Abstract
Objective: To evaluate the combined efficacy of high-intensity focused ultrasound (HIFU), gonadotropin-releasing hormone agonist (GnRH-a) and the levonorgestrel-releasing intrauterine system (LNG-IUS) for the treatment of severe adenomyosis.
Method: Four hundred and sixty-six patients with adenomyosis admitted to the Department of Gynecology of Shanghai First Maternity and Infant Hospital underwent HIFU treatment, and then were consecutively administered with GnRH-a 1 d, 1 month and 3 months after HIFU treatment. The uterine size was then measured with ultrasound or MRI 2–4 weeks after three cycles of GnRH-a injection. The LNG-IUS was then inserted when the uterine length less than 9 cm. The visual analog scale (VAS), verbal rating scale (VRS), menstrual volume score, uterus volume, MRI, serum levels of hemoglobin and CA125 were measured at pre and 3-, 6-, 12-month post-HIFU.
Results: Dysmenorrhea and menorrhagia significantly relieved after combined treatment with HIFU, GnRH-a and the LNS-IUS. The uterine volume shrank and returned to its normal size. The serum CA-125 level was reduced to the normal level after the combined treatment.
Conclusions: The combined therapeutic regimen of HIFU, GnRH-a and LNS-IUS is safe, effective and efficient for curing severe adenomyosis.
Introduction
Adenomyosis is a common benign gynecological disorder caused by ectopic endometrial glands and/or stroma tissues invading into the myometrium [Citation1]. Menorrhagia, dysmenorrhea and impaired reproduction are mainly symptoms of this disease [Citation2]. To facilitate surgical treatment, Grimbizis et al. classified adenomyosis into several subtypes: focal adenomyosis, namely, adenomyoma, defined as adenomyotic masses found within the myometrium; diffused adenomyosis, defined as adenomyotic tissues that are extensively scattered into the uterine myometrium; and other uncommon categories including polypoid adenomyomas and special types of adenomyosis [Citation3]. The clinical diagnostic criteria of severe adenomyosis include the following: an enlarged uterus whose size is more than that observed at 12 weeks of gestation; heavy menstrual bleeding and/or algomenorrhea; and sonography or MRI revealing that the outer 2/3 of the myometrium extends with heterotopic endometrial tissues [Citation4–6]. Traditionally, hysterectomy is the ‘golden standard’ for severe adenomyosis therapy, especially for women who do not need to preserve fertility [Citation7]. In recent years, several minimally invasive treatments have been performed [Citation8].
As a noninvasive thermal ablation technique, high-intensity focused ultrasonography (HIFU) has been introduced to treat adenomyosis and has had very good effects on both diffused and focal adenomyotic lesions [Citation9]. With ultrasound guidance, HIFU accurately triggers coagulation necrosis at the target lesion without damaging the adjacent normal tissue. Compared to open surgery, HIFU offers fewer complications, quicker recovery, shorter hospitalization and better quality of life [Citation10]. However, previous studies have also shown that approximately 20% of patients had recurrences soon after HIFU ablation [Citation11,Citation12]; thus, it is crucial to explore new therapeutic schedule to achieve lower recurrence rate.
Both gonadotropin-releasing hormone agonist (GnRH-a) and the levonorgestrel-releasing intrauterine system (LNG-IUS) are common conservative approaches for women with adenomyosis. GnRH-a alleviates dysmenorrhea and induces amenorrhea by suppressing gonadotropin secretion, which causes restrained ovarian function and is also defined as drug oophorectomy [Citation13]. The major side effects include premenopausal symptoms, bone loss and high relapse rate after medicine withdrawal. The LNS-IUS alleviates dysmenorrhea and hypermenorrhea by releasing progestin derivatives, which results in endometrial decidualization [Citation14]. However, the LNS-IUS is not suitable for women with an enlarged uterus especially a uterus the size of that observed at 3 months of gestation, due to high risk of prolapse or expulsion [Citation15].
