Abstract
The role of thermal ablation in the management of T1b renal masses is not well defined. The purpose of this review is to examine current evidence for cryoablation, radiofrequency ablation, and microwave ablation of T1b renal masses as well as review current AUA and EAU guidelines for thermal ablation of T1b masses. Given the size of these tumors, adjunctive maneuvers are often necessary to ensure patient safety and protect vital adjacent structures.
Introduction
The incidence of renal cell carcinoma (RCC) in the United States and Canada has been slowly increasing, albeit with corresponding decrease in related mortality [Citation1]. In part, this is almost certainly related to the growing number of incidental renal masses, which tend to be smaller and of lower stage, and greater emphasis on nephron sparing approaches for tumor management [Citation2].
The European Association of Urology guidelines for RCC, updated in 2014, maintains an emphasis on surgical management of localized renal tumors [Citation3]. Following a vigorous systematic review using multiple databases, the Guideline Panel quoted a lack of quality data to allow any definitive conclusions regarding oncologic outcomes to make regarding ablation of renal tumors. In turn, the EAU stated that cryoablation, radiofrequency ablation, and active surveillance can be offered to elderly patients and those patients with comorbid illness and limited life expectancy with small renal masses.
Current American Urological Association (AUA) 2017 guidelines recommend partial nephrectomy as standard of care in the management of localized renal cell cancer [Citation4]. Such localized extirpation is associated with excellent local tumor control. This nephron-sparing approach also minimizes the risk of new chronic kidney disease or progression of preexisting chronic kidney disease. The latest AUA guidelines do not specifically address the treatment of T1b renal masses (measuring 4.1–7.0 cm and localized to the kidney), but rather acknowledge controversy regarding the difficulty of partial nephrectomy in patients with such tumors.
While originally reserved for those patients with comorbid disease or contraindications to surgery, thermal ablation has slowly been incorporated into the management of patients with a small renal masse. Historical ablation outcomes, primarily using heat-based radiofrequency ablation (RFA), showed limitations in treating larger renal tumors. These less successful outcomes have tainted the overall efficacy of thermal ablation, as reflected in historical treatment algorithms [Citation5]. Nevertheless, expanding experience, has afforded the opportunity to appreciate the strengths and weaknesses of ablation modalities to optimize treatment of larger tumors. In this review, we will consider the three primary thermal ablation modalities of radiofrequency ablation, microwave ablation, and cryoablation in the management of T1b renal masses and the current clinical guidelines that address the role of ablation in managing patients with such tumors.
Radiofrequency ablation
Radiofrequency ablation (RFA) is the most established heat-based thermal ablation technique for treating small renal masses. RFA utilizes alternating current to create ionic agitation and frictional/resistive heating near the electrode while also relying on thermal conduction to create an ablation zone. Temperature monitoring is performed to ensure lethal heat is generated (>60 degrees C). Unfortunately, RFA is susceptible to tissue impedance factors, charring, and heat sink which can limit oncologic efficacy.
While RFA has been included in established treatment algorithms for small T1a renal masses, outcomes for T1b tumors remain unfavorable. In 2005, Gervais et al reported a six year experience treating 100 renal masses with RFA and found small size (p < 0.00001) and noncentral location (p = 0.0049) were independent predictors of complete necrosis during the initial treatment session [Citation6]. Nine tumors ranging in size from 4.0 to 8.9 cm in seven patients had local recurrence. No tumors less than 4 cm failed RFA ablation in the cohort [Citation6].
Zagoria et al published their experience treating 48 RCCs in 41 patients [Citation7]. Recurrence after one ablation session was noted in five tumors (12%) with the median size of 5.2 cm. No tumors less than 4 cm were locally recurrent in the study and the authors conclude that for larger RCC lesions, alternate treatments should be considered. Psutka et al examined long-term RFA outcomes in their cohort with a median follow up of 6.5 years [Citation8]. They found a 14.3% local recurrence rate for T1b lesions compared to 4.2% for T1a renal masses. Five-year disease-free survival (DFS) was 74.5% in the T1b cohort compared to 91.5% in the T1a group [Citation8]. Multiple additional studies have noted recurrence-free survival greater than 95% for small renal masses (less than 3 cm) treated with RFA with recurrence free survival (RFS) dropping to 60–85% for larger renal masses [Citation6–12]. Several studies have reported similar cancer specific and disease-free survival outcomes in patients undergoing RFA or nephrectomy (radical or partial) for T1b renal masses [Citation9,Citation12] with improved preservation of renal function with ablation. However, Takaki found significantly lower overall survival in their RFA cohort when compared to radical nephrectomy (RN) at ten years (48% vs. 97%) as well as primary local control of 81% after RFA [Citation12]. Based on the published experience, RFA outcomes for T1b renal masses are heterogeneous and alternate means of thermal ablation should be considered in this cohort.
