Abstract
Purpose: In this study, we evaluated the efficacy of microwave ablation-assisted laparoscopic hepatectomy (MLH) for the management of hepatocellular carcinoma (HCC) in cirrhotic patients.
Methods: Data from HCC patients with liver cirrhosis who underwent laparoscopic hepatectomy (LH) or MLH in Shengjing Hospital (Shenyang, China) were retrospectively analyzed from January 2013 to June 2017. The demographic characteristics, clinical features, intraoperative parameters and surgical outcomes were analyzed and compared. Propensity scores matching (PSM) analysis was used to minimize bias.
Results: A total of 54 patients were enrolled in the MLH group and 39 patients in the LH group. Following 1:1 matching by PSM analysis, 26 patients were selected from each group. Compared to the LH group, patients in the MLH group had significantly decreased intraoperative bleeding (48.0 vs. 203.9 ml, p < .0001) and reduced demand for hepatic inflow occlusion (0 vs. 6, p = .009). No significant difference was observed in average operation time (155.7 vs. 148.5 min) and postoperative hospitalization time (8.3 vs. 9.3 d) between the MLH and LH groups. Similarly, the 1-year and 3-year recurrence-free survival (RFS) rates as well as the 1-year and 3-year overall survival (OS) rates of the MLH and LH groups were not significantly different (83.1 vs. 82.4% and 64.6 vs. 36.6% as well as 100 vs. 95.8% and 93.8 vs. 59.1%, respectively: p > .05).
Conclusions: MLH significantly decreased intraoperative bleeding and reduced the need for hepatic occlusion without compromising the surgical outcome. Therefore, microwave ablation could be a valuable tool for LH in HCC patients with cirrhosis.
Introduction
In China, hepatocellular carcinoma (HCC) is the third most common cancer in males and the sixth in females, and HCC is the third leading cause of cancer-related death among males and females [Citation1]. To date, surgical resection by either open or laparoscopic surgery is the first choice for HCC management [Citation2]. Compared to open surgery, laparoscopic hepatectomy (LH) has several merits including reduced intraoperative blood loss, shorter operating time, fewer postoperative complications, shorter hospitalization period, and longer overall survival (OS) rate [Citation3–8].
In spite of technical advances, LH has its own set of challenges that impact on postoperative recovery and long-term outcome [Citation9–12]. Patients with cirrhotic liver usually present with impaired liver functions, portal hypertension and hypersplenism [Citation13]. The abundance of collateral circulation facilitates uncontrolled bleeding during liver resection, thereby increasing the risk of mortality and postoperative morbidity [Citation12]. Therefore, intraoperative hemorrhage remains one of the major concerns facing LH owing to the difficulty of achieving hemostasis by compression, suturing or hepatic occlusion [Citation9–11]. Additionally, in China, most HCC patients are susceptible to posthepatitic cirrhosis which further increases the risk of liver resection, especially in LH settings [Citation14]. Taken together, by developing bleeding-control regimens we will improve the outcomes of LH and the patients’ prognoses.
Precoagulation of the surgical margin by thermal ablation is a method to control bleeding during the operation. During thermal ablation, the generated heat is utilized to coagulate the surrounding blood vessels and liver tissues, thereby destroying the internal tumor directly and forming a blood-free zone [Citation15]. In 2002, the first precoagulation-assisted liver resection was performed by radiofrequency [Citation16]. After that, accumulating evidence suggests that precoagulation along the hepatic transection plane indeed minimizes intraoperative bleeding [Citation9,Citation17]. Simultaneously, the coagulative necrosis zone can also reduce the tumor-spreading risk during parenchymal resection [Citation12,Citation17]. This innovative technique combines the merits of liver resection and pre-thermal ablation leading to minimal blood loss during resection and favorable long-term survival [Citation9,Citation18,Citation19]. Microwave ablation is a faster technique that achieves higher ablation volume with lower heat injury to the blood vessels compared to radiofrequency ablation [Citation20]. The development of laparoscopic ultrasound devices has enabled microwave ablation to be employed along with laparoscopic liver surgery [Citation9]. In this study, we aimed to assess the short-term outcomes and oncologic efficiency of microwave ablation-assisted LH in cirrhotic HCC patients.