In this study, we investigated the therapeutic effects of HIFU in combination with GnRH-a and the LNG-IUS for patients with severe adenomyosis, to specifically evaluate the long-term efficacy of the combined treatment.
Materials and methods
Patients
From August 2015 to November 2017, 466 adenomyosis patients with moderate to severe dysmenorrhea and/or menorrhagia at Shanghai First Maternity Hospital were enrolled in this study. The inclusion criteria were as follows: (Citation1) clinical symptoms of progressive dysmenorrhea and/or menorrhagia and no dyspareunia; (Citation2) a large uterine size that was greater than that associated with 12 weeks pregnancy, an irregular spherical shape during gynecological examination or a uterine cavity deeper than 9 cm by hysteroscopy; (Citation3) enlargement of the uterus, an uneven echo of the myometrium and an unclear myometrium and lesion size by transvaginal ultrasound examination; (Citation4) no use of steroid hormones or GnRH-a 6 months before treatment and no contraindications for GnRH-a and an intrauterine contraceptive; (Citation5) refusal laparotomy or laparoscopic surgery; (Citation6) normal liver and kidney functions. The exclusion criteria were as follow: (Citation1) pregnancy, lactation or menstruation; (Citation2) acute pelvic inflammation; (Citation3) suspected gynecological malignancy; (Citation4) history of radiotherapy; (Citation5) inability to communicate with doctors during the procedure.
Preparation before HIFU ablation
Pelvic magnetic resonance imaging (MRI) was performed to evaluate the region, location and vascular supply of the lesion. The patients were required to consume liquid food and mild laxatives for 3 d for routine bowel preparations. Clyster was applied in the morning before the treatment day. The skin was degreased and degassed before treatment. A urinary catheter was inserted to adjust the bladder volume with warm saline irrigation.
HIFU treatment
HIFU ablation was performed with an ultrasound-guided HIFU therapeutic system (JC200, Haifu Medical Technology Co., Chongqing, China). Patients were kept on the treatment bed in a prone position, with the abdominal wall immersed in degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to prevent the bowels from entering into the acoustic pathway. Patients were then tranquilized to relieve any discomfort and prevent unnecessary body movements. SonoVue (Bracco Suisse SA, Cadempino, Switzerland) was injected intravenously at the beginning of HIFU treatment to assess the range and vascular perfusion of the lesion. Ultrasound imaging was used for real-time monitoring of lesion ablation and the status of neighboring normal tissues. The ultrasound evaluation of therapeutic efficacy was performed in accordance with general or greater grayscale changes inside the lesion. At the end of treatment, contrast-enhanced ultrasound was performed to measure the non-perfusion range of the lesion and thereby evaluate the ablation effect.
GnRH-a and LNG-IUS application
Triptorelin (Ipsen S.A., Paris, France), Goserelin (AstraZeneca, Cambridge, UK) or Leuprorelin (Livzon, Zhuhai, China) was injected on the second day, 1 month and 3 months after HIFU treatment. The uterine size was then measured by ultrasound or MRI 2–4 weeks after the third dose of GnRH-a. Patients with uterine lengths of less than 9 cm were implanted with the LNG-IUS (Mirena, Bayer Ag, Turku, Finland).
Clinical evaluation
MRI was performed on the day after HIFU treatment for the evaluation of lesion ablation.
The visual analog scale (VAS), verbal rating scale (VRS), menstrual volume score, uterine volume, MRI exam, and serum levels of hemoglobin and CA125 were measured before- and 3-, 6- and 12-month after HIFU ablation.
The VAS and VRS scores were used for dysmenorrhea evaluation. The left end of the VAS was scored as 0 to represent ‘no pain’ while the right end was scored as 100, representing the ‘most severe pain imagined’. Additionally, the 4-grade VRS was chosen to depict pain intensity: 0 = no pain, 1 = mild pain, 2 = moderate pain and 3 = severe pain [Citation16].
