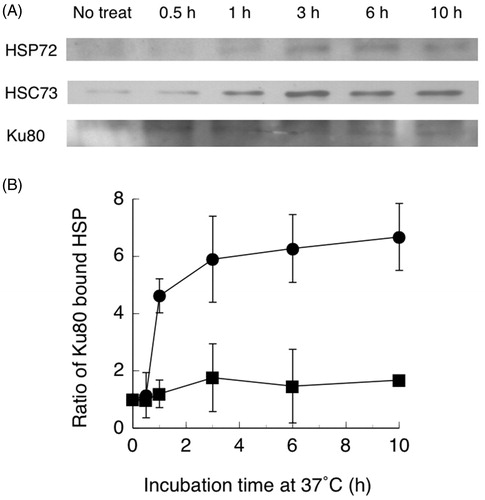
Nuclear proteins were prepared from heat-treated hybrid cells and precipitated with an anti-Ku80 antibody. Proteins that co-immunoprecipitated with Ku80 were separated by SDS-polyacrylamide gel electrophoresis. The separated proteins were analyzed by Western blotting using monoclonal anti-HSP72 and anti-HSC73 antibodies. As shown in , the amount of Ku80 bound HSC73 in control cells (0 h) without heat treatment was low. Ku80 bound HSC73 was observed within 0.5 h, increased at 1 h, reached a maximum at 3 h after heat treatment, and then increased gradually to 10 h. In the same membrane, only a small amount of Ku80 bound HSP72 was observed.
Figure 2. (A) Western blots of Ku80 bound HSP72, HSC73 and Ku80. (B) Ratios of Ku80 bound HSC73 (●) and HSP72 (■) following co-immunoprecipitation of nuclear extracts from hybrid cells with a Ku80 antibody. Hybrid cells were treated at 44 °C for 15 min, then cultured at 37 °C for the indicated time periods. Band densities were measured from photographs by a densitometer. Data are the average of two independent experiments. Error bars show the range.
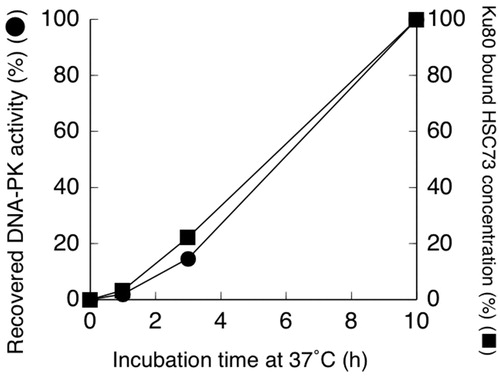
Relationship between recovered DNA-PK activity and the amount of Ku80 bound HSC73
The relationship between the time courses of recovery of heat-inactivated DNA-PK activity () and the amount of Ku80 bound HSC73 () was examined. In , 100% of DNA-PK activity and Ku80 bound HSC indicate the values determined after 10 h of incubation at 37 °C following heat treatment at 44 °C for 15 min. An excellent correlation was observed between the recovery of heat-inactivated DNA-PK activity and the amount of Ku80 bound HSC73.
Effect of overexpressing HSC73
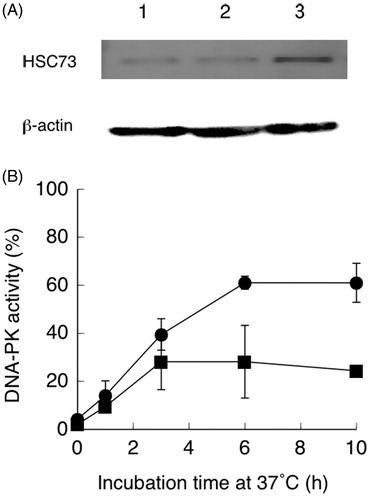
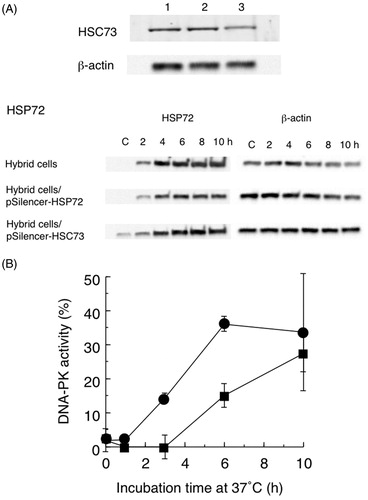
HSC73 cDNA was ligated to the eukaryotic expression vector, pcDNA3.1, and the resulting plasmid was transfected into hybrid cells. The content of HSC73 was increased in cells carrying the pcDNA-HSC73 plasmid compared with cells carrying vector alone (). As indicated in , over 60% of heat-inactivated DNA-PK activity was recovered in hybrid cells carrying the HSC73 expression vector. Maximum recovery was observed within 6 h after heat treatment.