Materials and methods
Patients
A total of 106 HCC patients with liver cirrhosis underwent laparoscopic surgery in Shengjing Hospital, China Medical University (Shenyang, China) between January 2013 and June 2017. In these cases, MLH or LH was determined based on whether the patient agreed to use a microwave probe that could not be reimbursed by medical insurance. All patients underwent contrast ultrasound, enhanced multidetector computed tomography (CT), and gadoxetic acid disodium-enhanced magnetic resonance imaging (MRI) scans along with detection of alpha-fetoprotein (AFP) levels in the preoperative period to achieve HCC diagnosis. Pathological examination of the resected tumor was then used to confirm the HCC diagnosis in all enrolled patients. Liver functions were evaluated by blood tests (complete blood panel and blood chemistry), using the 15-min indocyanine green retention rate (ICGR15) and Child–Pugh score according to the standard guidelines. Esophagogastroduodenoscopy was used to assess esophageal varices and gastric mucosa lesions caused by portal hypertension.
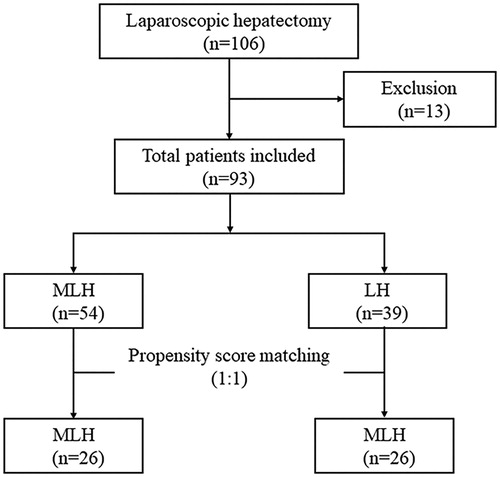
Patients were retrospectively enrolled using the following inclusion criteria: (i) the sole use laparoscopic technique for tumor resection; (ii) patients with liver cirrhosis; (iii) number of HCC nodules ≤ 3; (iv) tumor size ≤ 5 cm; and (v) the absence of vascular invasion and extrahepatic metastasis. A total of 93 patients were finally enrolled in our study then divided into a microwave ablation-assisted LH group (MLH group, n = 54) and a sole LH group (LH group, n = 39) (). Demographic characteristics and clinical features including the gender; age; American Society of Anesthesiologists physical status (ASA) score; body mass index (BMI); AFP; portal hypertension; tumor number, size, and location; operative time; intraoperative blood loss; and cut-surface recurrence were retrospectively collected from the patient files.
Surgical procedure
During LH, patients were placed in the supine position after general anesthesia according to the conventional protocols and CO2 pneumoperitoneum was established. Preoperative three-dimensional reconstruction and laparoscopic ultrasound (LUS; ALOKA ProSound α5, Aloka, Tokyo, Japan) were used to guide the selection of resection planes and ensure tumor-free margins. Using electrocautery under LUS guidance, the demarcation line was set 1–2 cm away from the tumor edge according to the degree of liver cirrhosis.
A total of 76 HCC cases underwent non-anatomic resection, while 17 patients underwent anatomical resection. Hepatic occlusion was necessary in 8 cases (6 patients in the LH group and 2 patients in the MLH group). Intermittent Pringle maneuver was undertaken to prevent massive bleeding by prior insertion of a silicone tube around the hepatoduodenal ligament (15-min intervals, with 5-min clamp-free resting periods between intervals [Citation21]).
