Abstract
Objective: The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in epithelial ovarian cancer (EOC) is still controversial. Present analysis aims to evaluate the survival benefit of HIPEC in treatment of EOC patients.
Methods: Articles related to ‘HIPEC’ and ‘ovarian cancer’ were comprehensively searched in four databases (PubMed, EMBASE, MEDLINE and Cochrane Library) up to 4 February 2018. Eligible studies were identified depending on the selection criteria. The survival outcome and adverse events were collected. The relationship between HIPEC and survival of EOC was assessed using random-effects models.
Results: A total of 1464 patients from 17 trials were subjected to analysis. The pooled results showed that HIPEC significantly improved overall survival (OS, HR = 0.50, 95% CI 0.36–0.69; p = 0.000) and progression-free survival (PFS, HR = 0.57, 95% CI 0.47–0.69; p = 0.000) among EOC patients when compared with no HIPEC controls. Similar results were observed in each year rate of survival. Subgroup analysis didn’t lead to the opposite results, except no significant increased 1-year of OS in primary EOC and 1- and 2-year of PFS in recurrent EOC treated with HIPEC were observed. No significant difference existed in the adverse events and mortality between HIPEC and no HIPEC.
Conclusions: HIPEC is associated with improved OS and PFS in both primary and recurrent EOC. However, no significant increased 1- and 2-year of PFS were reached in recurrent EOC treated with HIPEC. Further prospective randomized controlled trials are warranted.
1. Introduction
Epithelial ovarian cancer (EOC) is the most lethal malignancy of all gynecologic cancers. The majority of patients receive a diagnosis of advanced disease that has spread beyond the ovaries to the peritoneal surface [Citation1]. Primary cytoreduction surgery (CRS) combined with chemotherapy is the standard treatment for advanced EOC; however, more than half of the patients would recur [Citation2]. As a result, less than 40% of EOC are cured and 5-year survival of EOC is only about 46.5% [Citation3].
The peritoneum, including the omentum and pelvic/abdominal viscera, is the most common site for dissemination of EOC [Citation4]. Previous studies have demonstrated intraperitoneal (IP) chemotherapy enhances drug delivery at the peritoneal surface and may improve the prognosis of advanced EOC by eliminating residual microscopic peritoneal disease more efficiently than intravenous administration of chemotherapy [Citation1,Citation5–8]. Hyperthermic intraperitoneal chemotherapy (HIPEC) is described as IP chemotherapy delivered under hyperthermic conditions (37–43 °C), directly following surgery or as consolidation therapy at the end of standard therapy [Citation9]. As a single treatment, HIPEC could overcome the catheter-related problems associated with repetitive IP chemotherapy. Multiple studies validated that hyperthermia additive increased the cytotoxic effect of chemotherapeutic drugs such as platinum, even for drug-resistant tumor cells, by increasing drug-DNA adducts and increasing tumor penetration [Citation10–16]. And accumulating evidences suggested that HIPEC could improve the prognosis of the patients with various cancers [Citation17–20]. Even more, CRS and HIPEC was suggested as a standard treatment in pseudomyxoma peritonei, appendiceal tumors with peritoneal dissemination, peritoneal metastases from colorectal cancer and peritoneal mesothelioma at the 9th International Congress on Peritoneal Surface Malignancies in 2014 [Citation21].
However, the role of HIPEC plus CRS in the management of primary and recurrent EOC is still controversial [Citation22]. Chiva et al. [Citation23] concluded that HIPEC had only modest results concerning survival compared to other studies of primary EOC without HIPEC, and the survival after HIPEC in the recurrent cohort was much shorter than the results of classic studies about secondary CRS. However, the completeness statuses of CRS in this review were different from that of previous studies, which might affect the prognosis of EOC significantly [Citation24–26]. Moreover, no randomized controlled trial (RCT) was included in this review, which weakened the strength of the evidence. Huo et al. [Citation2] reported the first meta-analysis about EOC and HIPEC. They concluded that the addition of HIPEC to CRS and chemotherapy improves overall survival rates for both primary and recurrent EOC. But no information regarding progression-free survival (PFS) was collected in that meta-analysis.