Figure 4. (A) Western blot showing increased HSC73 and β-actin expression in hybrid cells carrying pcDNA3.1-HSC73. Lane 1: control hybrid cells; lane 2: hybrid cells carrying the pcDNA3.1 vector; lane 3: hybrid cells carrying pcDNA3.1-HSC73. (B) Recovery of DNA-dependent protein kinase (DNA-PK) activities during incubation at 37 °C after heat treatment at 44 °C for 15 min. Hybrid cells were transfected with pcDNA3.1-HSP73 (●) or the pcDNA3.1vector (■). Non-treated hybrid cell DNA-PK activity was set to 100%. Data are the average of two independent experiments. Error bars show the range.
Inhibition of HSP72 and HSC73
To assess the possibility that HSP72 also participated in the recovery of heat-inactivated DNA-PK activity, we used shRNAs to inhibit HSP72 and HSC73. The cellular content of HSC73 was reduced by HSC73 shRNA treatment (). The induction of HSP72 expression after heat treatment was reduced by HSP72 shRNA treatment (). As shown in , only 15% of heat-inactivated DNA-PK activity was recovered at 6 h of incubation after heat treatment in HSC73 shRNA-carrying hybrid cells. Because of the inhibition of HSC73 expression (), only HSP72 is active in this condition. In contrast, recovery of heat-inactivated DNA-PK activity was increased to 15% already at 3 h after heat treatment in HSP72 shRNA-carrying hybrid cells, and to over 35% at 6 h. In these cells, HSC73 is the major active HSP. These data are consistent with the time course of Ku80 bound HSP72 and HSC73 () and indicate that HSC73 is the primary factor in the recovery of heat-inactivated DNA-PK activity.
Figure 5. (A) Western blots showing reduced HSP expression in hybrid cells carrying pSilencer-HSC73 or pSilencer-HSP72. Anti-HSC73 and β-actin: lane l, control hybrid cells; lane 2, hybrid cells transfected with pSilencer-HSP72; lane 3, hybrid cells transfected with pSilencer-HSC73. Anti-HSP72 and β-actin: control hybrid cells, or cells transfected with pSilencer-HSP72 or pSilencer-HSC73 for 2–10 h at 37 °C after heat treatment at 44 °C for 15 min. C is control cells. (B) Recovery of heat-inactivated DNA-dependent protein kinase (DNA-PK) activity in hybrid cells transfected with pSilencer-HSC73 (■) or pSilencer-HSP72 (●). Non-treated hybrid cell DNA-PK activity was set to 100%. Data are the average of two independent experiments. Error bars show the range.
Discussion
In mammalian cells, NHEJ is the major repair pathway of DSBs, and DNA-PK plays an important role in this repair process [Citation10]. Previously, we reported that heat inactivation of DNA-PK resulted from the heat inactivation of Ku protein(s) [Citation11]. Burgman et al. first reported that heat treatment inactivated Ku 80 and Ku70 [Citation16]. From these results, we hypothesized that the recovery of heat-inactivated DNA-PK activity resulted from the recovery of heat-inactivated Ku protein(s).
In contrast to our hypothesis, Woudstra et al. reported that Ku80 or DNA-PKcs, and hence nonhomologous DSB end joining, did not play crucial roles in the enhancement of cellular radiosensitivity by hyperthermia [Citation21]. Dynlacht et al. also found that Ku80 was not essential for heat-radiosensitization based on the results of survival curves [Citation22]. However, Umeda et al. reported that heat sensitivity of DNA-PK differed among mouse, hamster, and human cells [Citation23]. This suggested that heat sensitivity of Ku was different among these cells. We used mouse cells that exhibited the most heat sensitivity while Dynlacht et al. used human cells that were heat resistant and Woudstra et al. used hamster cells that were moderately heat sensitive. These differences may explain this discrepancy.