In the MLH group, the microwave ablation probe (ECO-100AI10, ECO Microwave System Co, Nanjing, China) was inserted percutaneously through an additional tiny incision in the abdominal wall, and then consecutively inserted and spaced 2 cm apart along the marked transection line. LUS was used during probe insertion to avoid injury to the large vessels and the bile duct. Upon achieving the optimal insertion angle and depth, an emission power of 60 W for 3 min (duration) was routinely adopted to attain complete precoagulation [Citation22]. In order to control needle tract hemorrhage and prevent seeding metastases, the microwave ablation probe was pulled out slowly with the continuation of microwave energy. To achieve a complete coagulation belt, the time required for microwave ablation was directly associated with the tumor size, demarcation line length and lesion depth [Citation17]. For large-sized tumors (up to 5 cm), the precoagulation process was performed alternatively with liver resection, thus forming a curved resection plane to better preserve the liver parenchyma. Following precoagulation, the liver parenchyma was divided using a Harmonic scalpel (HARMONIC SYNERGY® Blades, Ethicon Inc., Cornelia, GA) (). LH was performed as detailed previously [Citation23]. Briefly, parenchymal separation was achieved using a Harmonic scalpel or Ethicon EndoSurgery device in the LH group. Small branches of the Glisson pedicles in the resection plane were clipped using a Hem-o-lock clip® to ensure complete homeostasis and biliostasis [Citation17,Citation24]. Routine abdominal drainage was used during the operation.
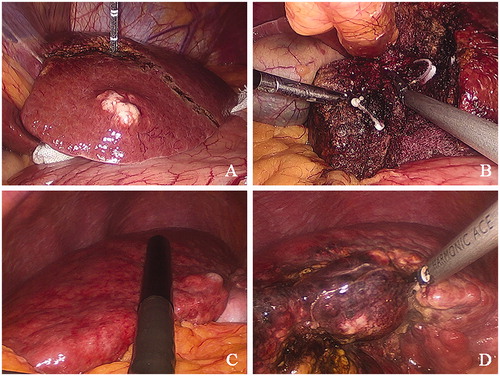
Figure 2. Photomicrographs representing main steps of microwave ablation-assisted laparoscopic hepatectomy. (A) Representative photo from a 57-year-old female patient who presented with mild liver cirrhosis and enough hepatic functional reserve demonstrating a pre-excision line spaced 2 cm from the tumor margin. Medical gauze was used to protect adjacent viscera from possible heat injury. (B) Representative photo from the same 57-year-old female patient representing the clipping of branches of the Glisson pedicles encountered in the process of liver resection using Hem-o-lock clips to ensure stump hemostasis and biliostasis. (C) Representative photo from a 42-year-old female patient who presented with severe liver cirrhosis and poor liver functional reserve. Laparoscopic ultrasound scanning was undertaken to determine the tumor margin and the transection line before parenchymal-sparing hepatectomy. (D) Representative photo from the same 42-year-old female patient who presented with severe liver cirrhosis. Precoagulation was performed closer to the tumor margin to preserve the liver parenchyma.
Postoperative care and follow up
On postoperative days 1, 3, 5 and 7, all patients underwent routine blood tests and liver function tests. Abdominal drainage was removed when serous fluid (i.e., thin and clear serum) without bile was observed. Ultrasound imaging or CT scans were performed before discharge. Following discharge, all patients were regularly followed up using the same oncological protocol. In particular, serum AFP levels and contrast-enhanced CT scans and/or MRI scans were investigated at 2 months postoperatively, then every 2–3 months thereafter. Hepatic recurrence was identified if tumor re-growth along the margin of resection or/and anywhere in the liver was detected by either CT or MRI. The OS time is defined as the elapsed interval from the initial liver resection to death or the last visit to the outpatient clinic (October 2017).
Propensity score matching (PSM) analysis
We conducted PSM analysis to overcome selection bias and confounding variables between the LH and MLH groups. Baseline variables including age, gender, BMI, ASA grade, portal hypertension, AFP, WBC, hemoglobin, platelet count, prothrombin time, albumin, total bilirubin, Child–Pugh classification, tumor number, tumor size and tumor location were matched in our model. A one-to-one nearest neighbor matching algorithm was applied with a caliper of 0.2 (Propensity Score Matching in SPSS®; SPSS Inc., Chicago, IL).