Here, we first analyzed the efficacy of HIPEC in improving both PFS and overall survival (OS) of EOC using meta-analysis, along with the first RCT about primary EOC and HIPEC reported in 2018 [Citation1]. Present study aims to evaluate the validity and rationale of HIPEC for the treatment of primary and recurrent peritoneal carcinomatosis of ovarian origin, through systematic review and meta-analysis of the current scientific literature available.
2. Materials and methods
2.1. Literature search strategy
Present study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, and registered at PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD420181109681). The authors searched the PubMed, MEDLINE, EMBASE and Cochrane Library databases for relevant studies published up to 4 February 2018, using the terms [‘Hyperthermic intraperitoneal chemotherapy’ OR ‘HIPEC’] AND [‘ovarian cancer’ OR ‘ovarian carcinoma’]. All retrieved abstracts were screened independently by two authors, and full texts of those relevant studies were further identified for inclusion eligibility independently by two authors. To include all potentially eligible studies, the references of identified articles were also checked by manual search. The disagreements were resolved by discussion and consensus.
2.2. Selection criteria
The following inclusive selection criteria were applied and only two-arm studies were included: (1) the study subjects included the patients with primary or recurrent EOC; (2) the intervention measure was HIPEC; (3) the objective of control group was the EOC patients treated without HIPEC and (4) the OS and/or PFS of EOC were reported.
There were no language restrictions. Review, statement, comment, letter and case report were excluded. Abstract would be included only if it provided all information needed. The duplicate publications would be removed. As for the studies that published multiple publications with accumulating numbers of patients or longer follow-up duration, only the most recent update or complete reports were included for assessment.
2.3. Data extraction and quality assessment
Two authors independently extracted the following data: first author, year of publication, number of patients, age, primary or recurrent EOC, study design, the completeness of cytoreduction (CC), follow-up duration, survival outcome (including the 1-, 2-, 3-, 4- and 5-year rate of survival, hazard ratio (HR), odds ratio (OR) and 95% confidence interval (CI)), morbidity (related to HIPEC or CRS) and mortality (within 30 days after surgery), the regimens, temperature and duration of HIPEC. Sugarbaker’s CC criteria were used [Citation27] as follows: CC-0, no macroscopic tumor visible; CC-1, largest residual tumor ≤2.5 mm; CC-2, largest residual tumor >2.5 mm and ≤2.5 cm and CC-3, largest residual tumor >2.5 cm. The data were calculated from Kaplan-Meier curves using Engauge Digitizer software 4.1 (sourceforge.net/projects/digitizer/) for those lack of 1-, 2-, 3-, 4- and 5-year rate of survival, HR/OR and 95% CI, except one author provided the data per our request by email. The Newcastle-Ottawa Scale (NOS) was employed for quality assessment of comparative studies. Studies with scores from 0 to 3, 4 to 6 and 7 to 9 were considered as low, moderate and high quality, respectively. Jadad scale was used to assess the quality of RCTs. Studies with scores 1 or 2 were considered as low quality, scores 3–5 as high quality.
2.4. Statistical analysis
HR and OR were used as the measure of the prognostic value. The relationship between HIPEC and survival of EOC was assessed using random-effects models. Heterogeneity was assessed by the Q test and expressed by I2 values. I2 values from 25% to 50%, from 50% to 75% and ≥75% were considered as low, moderate and high degrees of heterogeneity, respectively. Subgroup analyses were performed depending on the study design and primary or recurrent EOC. Publication bias was assessed by funnel plot and Egger’s linear regression test. If publication bias was indicated, trim-and-fill method was further used to estimate the effect of publication bias on interpretation of the results. Sensitivity analysis was performed to explore robustness of the findings. A p-value <0.050 was considered to be statistically significant. All statistical analyses were performed with the STATA 12.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Study selection and characteristics
The literature search and study selection procedure are summarized in . Totally 1,666 articles were screened and 80 studies were retrieved for further evaluation. Finally, a total of 1464 patients from 17 studies were subjected to meta-analysis (including one abstract [Citation28] and one article in Chinese [Citation29]). No additional study was identified by manual search.