DNA-PK activity recovered during incubation at 37 °C after heat treatment. Maximum recovery was observed at 3 h of incubation at 37 °C after heat treatment at 44 °C for 15 min (). Half of this recovery was inhibited in the presence of a cycloheximide concentration that completely inhibited de novo protein synthesis, including HSP72 (inducible HSP70). This result indicated that about half of the recovered DNA-PK activity was not dependent on de novo protein synthesis. Furthermore, the cycloheximide results indicate that de novo protein synthesis, including HSP72, was not necessary for the recovery of heat-inactivated Ku protein(s).
HSPs are known to reactivate thermally inactivated proteins through their function as molecular chaperones [Citation4–9]. Because cycloheximide treatment inhibits de novo protein synthesis, newly synthesized inducible HSP (HSP72) and Ku protein(s) may not play a part in these processes. Thus, we hypothesized that constitutive HSC73 should function as the molecular chaperone for the recovery of heat-inactivated Ku protein(s).
To study protein(s) binding with heat-inactivated Ku protein(s), we used co-immunoprecipitation with an anti-Ku80 antibody. As shown in , the maximum content of Ku80 bound HSC73 was observed at 3 h after heat treatment. This correlated with the maximum recovery of DNA-PK activity that was also detected 3 h after heat treatment. On the other hand, Ku80 bound HSP72 was not observed up to 10 h after heat treatment. These results suggested that heat-inactivated Ku protein(s) might be reactivated by the chaperone activity of HSC73. This conclusion was supported by the good correlation observed between the recovery of DNA-PK activity and the amount of HSC73 bound with Ku80 (). Kim et al. reported that constitutive heat shock element-binding factor (which is identical to Ku70/80) was heat sensitive and recovered during a subsequent incubation [Citation24]. In their experiments, 45 °C was used as the heat treatment. Because our heat treatment was performed at 44 °C, these results are directly comparable.
To confirm that HSC73 participated in the recovery of heat-inactivated DNA-PK activity as a molecular chaperone, we investigated the reactivation of heat-inactivated DNA-PK activity in hybrid cells overexpressing HSC73. In these cells, the recovery of heat-inactivated DNA-PK activity was rapid, with over 60% of the original activity being recovered by 6 h (). Consistent with this finding, less than 20% of DNA-PK activity was recovered during 10 h of incubation in cells treated with HSC73 shRNA. In these cells, only HSP72 is active and the poor recovery indicates that HSP72 is not involved. In cells treated with HSP72 shRNA, recovery of heat-inactivated DNA-PK activity was observed at 3 h, and over 35% of activity was recovered within 6 h of incubation after heat treatment. In these cells, the majority of active HSP was HSC73, while HSP72 was a minor component ().
Because the cellular content of HSP72 was very high, as shown in , it was difficult to inhibit HSP72 expression completely with shRNA. It was also possible that HSC73 shRNA inhibited HSP72 expression (). Nevertheless, the contribution of HSP72 to reactivation of heat-inactivated DNA-PKcs appears to be minimal. A good correlation was observed between the time course of recovery of heat-inactivated DNA-PK and the amount of Ku80 bound HSC73 (). In addition, accumulation of HSP72 was delayed compared with the recovery of DNA-PK activity. Together, these results indicated that HSC73 was the primary participant in the recovery of heat-inactivated DNA-PK activity.
HSC73 has multiple functions as a housekeeping chaperone. These include the folding of newly synthesized proteins and protein translocation [Citation25–27]. Because DSBs are critical forms of cell damage, rapid repair is needed. Cells cannot wait for HSP72 induction for survival. Thus, constitutive HSC73 should participate in the reactivation of heat-inactivated Ku protein(s). At present, we do not know how HSC73, but not HSP72, recognizes heat-inactivated DNA-PK specifically.