Complications associated with RFA are relatively rare and include hemorrhage, nerve injury, urothelial stricture, and urine leak. A review of 573 ablation procedures at the Mayo Clinic noted a 4.7% major complication rate with RFA [Citation13]. The most common complications were urothelial stricture (2.1%) and nerve injury (3.9%). The authors note that nerve injury is often avoidable with hydrodisplacement of the kidney from the adjacent psoas muscle or body wall.
Microwave ablation
Microwave ablation (MWA) is a second thermal ablation technique based on the generation of extreme heat at the distal end of an applicator using a dielectric heating mechanism. An alternating electromagnetic field emanating from the applicator causes polar molecules to continuously re-align, resulting in kinetic energy and secondary heat. Compared to RFA, the potential size of the ablation zone is larger due to limited impact of tissue impedance on energy deposition and higher temperatures created with secondary increased passive heating.
Early outcomes using MWA for the treatment of large renal tumors were unfavorable. Of the 4 T1b renal tumors in Castle et al’s experience, residual/recurrent tumor occurred in 2 [Citation14]. In a cohort of 8 T1b renal masses treated with MWA at the PLA General Hospital in Beijing, China, technical effectiveness (within one month of treatment) was achieved in 7 (87.5%) and local tumor progression was subsequently seen in 3 tumors measuring 5.5 cm, 5.8 cm, and 6.8 cm [Citation15]. Univariate analysis in this study showed tumor size to be significantly associated with disease free survival.
In 2016, Gao et al published their series of select patients with renal tumors adjacent the renal sinus [Citation16]. In this technically challenging cohort, initial ablation success (within 3 months) was achieved in 9 of 12 patients with T1b RCC; the three patients with residual tumor underwent successful retreatment. Ultimately, 4 patients with T1b RCC developed local tumor progression after 3 months, yielding a 3 year disease free survival of 60%.
Most recently, Wells et al published his experience in treating 30 consecutive renal tumors with MWA, including 7 T1b RCC [Citation17]. At mean and median follow-up of 6 months, there has been no local tumor progression. Recognizing the favorable outcomes, the authors called for larger cohorts and longer follow-up to validate oncologic efficacy.
Based on published experience, overall complications following MWA of larger renal masses appear to be similar to the treatment of T1a tumors. Complications occur in 3–17% of patients, and have been reported to include perirenal hematoma, urinoma formation, and skin dysesthesia [Citation18–21]. Importantly, the high heat generated by MWA can lead to significant urothelial injury. Secondary ureteropelvic strictures, including those remote from the ablation site, have been reported in multiple series [Citation14,Citation21,Citation22]. Thus, caution is needed in the thorough treatment of larger tumors.
Cryoablation
In the treatment of larger renal masses, cryoablation has demonstrated the greatest potential for effective treatment (). Taking advantage of multiple (8 or more) applicators, very large volumes of lethal ice can be generated such that effective outcomes may be achieved in the treatment of renal tumors measuring over 7 cm [Citation23].
Table 1. Ablation studies including the treatment of T1b renal masses.
It has been shown that the edge of the iceball based on CT imaging corresponds to a temperature of 0 °C [Citation24]. This ability to monitor the destructive ablation and grow the iceball beyond the tumor margin is a very important attribute that can provide a high level of treatment efficacy and secondary operator confidence. An additional important consideration related to the treatment of large renal masses is the size-related inherent encroachment of ice on critical structures such as small and large bowel. The ability to effectively monitor tissue destruction with CT allows such proximities to be navigated with relative safety, particularly when using adjunctive techniques such as hydrodissection. Such encroachment often includes the renal collecting system. In contrast to the heat-based techniques, lethal ice can be propagated into the collecting system with only remote risk of urine leak and urinoma [Citation25–28].