The menstrual volume score was analyzed with the pictorial blood loss assessment chart (PBAC). Patients were instructed to assess the same brand of sanitary napkins and 1/3, 1/3–3/5 and 1 totally drenched sanitary napkin was scored as 1 point, 5 points and 20 points, respectively. A PBAC score of 100 correlated with more than 80 ml of blood loss [Citation17].
The uterine volume was calculated with the following formula: 0.52 × length × anteroposterior diameter × transverse diameter [Citation18].
Statistical analysis
Statistical analysis was performed with Prism version 5.0 GraphPad Software (GraphPad Software Inc., La Jolla, CA). P< .05 was considered statistically significant.
Results
Efficacy of HIFU ablation
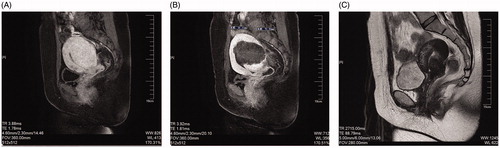
MRI was performed before and on the second-day post-HIFU treatment. As shown in , the enhanced MRI image showed that the myometrium of the posterior uterine wall was thickening with a rich vascular supply before treatment, while no perfusion area was found in the lesion on the second-day post-HIFU ablation. Furthermore, the MRI image revealed complete resolution of the glandular lesions and the uterus almost recovered to its normal size 6 months after HIFU ablation ().
Figure 1. Preoperative and postoperative MRI. (A) Enhanced MRI image of diffused adenomyosis before treatment, which shows that the myometrium of the posterior uterine wall was thickening with a rich vascular supply. (B) Enhanced MRI image acquired for a patient with adenomyosis 1 d after HIFU ablation, which shows a non-perfused region in the lesion. (C) T2WI image obtained 6 months after HIFU ablation for a patient with adenomyosis, which shows complete resolution of the glandular lesions and that the uterus almost recovered to its normal size.
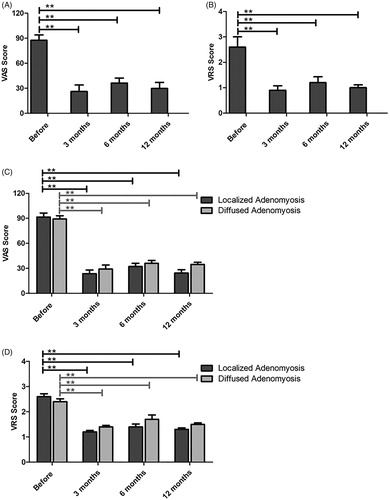
Dysmenorrhea was significantly alleviated after combined treatment with HIFU, GnRH-a and LNS-IUS
The VAS and VRS scores were compared before and after HIFU treatment combined with GnRH-a and LNS-IUS. As shown in and Supplement Table 1, both VAS () and VRS () decreased significantly after the combined treatment of HIFU, GnRH-a and LNS-IUS (p < .05) for all patients diagnosed with adenomyosis. Furthermore, significant pain alleviation for both localized and diffused adenomyosis was evident after the combined therapy (p < .05), with better therapeutic efficacy achieved in localized lesions (). However, no significant differences were found between 3-, 6- and 12-month post-treatment.
Figure 2. The VAS and VRS scores were compared before and after HIFU treatment. (A,B) The VAS (A) and VRS (B) scores decreased significantly after HIFU treatment (p < .05) for all patients diagnosed with adenomyosis. (C,D) Localized and diffused adenomyosis showed significant VAS (C) and VRS (D) score decreases after HIFU ablation (p < .05), with localized lesions achieving better therapeutic efficacy.