Statistical analyses
Chi-squared test or Fisher exact test was used to compare the categorical variables. Continuous variables were compared by independent t-test. Data were presented as mean ± standard deviation (SD). The OS and recurrence-free rates were estimated with Kaplan–Meier survival analysis using Prism version 6.0 software (GraphPad, La Jolla, CA) and the difference between curves was assessed using log-rank test. Statistical analyses were performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL). All statistical analyses were two-sided, and p < .05 was defined as statistically significant.
Results
Demographic characteristics
A total of 93 HCC patients (60 males and 33 females) were enrolled in this study. Patients were divided into a MLH group (n = 54) and LH group (n = 39), . As shown in , the baseline demographic characteristics and clinical features between the MLH and LH groups were not significantly different in gender, age, BMI, ASA grade, virus hepatitis, portal hypertension, AFP, WBC, hemoglobin, platelet count, prothrombin time, albumin level, total bilirubin level, Child–Pugh classification, and tumor number and location (p > .05). Following PSM analysis, a total of 26 patients were investigated in each group. Similarly, following 1:1 matching, there were no significant differences in the baseline characteristics between both groups (p > .05), .
Table 1. Demographic characteristics and clinical features of the enrolled patients before and after the propensity score matching analysis.
Surgical outcomes
All patients completed the surgical procedure as planned. Before PSM analysis, there was no significant difference in the mean operative time between the MLH and LH groups (157 ± 63 min vs. 149 ± 82 min, respectively; p > .05; ). Similarly, there was no significant difference in the mean operative time after PSM analysis between the MLH and LH groups (155 ± 60 vs. 148 ± 70 min, respectively; p > .05; ).
Table 2. Interoperative and postoperative parameters for the enrolled patients before and after propensity score matching analysis.
Before PSM analysis, we observed significant differences in the mean blood loss, the need for blood transfusion and hepatic inflow occlusion between the MLH and LH groups (p < .05; ). Following 1:1 matching analysis by PSM, the mean blood loss was significantly lower in the MLH group compared to the LH group (48 ± 74 vs. 204 ± 217 ml, respectively; p < .0001, ). Similarly, the hepatic inflow occlusion was significantly different between the MLH and LH groups (p < .05, ). Meanwhile, the need for blood transfusion was not significantly different between the LH and MLH groups following PSM analysis. Details for the surgical procedure and the postoperative outcomes are summarized in .
Following the surgical procedure, patients in the MLH group had lower cut-surface recurrence rates as well as a shorter hospitalization period although they did not reach statistical significance (). The rate of postoperative hemorrhage was higher in the LH group than the MLH group, but the difference did not reach statistical significance before or after PSM analysis. Particularly, two patients in the LH group had postoperative hemorrhage while no patients in the MLH group were presented with postoperative hemorrhage. Postoperative complications including bile leakage, ascites, infection, and perihepatic effusion did not differ significantly between both groups.
Postoperative recurrence and survival rates
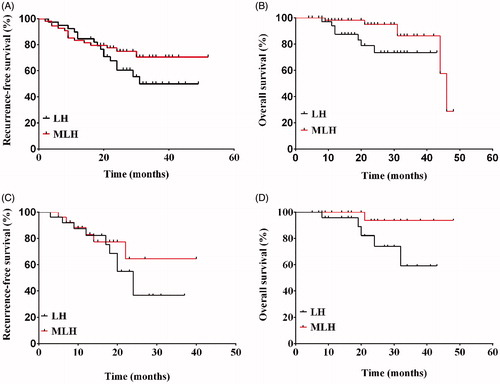
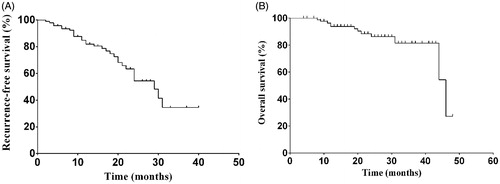
In this study, patients were followed up for a median duration of 21 months (range 4–48 months). For all patients, the 1-, 2- and 3-year recurrence-free survival (RFS) rates were 82.0, 54.4 and 34.5%, respectively, while the 1-, 2- and 3-year OS rates were 93.9, 86.3 and 81.5%, respectively (). During the follow-up period, there were 30 cases of HCC recurrences (14 cases in the MLH group and 16 in the LH group) without extrahepatic malignancies. Among those cases, three patients had HCC recurrence along the cut-surface in the LH group ().