The Mayo Clinic group published their experience in treating 46 patients with T1b renal cell carcinoma using percutaneous cryoablation [Citation29]. Adjunctive techniques were frequently used, including prophylactic embolization to minimize bleeding (15% patients), retrograde irrigation of the collective system via externalized ureteral stent (15% patients), and hydrodissection (35%). A single (2%) technical failure was identified within 3 months of treatment and local recurrence-free survival at 3 years was 96%. However, the complication rate in this series was 15%, and the authors emphasized the need for multidisciplinary management in pursuing ablative treatment of such large tumors.
Blute et al reviewed their experience in the treatment of 139 patients with percutaneous cryoablation [Citation30]. Patients had tumors of mean size of 2.4 cm, but ranging up to 6.5 cm, including 44 patients with t1b renal masses. Recognizing that local tumor progression only developed in 7% of patients, the authors found that tumor size was not associated with such tumor recurrence; the specific number of T1b tumors with recurrence was not provided.
In a large series of patients treated with cryoablation, Aoun et al included a cohort of 67 patients with T1b RCC [Citation31]. With reported overall mean follow-up of 32 months, successful treatment (i.e. no local recurrence) was achieved in 94% in these patients. An interesting observation recorded in this series was a mean iceball size of 5 cm in the treatment of 382 tumors overall, including iceballs up to 10.5cm in mean diameter. Such information describes the potential of cryoablation to treat large neoplasms.
Even more recently, Hebbadj et al described the cryoablation of T1b renal masses in 27 patients over 8 years [Citation32]. Complexity of treatment was evident in the need to displace adjacent anatomic structures during 21 of the procedures. In their experience, technical efficacy was achieved in 87.5% of patients and 3 additional patients went on to develop local tumor progression. A single patient died due to evolution of RCC metastases, yielding a cancer-specific survival in their study cohort of 95.7% at 3 years.
Others have had less success in the treatment of T1b renal masses. Caputo et al reported the Cleveland Clinic experience in treating 31 T1b renal tumors (71% shown to be RCC) [Citation33]. In comparing cryoablation with partial nephrectomy, they found the rate of local tumor progression to be much greater with ablation, specifically 23% versus none. In reviewing the methods of ablation, it is worth noting that 25 of the 31 cryoablation procedures were performed laparoscopically using intraoperative ultrasound guidance, with inherent limitations of ultrasound in imaging through solid ice. In addition, the authors reported a mean 2 cryoprobes (of 2.4 mm, 3.8 mm, and 4.6 mm outer diameter) used per treatment. Although additional details regarding probe use were not provided, others have shown that the maximum volume of lethal ice using two cryoprobes of 2.4mm diameter is 3.3cm [Citation34], and using two probes of 3 mm is 3.5–4.0 cm [Citation35].
The successful treatment of large renal masses using cryoablation requires aggressive placement of multiple cryoprobes; as a general rule, one cryoprobe of 2 mm or larger in outer diameter is placed per cm of tumor. As above, the sheer size of the tumor often includes extension of the tumor centrally in the kidney. This requires cryoprobe placement in the region of the vascular renal pelvis, with probes often in close proximity, to overcome convection vascular warming effects (). Several studies have shown a relationship of bleeding complications with tumor size, number of cryoprobes, and central location of the tumor [Citation13,Citation31,Citation36]. Thus, diligent periprocedural management of patients undergoing cryoablation of larger tumors is needed, often including collaboration between radiologists, urologists, and anesthesiologists.
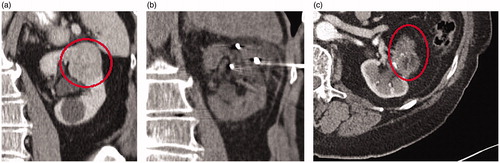
Figure 1. Seventy-two-year-old male presents with a large 7 cm right renal mass. Due to extensive medical comorbidities, he was referred for cryoablation. Preablation contrast-enhanced axial CT demonstrates a 7 cm solid exophytic mass arising from the lower pole right kidney (A). Seven cryoablation needles were placed into the mass using US/CT guidance and a 12 min freeze-5 min passive thaw-12 min refreeze was performed (B). Contrast enhanced CT at 28 months post ablation demonstrates no evidence of residual disease (C).