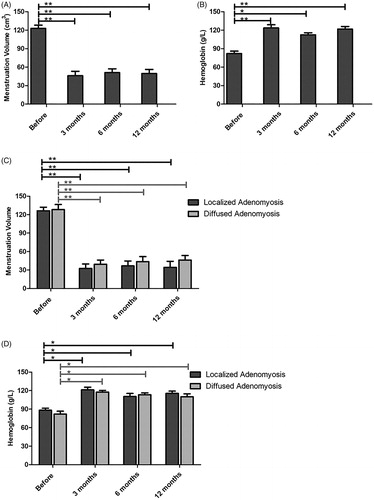
Menstrual volume significantly decreased after combined treatment of HIFU, GnRH-a and LNS-IUS
Menstrual blood loss was evaluated with PBAC, which is considered a valid tool with high specificity and sensitivity [Citation17]. As shown in and Supplement Table 2, patients suffered severe menorrhagia before treatment, based on the menstrual volume score calculated by PBAC, which was 122.9 ± 9.1. The score was reduced to 46.2 ± 12.3, 51.2 ± 10.6 and 49.7 ± 11.4 at 3-, 6- and 12-month post-HIFU treatment respectively, indicating significant improvement in hypermenorrhea. Excessive menstrual bleeding commonly leads to anemia; thus, the hemoglobin level reflects the degree of menstrual bleeding. As shown in and Supplement Table 2, the hemoglobin concentration increased significantly 3-, 6- and 12-month after HIFU treatment, indicating that the combined treatment of HIFU, GnRH-a and LNS-IUS significantly decreased menstrual blood loss. The menstrual volume score and hemoglobin level improved in both the localized and diffused adenomyosis groups, but there was no significant difference between the two groups ().
Figure 3. The menstrual volume score and hemoglobin level were compared before and after HIFU treatment. (A) The menstrual volume score decreased after HIFU treatment. (B) The hemoglobin level increased followed by HIFU ablation. (C,D) Localized and diffused adenomyosis showed significant menstrual volume decreases (C) and hemoglobin increases (D) after HIFU ablation (p < .05) and localized lesions exhibited better treatment outcomes.
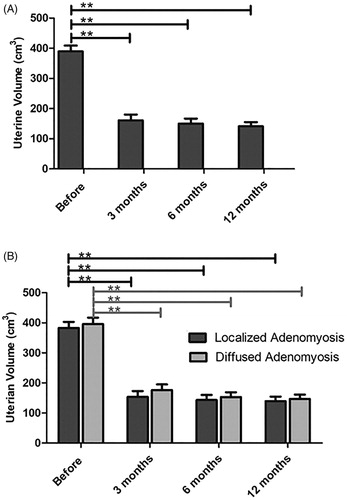
Efficacy of combined treatment of HIFU, GnRH-a and LNS-IUS on the uterine volume
The uterine volume was analyzed by MRI or transvaginal ultrasound. As shown in and Supplement Table 3, the uterine volume was significantly reduced at the third month and almost recovered to its normal size at the 12th month after HIFU ablation, but there was no significant difference between the two groups of adenomyosis patients.
Figure 4. The uterine volume was compared before and after HIFU treatment. (A) The uterine volume was significantly reduced after HIFU ablation. (B) Localized and diffused adenomyosis showed significant uterine volume decreases after HIFU treatment, while localized lesions revealed superior clinical effectiveness.
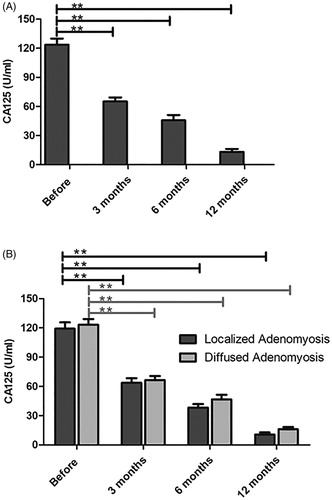
CA-125 decreased to normal levels after the combined treatment
The serum CA-125 level decreased progressively after the combined treatment. As shown in and Supplement Table 4, CA-125 levels decreased significantly 3 months after HIFU ablation. Furthermore, the CA-125 value was within the normal range 12 months after HIFU. Both localized and diffused adenomyosis groups revealed an obvious decline in CA-125 levels, although no significant difference was observed between the two groups.