Figure 3. Recurrence-free survival rate (A) and overall survival rate (B) for all enrolled patients (n = 93).
Before PSM, the 1- and 3-year RFS rates were 82.3 and 43.2% in the MLH group, and 81.9 and 26.7% in the LH group, respectively (p = .427). The 1- and 3-year OS rates were 98.1 and 86.4% in the MLH group, and 87.5 and 73.5% in the LH group, respectively (p = .035; ). Following PSM analysis, the 1- and 3-year RFS rates were 83.1 and 64.6% in the MLH group, and 82.4 and 36.6% in the LH group, respectively (p =.457). Meanwhile, the 1- and 3-year OS rates were 100.0 and 93.8% in the MLH group, and 95.8 and 59.1% in the LH group, respectively (p = .058; ).
Discussion
Despite significant advancements in laparoscopic instruments and the expansion of LH application in HCC management, intraoperative hemorrhage is still a major complication and a main cause of conversion to open surgery [Citation23,Citation25]. In addition, perioperative hemorrhage and blood transfusions are considered to be independent risk factors for tumor recurrence and an unfavorable HCC prognosis, especially for patients with liver cirrhosis [Citation9,Citation26,Citation27]. Owing to the coagulation of small vessels by microwave ablation, patients in the MLH group had minimal amount of blood in the resection field which greatly reduced the use of clamps and laparoscopic suturing compared to patients in the LH group. Indeed, the mean blood loss in the MLH groups was significantly lower than the LH group (p < .0001). Moreover, blood transfusion was not required in the MLH group while four patients in the LH group needed blood transfusion. Consequently, in agreement with previous findings, all patients in the MLH group underwent laparoscopic surgery without major complications or conversion to open hepatectomy [Citation9].
The intraoperative hepatic occlusion was not required in the MLH group. This could be attributed to the bloodless resection plane created by precoagulation, thereby leading to a faster and more straightforward liver resection. In contrast, Pringle maneuver was undertaken to control hemorrhage at the cut-surface as well as to obtain a clear surgical field in six patients of the LH group. Although Pringle maneuver is a commonly used technique to control bleeding, ischemia-reperfusion injury remains a major complication [Citation12]. Furthermore, blockage of the mesenteric venous drainage could elevate the intestinal microvascular network pressure, which could induce bacterial translocation and result in gastrointestinal complications [Citation28]. Moreover, Pringle maneuver has been associated with increased postoperative ascites and pleural effusion [Citation29].
On the other hand, microwave ablation can coagulate the liver tissue and blood vessels around the tumor without blocking the liver blood flow [Citation30]. Reuter and Martin reported that microwave ablation had a coagulation diameter of at least 8 mm, which can significantly reduce bleeding during hepatectomy [Citation18]. Compared to the LH group, the 1- and 3-year OS rates were significantly improved in the MLH group. However, following 1:1 matching with the PSM analysis, we observed a tendency to a longer OS rate in the MLH group without attaining statistical significance. This could be due to the relatively small sample size. Taken together, the use of microwave ablation in combination with LH can improve the surgical outcomes and patient prognosis compared to the use of LH alone.
Our results demonstrated that a shorter postoperative hospitalization period was observed in the MLH group (7.4 vs. 8.6 d, p = .045) before PSM analysis. However, the difference was no longer significant following PSM analysis. Likewise, the postoperative complications did not significantly differ between the two groups. We did not observe any postoperative bile leakage in the MLH group. This could be due to the shrinkage and occlusion of microvessels as well as the bile duct following microwave-induced precoagulation of liver tissues. Therefore, the use of MLH created a relatively bloodless resection surface and reduced bile leakage [Citation31]. In agreement with previous findings, we limited the ablation time to around 3 min because over-coagulation may result in liver damage and complications, such as abscess formation [Citation17].