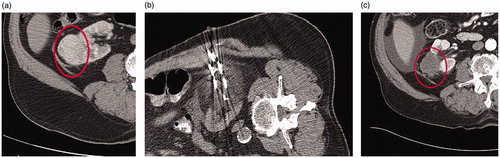
Figure 2. Forty-nine-year-old male presents with a 5 cm interpolar left renal mass. Preablation contrast-enhanced coronal CT demonstrates a heterogeneous, enhancing 5 cm mass in the interpolar left kidney (A). A biopsy during ablation revealed grade 2 clear cell renal cell carcinoma. Six cryoablation needles were used to perform a 8 min freeze- 5 min passive thaw- 10 min refreeze (B). Contrast enhanced CT five years after ablation demonstrates no evidence of recurrent RCC (C).
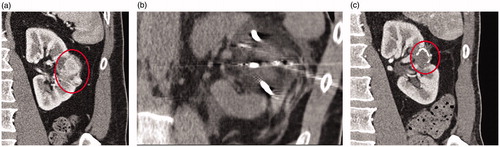
Figure 3. Eighty-year-old male presents with a 4.9 cm upper pole left renal mass. Preablation contrast enhanced coronal CT demonstrates a enhancing 4.9 cm mass in the upper pole left kidney (A). Pre ablation biopsy revealed grade 2 clear cell renal cell carcinoma. Four cryoablation needles were placed into the mass using ultrasound guidance and a 12 min freeze- 5 min passive thaw- 12 min refreeze was performed (B). Contrast enhanced coronal CT 72 months after ablation demonstrates no evidence of recurrent tumor (C).
Adjunctive maneuvers
Particularly related to cryoablation of larger tumors, significant bleeding can result due to the use of multiple applicators and/or central placement of the applicators [Citation13,Citation36]. Cracking of the iceball, presumably due to differential expansion/contraction of tissue during dynamic temperature fluctuations, may also occur and result in significant hemorrhage [Citation37,Citation38]. Accordingly, prophylactic selective renal artery embolization should be considered to minimize this risk of bleeding. In one small study, patients treated with such embolization had smaller post-procedural hematomas compared to those who did not have such treatment [Citation39]. Embolization should also be considered prior to ablation in those patients with need for aggressive anticoagulation following ablation (e.g. recent coronary stent placement or arterial embolus). Peri-procedural arterial blood pressure monitoring is helpful for real-time assessment of patient hemodynamics to help differentiate normal post-procedural bleeding from that warranting urgent intervention. Close observation following treatment, often including brief hospitalization, is warranted particularly for frail patients.
As stated above, larger renal masses inherently encroach on adjacent structures with related risk of thermal injury. Image-guided infusion of fluid, gas, or gel can be used to provide mechanical displacement between the index renal mass and such critical structures [Citation40–42]. Such displacement techniques allow necessary aggressive treatment of large renal masses. Additionally, CT or magnetic resonance imaging (MRI) of the iceball is needed to ensure patient safety and monitoring of the treatment as it relates to adjacent critical structures [Citation43].
Thermal ablation and the T1b renal mass – current guidelines
Thermal ablation represents a valuable management strategy in the treatment armamentarium for clinically localized renal masses, particularly among patients with an unfavorable comorbidity profile in whom extirpation is not feasible. The American Urologic Association (AUA) guideline panel currently endorses the elective use of percutaneous thermal ablative techniques, including both radiofrequency ablation (RFA) and cryoablation, for renal masses <3 cm in greatest diameter [Citation4]. This recommendation, which implicitly advocates against use of ablative strategies for lesions exceeding the 3 cm size cutoff, is premised primarily on studies with relatively longer follow-up (>36 months) that cite a higher risk for locoregional relapse when compared to partial nephrectomy [Citation44–46].
The AUA guideline committee does recognize, however, that surgical and ablative treatments offer comparable metastasis-free and cancer-specific survival for the small renal mass, which likely reflects the ability to successfully salvage local recurrence after ablative therapy with repeat ablation [Citation4,Citation47]. Moreover, current evidence guiding the use of ablative therapies occurs mainly in the form of retrospective single-institutional studies, the results of which are further qualified by inconsistencies in treatment delivery (i.e. the number of probes utilized; use of adjuvant maneuvers to improve tumor accessibility) and approach to intra-procedural monitoring – variables that profoundly influence treatment success. Indeed, several experienced centers have reported favorable oncologic outcomes after percutaneous cryoablation for biopsy-proven T1b RCC with intermediate follow-up, suggesting broader applicability of thermal ablation through refinements in technique [Citation29,Citation31].