Figure 5. The serum CA-125 value was compared before and after treatment with HIFU. (A) The serum CA-125 level was significantly reduced with the combined treatment and within the normal range at 12 months after HIFU. (B) Localized and diffused adenomyosis revealed an obvious decline in CA-125 levels and better efficacy was observed for localized lesions.
Discussion
Adenomyosis mainly affects women of reproductive age and leads to infertility and poor quality of life. Currently, there is a lack of accurate treatment guidelines, especially for severe adenomyosis. Hysterectomy is the most effective tool for management, but removing the uterus is crucial for patients with fertility aspirations. Uterine-sparing surgery remains controversial due to difficulties in sufficiently isolating the adenomyotic lesion thus increasing the risk of uterine rupture during pregnancy [Citation19]. Healthcare guidelines aim to relieve clinical symptoms and restore fertility; however, neither hormonal nor non-hormonal drugs are cytoreductive; thus, the high risk of recurrence after discontinuation of medicine remains a vexing question. Furthermore, all medicines have various side effects that impact their long-term use [Citation7].
HIFU therapy is a relatively new noninvasive management tool and has been approved based on its efficacy and reliability in the treatment of adenomyosis [Citation8]. Focused ultrasound waves are targeted to the lesion which leads to an instant temperature rise and other biological effects that cause coagulation necrosis [Citation20]. In our study, every patient underwent an MRI diagnosis for the assessment of location and size before and after HIFU ablation. As shown in , a non-perfusion area was observed by MRI after HIFU treatment. Post-HIFU performance, more than 80% of the adenomyotic lesion was ablated.
Adenomyosis is an estrogen-dependent benign disorder; hence, hormone therapy has been applied and is universally accepted as a supplement for adenomyosis treatment. GnRH-a downregulates estrogen by suppressing gonadotropin secretion due to inhibition of the hypothalamic-pituitary-ovarian axis [Citation21]. Our study showed that GnRH-a treatment following HIFU ablation significantly diminished the adenomyotic lesions and reduced the uterine volume. HIFU combined with 3–4 doses of GnRH-a treatment decreased the uterine volume by 60% and the uterine cavity depth by less than 9 cm. Thus, almost all patients achieved an appropriate uterine size for the implantation of the LNG-IUS, 3 months after GnRH-a application. Local levonorgestrel release from the LNG-IUS led to endometrial atrophy and further shrinkage of the adenomyotic lesions [Citation22]. After further follow-up, the uterine size had become much smaller at 6 months after HIFU ablation and almost recovered to its normal size by 12 months after HIFU treatment.
We have established that combined treatment with HIFU, GnRH-a and the LNG-IUS effectively improves clinical symptoms. The VAS and VRS are the most widely used scales for evaluating pain intensity because of their high sensitivity for assessing pain intensity [Citation16]. Both the VAS and VRS scores significantly decreased after the combined treatment and were maintained at a very low level according to our follow-up evaluation. The PBAC is commonly used to evaluate heavy menstrual bleeding. We found that the PBAC was remarkably decreased and remained at low levels throughout with the three-step treatment. Hemoglobin is strongly correlated with menstrual flow. Our study measured the hemoglobin level and found that most patients suffered varying degrees of anemia before HIFU ablation. The hemoglobin level was clearly elevated after the combined therapy indicating the good therapeutic efficacy for adenomyosis-induced hypermenorrhea.
Several studies have verified the intimate connection between serum CA-125 and adenomyosis [Citation4,Citation23,Citation24]. The serum CA-125 level is regarded as a marker for distinguishing adenomyosis from leiomyoma [Citation23], predicts the development and prognosis of adenomyosis [Citation24] and is used to identify severe adenomyosis [Citation4]. In our study, the average serum CA-125 level was 123.5 ± 11.2 in patients with severe adenomyosis. The CA-125 value decreased significantly with HIFU ablation and subsequent GnRH-a treatment and was maintained at normal levels after the implantation of LNG-IUD.