Indocyanine green is a tricarbocyanine dye that is exclusively excreted by the liver and its clearance rate can be used as an indicator of the liver function and hepatic blood flow [Citation32]. Yamamoto et al. previously demonstrated that a higher ICGR can indicate reduced risk of postoperative liver decompensation and improved OS in cirrhotic patients [Citation33]. In addition, parenchymal-preserving strategies also allow for repeated hepatectomy in the case of tumor recurrence [Citation34,Citation35]. Therefore, in this study, we opted for parenchymal-sparing hepatectomy instead of the anatomical hepatectomy in the majority of patients (76/93). However, parenchymal-sparing hepatectomy is a double-edged sword. On one hand, it can be a superior approach for preserving the liver function, but on the other parenchymal-sparing hepatectomy can enhance postoperative recurrence due to the underlying micrometastasis [Citation36]. Interestingly, the three cases of cut-surface-recurrence were observed in the LH group, whereas no recurrence was observed in microwave ablation-assisted parenchymal-sparing hepatectomy. Moreover, there was no significant difference in the RFS rates between both groups. The absence of surgical margin recurrence in the MLH group could be caused by the eradication of possible satellite cancer nodules and small tumor emboli along the surgical margin by microwave ablation [Citation37,Citation38]. Alternatively, the closure of small hepatic and portal vein branches by precoagulation can also block potential metastases. Otherwise, microwave ablation can reduce the levels of inflammatory cytokines, and enhance the local anti-tumor immune response [Citation10,Citation39]. Taken together, MLH can achieve a complete tumor-negative margin and block the intrahepatic metastasis via the microvessels [Citation9,Citation12,Citation17]. Therefore, it is plausible to hypothesize that laparoscopic microwave ablation-assisted parenchymal-sparing liver resection can be more suitable for cirrhotic patients with poor liver function.
In conclusion, compared to LH, we demonstrated that the combination of microwave ablation with LH decreased interoperative bleeding and prevented hepatic inflow occlusion without compromising the postoperative morbidity rate. Nevertheless, this study has few limitations that should be resolved in future research. First, despite using PSM analysis, this study was retrospectively designed and, therefore, our results could be affected by factors like selection bias and single-center analysis. Second, the follow-up period was relatively short which limited our ability to investigate the long-term oncological outcomes in the MLH and LH groups. Finally, our sample size after matching was relatively small (n = 26), which could affect the reliability of our results, Therefore, a multicenter, randomized and controlled clinical trial with a longer follow-up period will be essential to confirm our results.
Ethical approval
This study was approved by the Ethics Committee of Shengjing Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380.
- Franken C, Lau B, Putchakayala K, et al. Comparison of short-term outcomes in laparoscopic vs. open hepatectomy. JAMA Surg. 2014;149:941–946.
- Cheung TT, Dai WC, Tsang SH, et al. Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg. 2016;264:612–620.
- Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–511.
- Bryant R, Laurent A, Tayar C, et al. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111.
- Ratti F, Barkhatov LI, Tomassini F, et al. Learning curve of self-taught laparoscopic liver surgeons in left lateral sectionectomy: results from an international multi-institutional analysis on 245 cases. Surg Endosc. 2016;30:3618–3629.
- Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs. open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356.
- Francone E, Muzio E, D’Ambra L, et al. Precoagulation-assisted parenchyma-sparing laparoscopic liver surgery: rationale and surgical technique. Surg Endosc. 2017;31:1354–1360.
- Jiao LR, Ayav A, Navarra G, et al. Laparoscopic liver resection assisted by the laparoscopic Habib Sealer. Surgery. 2008;144:770–774.