Nevertheless, until such data matures, the current consensus among the AUA guideline panel is that individuals presenting with a T1b tumor be preferentially managed by partial or radical nephrectomy if they are deemed fit for surgery [Citation4]. Ablation for larger tumors may be considered in the presence of comorbidities that preclude the use of surgery; however, even in this context, patients should be counseled on the higher perioperative risks [Citation13,Citation29,Citation48] and potential for relapse [Citation4,Citation47].
The European Association of Urology (EAU) offers a more restricted endorsement of thermal ablation for localized renal masses, irrespective of size, due mainly to the lack of high-quality evidence supporting its use in comparison to more established surgical techniques. The most recent guideline statement suggests that ablative techniques primarily in the form of cryoablation and RFA be reserved for patients with the small renal mass who are either elderly and thus limited in their actuarial life expectancy, or those with competing health risks that would more substantially impact longevity [Citation3]. The guideline panel cites marked heterogeneity in reported oncologic outcomes as well as postprocedural complication risk and renal functional change for ablation compared to surgery; specifically, it highlights that no study to-date demonstrates superior cancer-control for ablation versus surgery [Citation49,Citation50] and that any perioperative or renal functional benefit derived from ablation appears to be of minimal clinical significance [Citation51,Citation52]. As such, by extension, the EAU does not acknowledge a role for thermal ablation in tumors >4 cm in size.
Conclusion
Thermal ablation of T1b RCC is a promising emerging treatment for select patients when properly applied. Current evidence is heterogeneous and limited by thermal modality utilized, number of probes/antennae used, and operator technique (laparoscopic vs. percutaneous, method of intraprocedural monitoring). Cryoablation and microwave ablation likely yield the best opportunity for durable oncologic efficacy. Further studies are needed to assess longitudinal oncologic outcomes in this cohort.
Disclosure statement
All authors have no conflict of interest.
References
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530.
- Palsdottir HB, Hardarson S, Petursdottir V, et al. Incidental detection of renal cell carcinoma is an independent prognostic marker: results of a long-term, whole population study. J Urol. 2012;187:48–53.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924.
- Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529.
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279.
- Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol. 2005;185:64–71.
- Zagoria RJ, Pettus JA, Rogers M, et al. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77:1393–1397.
- Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486–492.
- Chang X, Zhang F, Liu T, et al. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: long-term clinical and oncologic outcomes. J Urol. 2015;193:430–435.
- Iannuccilli JD, Dupuy DE, Beland MD, et al. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol. 2016;26:1656–1664.
- Pantelidou M, Challacombe B, McGrath A, et al. Percutaneous radiofrequency ablation versus robotic-assisted partial nephrectomy for the treatment of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2016;39:1595–1603.
- Takaki H, Soga N, Kanda H, et al. Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage T1b renal cell carcinoma. Radiology. 2014;270:292–299.
- Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. 2012;23:48–54.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77:792–797.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012;263:900–908.
- Gao Y, Liang P, Yu X, et al. Microwave treatment of renal cell carcinoma adjacent to renal sinus. Eur J Radiol. 2016;85:2083–2089.
- Wells SA, Wheeler KM, Mithqal A, et al. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol. 2016;41:1203–1211.
- Chan P, Velasco S, Vesselle G, et al. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol. 2017;72:786–792.
- Ierardi AM, Puliti A, Angileri SA, et al. Microwave ablation of malignant renal tumours: intermediate-term results and usefulness of RENAL and mRENAL scores for predicting outcomes and complications. Med Oncol. 2017;34:97.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284:272–280.
- Mansilla AV, Bivins EE, Jr., Contreras F, et al. CT-guided microwave ablation of 45 renal tumors: analysis of procedure complexity utilizing a percutaneous renal ablation complexity scoring system. J Vasc Intervent Radiol. 2017;28:222–229.
- Thompson SM, Schmitz JJ, Thompson RH, et al. Introduction of microwave ablation into a renal ablation practice: valuable lessons learned. AJR.2018;211:1381–1389.
- Moynagh MR, Schmit GD, Thompson RH, et al. Percutaneous cryoablation of clinical T2 (> 7 cm) renal masses: technical considerations, complications, and short-term outcomes. J Vasc Intervent Radiol. 2015;26:800–806.
- Saliken JC, McKinnon JG, Gray R. CT for monitoring cryotherapy. AJR Am J Roentgenol. 1996;166:853–855.