The characteristics of included studies are presented in . In brief, seven studies of primary EOC, nine of recurrent EOC and one of both primary and recurrent EOC were included. Only two studies were RCTs. Sixteen studies compared the survival of EOC treated by CRS plus HIPEC with those by CRS, while one study compared CRS plus HIPEC with systemic chemotherapy only. The reported mean or median age of HIPEC group ranged from 46.1 to 61.0 years, while no HIPEC group ranged from 45.8 to 65.0 years. Fourteen studies (n = 1227) investigated the relationship between HIPEC and OS of EOC, of which five studies (n = 591) for primary EOC, eight studies (n = 525) for recurrent EOC and one study (n = 111) for both primary and recurrent EOC. Ten studies (n = 1031) assessed the relationship between HIPEC and PFS of EOC, of which six studies (n = 726) for primary EOC and four studies (n = 287) for recurrent EOC. Except for one study [Citation28] with moderate quality, all others studies had high quality assessment according to the NOS and Jadad. HIPEC-related morbidity included leucopenia, abdominal pain, infection, fistula and ileus, which was reported in 12 studies. The adverse events between HIPEC and no HIPEC were compared in 10 studies and no significant difference existed except one study [Citation31]. HIPEC-related mortality was evaluated in nine studies and no death reported in HIPEC group. The detailed CC score, HIPEC procedure and survival rate of the included studies are listed in Supplemental Table 1. The majority of patients got CC score of 0–1. The 100% rate of complete cytoreduction (CC-0) was reported in four studies [Citation33–35,Citation43]. The temperature of HIPEC ranged from 40 °C to 44 °C and the duration ranged from 30 to 120 min.
Table 1. The characteristics of the included studies.
3.2. Main analysis
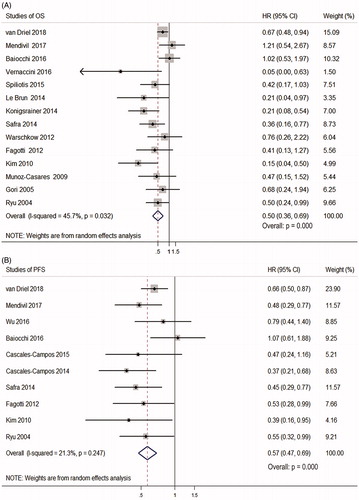
Random-effects models showed that HIPEC was significantly associated with longer OS/PFS of EOC (OS: HR = 0.50, 95% CI 0.36–0.69; p = 0.000; PFS: HR = 0.57, 95% CI 0.47–0.69; p = 0.000) (. However, there existed low heterogeneity in OS between the studies (I2=45.7%, p = 0.032) ().
Figure 2. Forest plots showing the effect of HIPEC on survival of EOC. (A) The effect of HIPEC on OS of EOC. (B) The effect of HIPEC on PFS of EOC.
We further analyzed each year rate of survival, since the median follow-up period was varied from 18.0 to 80.2 months in the studies. Compared with no HIPEC group, CRS plus HIPEC significantly improved 1-, 2-, 3-, 4- and 5-year OS (OR = 2.82, 95% CI 1.54–5.18; 2.67, 1.83–3.91; 3.02, 1.82–4.99; 2.33, 1.50–3.63 and 2.48, 1.71–3.61. p = 0.001, 0.000, 0.000, 0.000 and 0.000 respectively) (). In addition, CRS plus HIPEC had significantly better 1-, 2-, 3-, 4- and 5-year PFS compared with no HIPEC group (OR = 1.87, 95% CI 1.23–2.82; 2.79, 1.91–4.07; 2.52, 1.78–3.57; 3.12, 1.84–5.30 and 2.19, 1.45–3.31. p = 0.003, 0.000, 0.000, 0.000 and 0.000 respectively) ().
Table 2. The effect of HIPEC on each year OS/PFS rate of EOC and subgroup analysis according to primary or recurrent EOC.