We also tried to compare the treatment efficacy between localized and diffused adenomyosis. It seems that a better curative effect was observed for localized adenomyosis, because the VAS/VRS scores, PBAC and serum CA-125 levels were decreased more noticeably for diffused lesions. However, no significant statistical difference was observed between the two groups.
In conclusion, the three-step approach of HIFU, GnRH-a and LNG-IUD lowers the risk of hysterectomy and thus improves quality of life, especially for patients with localized adenomyosis. This combined therapeutic regimen is safe, effective and efficient for curing severe adenomyosis.
Supplemental Material
Download MS Excel (8.7 KB)Supplemental Material
Download MS Excel (9.4 KB)Supplemental Material
Download MS Excel (9 KB)Supplemental Material
Download MS Excel (9.1 KB)Acknowledgments
We thank Vishnu Prasad Adhikari for language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Taneichi A, Fujiwara H, Takahashi Y, et al. Influences of uterine adenomyosis on muscle invasion and prognosis of endometrioid adenocarcinoma. Int J Gynecol Cancer. 2014;24:1429–1433.
- Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril. 2018;109:380–388.e1.
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril. 2014;101:472–487.
- Sheth SS, Ray SS. Severe adenomyosis and CA125. J Obstet Gynaecol. 2014;34:79–81.
- Abbott JA. Adenomyosis and Abnormal Uterine Bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Prac Res Clin Obstet Gynaecol. 2017;40:68–81.
- Hulka CA, Hall DA, McCarthy K, et al. Sonographic findings in patients with adenomyosis: can sonography assist in predicting extent of disease? Am J Roentgenol. 2002;179:379–383.
- Tsui KH, Lee WL, Chen CY, et al. Medical treatment for adenomyosis and/or adenomyoma. Taiwan J Obstet Gynecol. 2014;53:459–465.
- Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018;51:119–137.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96:707–714.
- Elhelf IAS, Albahar H, Shah U, et al. High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018;99:349–359.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine. 2016;95:e2443.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Rickes D, Nickel I, Kropf S, et al. Increased pregnancy rates after ultralong postoperative therapy with gonadotropin-releasing hormone analogs in patients with endometriosis. Fertil Steril. 2002;78:757–762.
- Grandi G, Farulla A, Sileo FG, et al. Levonorgestrel-releasing intra-uterine systems as female contraceptives. Exp Opin Pharmacother. 2018;19:677–686.
- Zhang P, Song K, Li L, et al. Efficacy of combined levonorgestrel-releasing intrauterine system with gonadotropin-releasing hormone analog for the treatment of adenomyosis. Med Princ Pract. 2013;22:480–483.
- Aicher B, Peil H, Peil B, et al. Pain measurement: visual analogue scale (VAS) and verbal rating scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgia. 2012;32:185–197.
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–739.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198:373.e1–377.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406–417.
- Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95:900–905.
- Khan KN, Kitajima M, Hiraki K, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25:2878–2890.
- Rose S, Chaudhari A, Peterson CM. Mirena (Levonorgestrel intrauterine system): a successful novel drug delivery option in contraception. Adv Drug Deliv Rev. 2009;61:808–812.
- Mu Y, Hu X, He J, et al. Serum levels of vascular endothelial growth factor and cancer antigen 125 are related to the prognosis of adenomyosis patients after interventional therapy. Int J Clin Exp Med. 2015;8:9549–9554.
- Kil K, Chung JE, Pak HJ, et al. Usefulness of CA125 in the differential diagnosis of uterine adenomyosis and myoma. Eur J Obstet Gynecol Reprod Biol. 2015;185:131–135.
