Abstract
Background: Isolated limb perfusion (ILP) is a treatment option for unresectable in-transit melanoma metastases of the extremities. Approximately two-thirds of the patients have a complete response, and known predictive factors mainly regard tumor burden. In an attempt to identify subgroups with higher response rates, we retrospectively analyzed the predictive value of the BRAF V600E/K mutation for response at our institution.
Methods: Between January 2012 and December 2017, 98 consecutive patients underwent first-time ILP with melphalan for melanoma in-transit metastases and were included in the study. Data was retrieved from our prospectively kept database. Tumor burden was assessed preoperatively as number of lesions and largest tumor diameter. BRAF status was determined according to clinical routine. Response rates were classified according to WHO criteria.
Results: Of the 98 patients included in the analysis, 32 patients had a BRAF V600E/K mutation (33%) and 66 patients were BRAF wild type (wt). There was no difference in age, sex or tumor burden between the groups. Comparing response between BRAF V600E/K mutation and BRAF wt, the overall response rate was 69% vs. 77% (p=.36) and the complete response rate was 47% vs. 52% (p=.67). There was no difference in survival, with a median survival of 47 months.
Conclusion: In this consecutive series of patients, BRAF V600E/K mutation was not found to be a significant factor for response or survival following ILP.
Introduction
Cutaneous melanoma is a cancer with a constantly rising incidence in the world population with estimated 288,000 new cases per year (3.1 per 100,000 age standardized rate) as reported by the GLOBOCAN registry in 2018 [Citation1]. In-transit metastases develop in 4-7% of patients [Citation2,Citation3]. Surgery is still the gold standard, but when intervals between new lesions shortens or lesions are no longer possible to treat with simple excisions, the therapeutic options include different locoregional treatments. Some of these are more recently introduced into the clinical routine, like electrochemotherapy (ECT), talimogene laherparepvec (T-VEC) and Rose Bengal, while isolated limb infusion (ILI) or isolated limb perfusion (ILP) have been used since the 1990s and the late 1950s [Citation4].
In cutaneous melanoma different mutations in oncogenes such as BRAF, MEK, NRAS or tumor suppressors such as NF-1, can promote cell proliferation acting on the mitogen-activated protein kinase pathway (MAPK) [Citation5,Citation6]. Activation of the MAPK pathway is a common and early event in tumor development. About 50% of patients with cutaneous melanoma have an activation of the BRAF mutation. There are two main variants, BRAF V600E and V600K, with the former being the most frequent while the K mutation is associated with earlier metastases and shorter survival[Citation7]. Both types of mutations are associated to high response rates to systemic treatment with BRAF inhibitors [Citation8].
Several predictive factors for response following ILP and ILI have been identified earlier, where the size and the total number of metastases are negative factors for response [Citation9,Citation10]. In 2008, Gallagher et al. reviewed 30 patients that underwent ILI and analyzed the correlation between their mutational status and the clinical response rate. Fifty per cent of the patients carried a BRAF V600E/K mutation and they showed a lower response rate. The overall response rate (ORR) was 27% including a complete response (CR) rate of 7%, compared to the BRAF wt group where the ORR was 43% with a CR rate of 10% [Citation11]. In 2018 Li et al. reported the response results on a series of 150 patients treated with ILI, without, however, any difference in response in relation to their BRAF status [Citation12].
The predictive value of BRAF mutation status in association with response after ILP has not been investigated so far. The aim of this study was to retrospectively analyze a consecutive series of patients with melanoma in-transit metastases, treated with first-time ILP in order to investigate if BRAF mutational status is a predictive factor for response and also to identify any potential impact on survival.
Methods
Patients
A consecutive series of patients (n = 111) with melanoma in-transit metastases treated with first-time ILP at Sahlgrenska University Hospital between January 1st 2012 and December 31st 2017 were identified from a prospectively kept database. Data for both response and BRAF-mutational status were available for 98 patients (88%) and these were included into the final analysis. The study was approved by the Regional Ethical Review Board at the University of Gothenburg (Dnr. 721-08).