Conclusion
In conclusion, heat inactivation of DNA-PK resulted from heat inactivation of Ku protein(s). Recovery of DNA-PK activity occurred after heat inactivation, and at least half of this recovery did not involve de novo protein synthesis. The recovery of heat-inactivated DNA-PK activity within 3 h might result from the reactivation of heat-inactivated Ku protein(s) by HSC73 chaperone activity. This information will be useful to improve the effectiveness of anticancer therapy utilizing a combination of radiation and hyperthermia by suggesting a new means to depress the reactivation of heat-inactivated proteins by HSC73 molecules.
Disclosure statement
The authors alone are responsible for the content and writing of this article and have no competing interests.
Additional information
Funding
References
- Chicheł A, Skowronek J, Kubaszewska M, et al. Hyperthermia-description of a method and a review of clinical applications. Rep Pract Oncol Radiother. 2007;12:267–275.
- El-Awady RA, Dikomey E, Dahm-Daphi J. Heat effects on DNA repair after ionising radiation: hyperthermia commonly increases the number of non-repaired double-strand breaks and structural rearrangements. Nucleic Acids Res. 2001;29:1960–1966.
- Ostapenko VV, Wang X, Ohnishi K, et al. Increased resistance of the radiosensitive M10 mutant cells of the L5178Y mouse lymphoma cell line to heat-induced apoptosis. Radiat Res. 1999;152:321–327.
- Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710.
- Kim YE, Hipp MS, Bracher A, et al. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355.
- Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999;29:748–751.
- Silver JT, Noble EG. Regulation of survival gene hsp70. Cell Stress Chaperones. 2012;17:1–9.
- Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40-a review. Int J Hyperthermia. 2000;16:231–245.
- Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012;136:354–374.
- Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst). 2004;3:909–918.
- Ihara M, Takeshita S, Okaichi K, et al. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia. 2014;30:102–109.
- Davis AJ, Chen BPC, Chen DJ. DNA-PK: a dynamic enzyme in a versatile DSB repair pathway. DNA Repair (Amst). 2014;17:21–29.
- Collis SJ, DeWeese TL, Jeggo PA, et al. The life and death of DNA-PK. Oncogene. 2005;24:949–961.
- Okayasu R, Suetomi K, Yu Y, et al. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345.
- Ihara M, Suwa A, Komatsu K, et al. Heat sensitivity of double-stranded DNA-dependent protein kinase (DNA-PK) activity. Int J Radiat Biol. 1999;75:253–258.
- Burgman P, Ouyang H, Peterson S, et al. Heat inactivation of Ku autoantigen: possible role in hyperthermic radiosensitization. Cancer Res. 1997;57:2847–2850.
- Komatsu K, Ohta T, Jinno Y, et al. Functional complementation in mouse-human radiation hybrids assigns the putative murine scid gene to the pericentric region of human chromosome 8. Hum Mol Genet. 1993;2:1031–1034.
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–1489.
- Suwa A, Hirakata M, Takeda Y, et al. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci USA. 1994;91:6904–6908.
- Yamane M, Hattori H, Sugito K, et al. Cotranslocation and colocalization of hsp40 (DnaJ) with hsp70 (DnaK) in mammalian cells. Cell Struct Funct. 1995;20:157–166.
- Woudstra EC, Konings AWT, Jeggo PA, et al. Role of DNA-PK subunits in radiosensitization by hyperthermia. Radiat Res. 1999;152:214–218.
- Dynlacht JR, Bittner ME, Bethel JA, et al. The non-homologous end-joining pathway is not involved in the radiosensitization of mammalian cells by heat shock. J Cell Physiol. 2003;196:557–564.
- Umeda N, Matsumoto Y, Yin HL, et al. Difference in the heat sensitivity of DNA-dependent protein kinase activity among mouse, hamster and human cells. Int J Radiat Biol. 2003;79:671–680.
- Kim D, Ouyang H, Yang SH, et al. A constitutive heat shock element-binding factor is immunologically identical to the Ku autoantigen. J Biol Chem. 1995;270:15277–15284.
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451.
- Rios PDL, Ben-Zvi A, Slutsky O, et al. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc Natl Acad Sci. 2006;103:6166–6171.
- Mattoo RUH, Goloubinoff P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci. 2014;71:3311–3325.