- Hirokawa F, Hayashi M, Miyamoto Y, et al. Short- and long-term outcomes of laparoscopic versus open hepatectomy for small malignant liver tumors: a single-center experience. Surg Endosc. 2015;29:458–465.
- Abdelraouf A, Hamdy H, El Erian AM, et al. Initial experience of surgical microwave tissue precoagulation in liver resection for hepatocellular carcinoma in cirrhotic liver. JESP. 2014;44:343–350.
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761.
- Gaillard M. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol. 2014;20:4892.
- Yu J, Liang P. Status and advancement of microwave ablation in China. Int J Hyperthermia. 2016;33:278–287.
- Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563.
- Chen ZB, Qin F, Ye Z, et al. Microwave-assisted liver resection vs. clamp crushing liver resection in cirrhosis patients with hepatocellular carcinoma. Int J Hyperthermia. 2018;34:1359–1366.
- Reuter NP, Martin RC. 2nd. Microwave energy as a precoagulative device to assist in hepatic resection. Ann Surg Oncol. 2009;16:3057–3063.
- Fioole B, van der Bilt JD, Elias SG, et al. Precoagulation minimizes blood loss during standardized hepatic resection in an experimental model. Br J Surg. 2005;92:1409–1416.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
- Liu S, Li X, Li H, et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol. 2016;114:112–118.
- Amabile C, Ahmed M, Solbiati L, et al. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33:34–42.
- Beard RE, Wang Y, Khan S, et al. Laparoscopic liver resection for hepatocellular carcinoma in early and advanced cirrhosis. HPB (Oxford). 2018;20:521–529.
- Sasaki K, Matsuda M, Hashimoto M, et al. Liver resection for hepatocellular carcinoma using a microwave tissue coagulator: experience of 1118 cases. World J Gastroenterol. 2015;21:10400–10408.
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592–2597.
- Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623.
- Tralhao JG, Kayal S, Dagher I, et al. Resection of hepatocellular carcinoma: the effect of surgical margin and blood transfusion on long-term survival. Analysis of 209 consecutive patients. Hepatogastroenterology. 2007;54:1200–1206.
- Li BC, Xia ZQ, Li C, et al. The incidence and risk factors of gastrointestinal complications after hepatectomy: a retrospective observational study of 1329 consecutive patients in a single center. J Surg Res. 2014;192:440–446.
- Lee KF, Wong J, Cheung SYS, et al. Does intermittent pringle maneuver increase postoperative complications after hepatectomy for hepatocellular carcinoma? A randomized controlled trial. World J Surg. 2018;42:3302–3311.
- Dong X, Sun Z, Wu T, et al. 915-MHz microwave-assisted laparoscopic hepatectomy: a new technique for liver resection. Surg Endosc. 2019;33:395–400.
- Itano O, Ikoma N, Takei H, et al. The superficial precoagulation, sealing, and transection method: a “bloodless” and “ecofriendly” laparoscopic liver transection. Tech Surg Laparosc Endosc Percutan Tech. 2015;25:e33–e36.
- Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373.
- Yamamoto Y, Ikoma H, Morimura R, et al. Clinical analysis of anatomical resection for the treatment of hepatocellular carcinoma based on the stratification of liver function. World J Surg. 2014;38:1154–1163.
- Mise Y, Aloia TA, Brudvik KW, et al. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263:146–152.
- Montalti R, Tomassini F, Laurent S, et al. Impact of surgical margins on overall and recurrence-free survival in parenchymal-sparing laparoscopic liver resections of colorectal metastases. Surg Endosc. 2015;29:2736–2747.
- Finch RJ, Malik HZ, Hamady ZZ, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg. 2007;94:1242–1248.
- Satoi S, Matsui Y, Kitade H, et al. Long-term outcome of hepatocellular carcinoma patients who underwent liver resection using microwave tissue coagulation. HPB (Oxford). 2008;10:289–295.
- Sadot E, Groot Koerkamp B, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–485.
- Wu F. Heat-based tumor ablation: role of the immune response. Adv Exp Med Biol. 2016;880:131–153.