Isolated limb perfusion (ILP)
ILP was performed via the brachial (n = 15), external iliac (n = 7) and femoral approach (n = 76). Limb isolation was achieved through clamping and cannulation of the major artery and vein for the extremity under treatment. Isolation of the collateral flow was achieved be means of an inflatable tourniquet or an Esmarch bandage applied proximally on the treated limb. The cannulas were connected to an oxygenated extracorporeal circuit. Continuous leakage monitoring was carried out using a precordial scintillation probe (Medic View, Sweden) to detect and measure leakage of technetium-99m labeled human serum albumin (Vasculosis, Cis-Bio International, Gif-sur-Yvette, France), which was injected into the perfusion circuit. The dose of melphalan was calculated as 13 mg/L perfused tissues for upper limb and 10 mg/L perfused tissues for lower limb.
Response
Clinical responses are reported as the response 3 months after the perfusion, according to WHO criteria [Citation13]. Complete response (CR) is defined as the disappearance of all lesions, partial response (PR) as a decrease of more than 50% of the tumor burden, progressive disease (PD) as a 25% increase in existing lesions or the appearance of new tumors and stable disease (SD) when criteria for neither PR or PD is met.
BRAF mutation analysis
BRAF gene mutation analysis uses DNA sequencing to detect mutations in the BRAF oncogene. The test is used as a clinical routine in Sweden for patients with melanoma stage III and IV, and the analysis is performed by six different clinical molecular pathology laboratories in the different regions of Sweden. Mutational analyses were performed on the primary tumor except in ten cases where the analysis was on lymph nodes resected at the same time as ILP. Data concerning BRAF-mutational status were retrieved from the clinical molecular pathology departments of the referring hospitals.
Toxicity and complications
Local toxicity was evaluated according to the Wieberdink scale up to three months after treatment and recorded as the worst toxicity during that period [Citation14]. Complications were classified according to the Clavien-Dindo scale within 30 days post-operatively [Citation15].
Statistics
IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, New York, USA) was used for statistical analysis. Univariate and multivariate logistic regression using the enter method was used to analyze the role of BRAF status as an independent predictive factor for response. Survival estimates were calculated using the Kaplan Meier method and the log-rank test. Cox-regression analysis using the enter method was used for multivariate analysis for survival. A p-value of < 0.05 was regarded as significant.
Results
Patients
There were 32 patients with a BRAF V600E/K mutation (33%) and 66 patients were BRAF wt (66%), equally distributed between males and females. Data regarding exact type of mutation (V vs K) were not available for 10 patients, mainly due to different laboratory routines performed in the early study period. Only 1 of the remaining 22 patients had a V600 K mutation. For this reason, no further analysis was performed considering specific mutation type. The median age was 72 years (range 40–93 years) with a trend towards lower age in patients with a BRAF V600E/K mutation (p=.09). The majority of the patients (52%) had isolated in-transit metastases (AJCC 7, N2c), 39% had also regional lymph nodes (AJCC7 N3) and nine patients (9%) had limited stage IV disease along with in-transit metastases treated with ILP ().
Table 1. Patient and tumor characteristics.
Response
The overall response rate (ORR) was 69% for BRAF V600E/K and 77% for BRAF wt (p=.36), while the complete response (CR) rate was 47% for BRAF V600E/K patients and 52% for BRAF wt (p=.67), respectively (). In univariate analysis, stage and tumor size were significant predictive factors for complete response, but in the multivariate logistic regression analysis, no independent predictive factor was identified ().
Table 2. Response rates based on BRAF mutational status.
Table 3. Univariate and multivariate logistic regression for complete response.
Table 4. Prognostic factors for survival.
Toxicity and complications
The local toxicity was evaluable in the 97% of patients and showed a grade I toxicity in 12 patients (13%), a grade II toxicity in 51 patients (54%), a grade III toxicity in 28 patients (29%), a grade IV toxicity in four cases (4%) and no grade V toxicity. There was no difference in toxicity between patients with BRAF V600E/K and BRAF wt (p=.41). According to the Clavien-Dindo scale three patients (3%) had a grade I and five patients (5%) a grade II complication.