- Brashears JH, 3rd, Raj GV, Crisci A, et al. Renal cryoablation and radio frequency ablation: an evaluation of worst case scenarios in a porcine model. J Urol. 2005;173:2160–2165.
- Janzen NK, Perry KT, Han KR, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol. 2005;173:1368–1374.
- Rosenberg MD, Kim CY, Tsivian M, et al. Percutaneous cryoablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of hemorrhagic and collecting system complications. AJR Am J Roentgenol. 2011;196:935–939.
- Sung GT, Gill IS, Hsu TH, et al. Effect of intentional cryo-injury to the renal collecting system. J Urol. 2003;170:619–622.
- Atwell TD, Vlaminck JJ, Boorjian SA, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Intervent Radiol. 2015;26:792–799.
- Blute ML, Jr., Okhunov Z, Moreira DM, et al. Image-guided percutaneous renal cryoablation: preoperative risk factors for recurrence and complications. BJU Int. 2013;111:E181–E185.
- Aoun HD, Littrup PJ, Jaber M, et al. Percutaneous cryoablation of renal tumors: is it time for a new paradigm shift?. J Vasc Interv Radiol. 2017;28:1363–1370.
- Hebbadj S, Cazzato RL, Garnon J, et al. Safety considerations and local tumor control following percutaneous image-guided cryoablation of T1b renal tumors. Cardiovasc Intervent Radiol. 2018;41:449–458.
- Caputo PA, Zargar H, Ramirez D, et al. Cryoablation versus partial nephrectomy for clinical T1b renal tumors: a matched group comparative analysis. Eur Urol. 2017;71:111–117.
- Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351.
- Auge BK, Santa-Cruz RW, Polascik TJ. Effect of freeze time during renal cryoablation: a swine model. J Endourol. 2006;20:1101–1105.
- Kakarala B, Frangakis CE, Rodriguez R, et al. Hemorrhagic complications of percutaneous cryoablation for renal tumors: results from a 7-year prospective study. Cardiovasc Intervent Radiol. 2016;39:1604–1610.
- Hruby G, Edelstein A, Karpf J, et al. Risk factors associated with renal parenchymal fracture during laparoscopic cryoablation. BJU Int. 2008;102:723–726.
- Schmit GD, Atwell TD, Callstrom MR, et al. Ice ball fractures during percutaneous renal cryoablation: risk factors and potential implications. J Vasc Interv Radiol. 2010;21:1309–1312.
- Woodrum DA, Atwell TD, Farrell MA, et al. Role of intraarterial embolization before cryoablation of large renal tumors: a pilot study. J Vasc Interv Radiol. 2010;21:930–936.
- Farrell MA, Charboneau JW, Callstrom MR, et al. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181:1315–1317.
- Kam AW, Littrup PJ, Walther MM, et al. Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2004;15:753–758.
- Wang X, Zhao X, Lin T, et al. Thermo-sensitive hydrogel for preventing bowel injury in percutaneous renal radiofrequency ablation. Int Urol Nephrol. 2016;48:1593–1600.
- Cazzato RL, Garnon J, Shaygi B, et al. How to perform a routine cryoablation under MRI guidance. Top Magn Reson Imaging. 2018;27:33–38.
- Best SL, Park SK, Yaacoub RF, et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol. 2012;187:1183–1189.
- Gervais DA, Arellano RS, McGovern FJ, et al. Radiofrequency ablation of renal cell carcinoma: part 2, Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:72–80.
- Tanagho YS, Roytman TM, Bhayani SB, et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology. 2012;80:307–314.
- Pierorazio PM, Johnson MH, Patel HD, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-analysis. J Urol. 2016;
- Sidana A, Aggarwal P, Feng Z, et al. Complications of renal cryoablation: a single center experience. J Urol. 2010;184:42–47.
- Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252–259.
- Whitson JM, Harris CR, Meng MV. Population-based comparative effectiveness of nephron-sparing surgery vs ablation for small renal masses. BJU Int. 2012;110:1438–1443.
- Guillotreau J, Haber GP, Autorino R, et al. Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol. 2012;61:899–904.
- Klatte T, Mauermann J, Heinz-Peer G, et al. Perioperative, oncologic, and functional outcomes of laparoscopic renal cryoablation and open partial nephrectomy: a matched pair analysis. J Endourol. 2011;25:991–997.