Survival
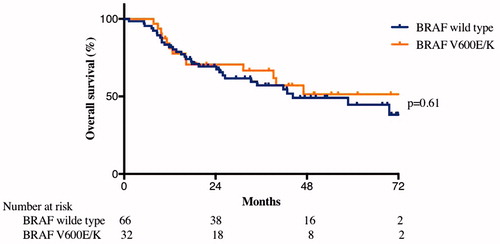
The median survival for all included patients was 47 months. For the BRAF V600E/K patients the median survival was not reached, while it was 44 months for the BRAF wt group. The 2-year OS was 51% for the BRAF V600E/K group and 49% for the BRAF wt group. There was no significant difference in overall survival (p=.61) (). In univariate Cox regression analysis for survival, age, sex, stage and tumor size were identified as prognostic factors, but in the multivariate analysis the only independent factors were age (OR 1.03 p=.02) and stage (N2c vs. N3, OR 2.6 p=.008; N2c vs. M1, OR 18.4 p<.001). Excluding M1 patients from the analysis, the only independent prognostic factors were still only age (OR 1.03, p=.04) and stage (N2c vs. N3, OR 2.1 p=.04) ().
Discussion
The aim of this study was to retrospectively analyze all patients with melanoma in-transit metastases that underwent first time ILP at our institution during a six-year time period and to evaluate the role of BRAF mutation as a predictive factor for response and survival. The indications for ILP were melanoma in-transit metastases (stage N2C, N3) and also stage IV patients with in-transit disease that did not respond to systemic treatment and were not amenable to surgical excision. All data concerning BRAF mutation were collected to test the hypothesis that a MAP kinase pathway activation could represent a factor for higher sensitivity to alkylating drugs like melphalan used under ILP, thus impacting the response rate.
In our analysis, the BRAF status of melanoma patients did not show any influence on response after ILP. Stage and tumor size were the only positive factors at univariate analysis for CR, but at the multivariate analysis it was not possible to identify any independent significant predictive factor. Our results are in accordance with results presented by Li et al. in 150 patients where BRAF status was not found to be predictive for response after ILI [Citation12]. On the contrary, that was not the case when comparing our results to those in the study by Gallagher et al. including 30 patients treated with ILI, where BRAF V600E/K was reported as a strong predictive factor for a worse response [Citation11]. Some data suggests that patients with BRAF mutations are more likely to present with more advanced stage of disease [Citation16], and also in this study there were more patients having N3 disease in the BRAF-mutated group (53% vs. 32%), but without statistical significance.
In the literature, there has been conflicting reports about the prognostic role of BRAF mutational status for survival. An analysis including 197 patients with unresectable stage IIIC and stage IV melanoma showed a worse prognosis for patients carrying a BRAF V600E/K mutation, with overall survival of 5.7 months compared to 8.5 months for non-carriers [Citation17]. These findings can be compared to a study by Carlino et al. including 192 patients that were not treated with BRAF-inhibitors, where BRAF mutational status was not identified as a prognostic factor in advanced melanoma, in that study [Citation18].
Unsurprisingly, there was no difference between BRAF V600E/K and BRAF wt patients concerning neither local toxicity or rates of postoperative complications. Furthermore, only five patients in the BRAF wt group underwent ILP with melphalan and TNF-alpha in our cohort, so it was not possible to evaluate any effect of TNF-alpha. These aspects have never been systematically studied so far, to our knowledge, but it could be interesting to keep track of in future prospective studies.
The main limitation of our study was related to the absence of data regarding NRAS mutational status. NRAS mutation has been reported to be more frequent in extremity melanoma [Citation16] and with a more aggressive behavior [Citation19]. NRAS has been described as a prognostic factor with contrasting data in different studies. In the study of Ugurel et al. [Citation20] NRAS mutation was compared with BRAF V600 E/K and was associated with an improved survival, but in a study by Jacob et al. the NRAS mutation was associated with a worse prognosis [Citation21]. The NRAS mutational status was investigated as a predictive factor for response after ILI by both Gallagher and Li, but without any significance [Citation11,Citation12].
In the last decade, the new targeted therapies using BRAF inhibitors (vemurafenib and dabrafenib) have shown great improvements in overall survival for patients with a BRAF V600E/K mutation both as monotherapy or in combination with MEK inhibitors (cobimetinib and trametinib) [Citation22–28]. Even if patients with a BRAF mutation seems to have worse initial characteristics of the primary lesion (presence of ulceration and a higher mitotic rate), there is no correlation with response to systemic chemotherapy [Citation18]. The response rates after ILP still remain higher in comparison to systemic therapies using BRAF and MEK inhibitors, with an ORR up to 90% and a CR of 60% [Citation29], and still offers an opportunity to gain local control for patients with acquired resistance to MAPK kinase pathway inhibitors [Citation30]. There is a scarce amount of data available on response rates after systemic treatment for in-transit metastases, and to our knowledge no data exists concerning modern immunotherapy. At most units, systemic therapies constitute the first line of treatment, but there are still a large proportion of patients that do not respond and loco-regional therapies still has a valid and highly efficient role to play. However, in the rapidly changing landscape of systemic therapies for melanoma the exact role will most probably have to be redefined in the near future.
Taken together, in this consecutive series of patients with melanoma in-transit metastases treated with first time ILP, BRAF mutational status was not a significant factor for response or survival. It would be interesting to perform wider genomic analyses investigating the potential correlations with response after both ILP and ILI in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Figure 1. Overall survival. There was no significant difference in overall survival depending on if the patients were BRAF V600E/K or BRAF wild type (p = 0.61). Median survival for BRAF wild type patients was 44 months, while for patients with BRAF V600E/K mutation, the median survival was not reached.
Additional information
Funding
References
- World Health Organization. International Registry for Research on Cancer GLOBOCAN 2018. Estimated number of new cases in 2018, w., both sexes, all ages catecorized by type of tumor. Available from: http://gco.iarc.fr Last update September 2018.
- Read RL, Haydu L, Saw RP, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22(2): 475–481.
- Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–596.
- Testori A, Ribero S, Bataille V. Diagnosis and treatment of in-transit melanoma metastases. Eur J Surg Oncol. 2017;43(3):544–560.
- Goel VK, Lazar AJ, Warneke CL, et al. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126(1):154–160.
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696.
- Li Y, Umbach DM, Li L. Putative genomic characteristics of BRAF V600K versus V600E cutaneous melanoma. Melanoma Res. 2017;27(6):527–535.
- Forbes SA, Bhamra G, Bamford S, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet. 2008; Chapter 10:Unit 10,11.
- Muilenburg DJ, Beasley GM, Thompson ZJ, et al. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin d in melanoma. Ann Surg Oncol. 2015;22(2):482–488.
- Olofsson R, Mattsson J, Lindner P. Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hyperthermia. 2013;29(6):551–557.
- Gallagher SJ, Thompson JF, Indsto J, et al. p16INK4a expression and absence of activated B-RAF are independent predictors of chemosensitivity in melanoma tumors. Neoplasia. 2008;10(11):1231–1239.
- Li S, Sheng X, Si L, et al. Outcomes and predictive factors of isolated limb infusion for patients with in-transit melanoma in China. Ann Surg Oncol. 2018;25(4):885–893.
- World Health Organization. WHO handbook for reporting results of cancer treatment. WHO offset publication no 48. Geneva Albany, N.Y.: World Health Organization; sold by WHO Publications Centre USA; 1979. p. 45.
- Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18(10):905–910.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Ellerhorst JA, Greene VR, Ekmekcioglu S, et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res. 2011;17(2):229–235.
- Long GV, Menzies AM, Nagrial AM, et al., Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246.
- Carlino MS, Haydu LE, Kakavand H, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br J Cancer. 2014;111(2):292–299.
- Adler NR, Wolfe R, Kelly JW, et al. Tumour mutation status and sites of metastasis in patients with cutaneous melanoma. Br J Cancer. 2017;117(7):1026–1035.
- Ugurel S, Thirumaran RK, Bloethner S, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS One. 2007;2(2):e236.
- Jakob JA, Bassett RL, Jr., Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–4023.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365.
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–332.
- Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754.
- Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–1639.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823.
- Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, et al. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 2010;15(4):416–427.
- Kakadia S, Yarlagadda N, Awad R, et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095–7107.