3.3. Subgroup analysis
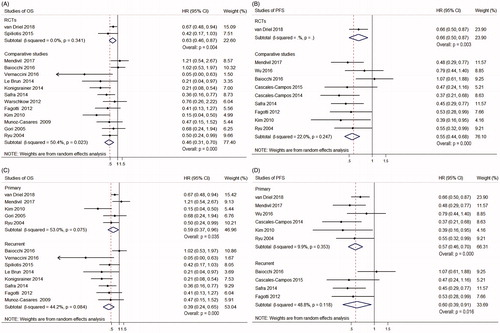
Subgroup analysis was conducted due to the quality difference between RCTs and comparative studies, which did not lead to the opposite results (OS: HR = 0.63, 95% CI 0.46–0.87 and 0.46, 0.31–0.70; p = 0.004 and 0.000 respectively; PFS: HR = 0.66, 95% CI 0.50–0.87 and 0.55, 0.44–0.68; p = 0.003 and 0.000 respectively) (). The heterogeneity of OS among the RCTs were eliminated as expected (I2 = 0 vs I2 = 45.7%) (). Further subgroup analysis according to primary or recurrent EOC also indicated that HIPEC improved both OS and PFS (OS: HR = 0.59, 95% CI 0.37–0.96 and 0.39, 0.24–0.65; p = 0.035 and 0.000 respectively; PFS: HR = 0.57, 95% CI 0.46–0.70 and 0.60, 0.39–0.91; p = 0.000 and 0.016 respectively) ().
Figure 3. Forest plots showing the effect of HIPEC on survival of EOC after subgroup analysis. (A) The effect of HIPEC on OS of EOC according to different study design. (B) The effect of HIPEC on PFS of EOC according to different study design. (C) The effect of HIPEC on OS of EOC according to primary or recurrent EOC. (D) The effect of HIPEC on PFS of EOC according to primary or recurrent EOC.
In primary EOC, HIPEC significantly improved 2-, 3-, 4- and 5-year OS (OR = 2.11, 95% CI 1.33–3.35; 1.99, 1.37–2.88; 1.99, 1.15–3.44 and 2.14, 1.50–3.06; p = 0.001, 0.000, 0.013 and 0.000 respectively) and 1-, 2-, 3-, 4- and 5-year PFS of primary EOC (OR = 2.11, 95% CI 1.49–2.98; 3.01, 2.16–4.19; 2.56, 1.66–3.94; 3.28, 1.59–6.76 and 2.10, 1.30–3.40; p = 0.000, 0.000, 0.000, 0.001 and 0.003 respectively) compared with no HIPEC (). While the 1-year OS (OR = 1.78, 95% CI 0.86–3.68, p = 0.120) of primary EOC with HIPEC showed a trend of better survival, although the difference was not significant.
In recurrent EOC, HIPEC significantly improved 1-, 2-, 3-, 4- and 5-year OS (OR = 3.17, 95% CI 1.16–8.66; 3.36, 1.56–7.26; 4.32, 1.59–11.73; 2.55, 1.06–6.17 and 2.75, 1.09–6.91; p = 0.025, 0.002, 0.004, 0.037 and 0.031 respectively) and 3-, 4- and 5-year PFS (OR = 2.57, 95% CI 1.25–5.25; 2.70, 1.26–5.78 and 2.59, 1.08–6.21; p = 0.010, 0.011, and 0.033 respectively) compared with no HIPEC (). While the 1- and 2-year PFS (OR = 1.46, 95% CI 0.50–4.28 and 2.48, 0.88–7.04; p = 0.495 and 0.087 respectively) of recurrent EOC with CRS plus HIPEC showed a trend of better survival, although the difference was not significant ().
3.4. Sensitivity analysis and publication bias
Due to the variation of the inclusion criteria, the influence of each study on the pooled HR of OS/PFS and OR of each year survival rate was evaluated by repeating meta-analysis after excluding one study at a time. The results showed that the association of HIPEC with OS, PFS and each year survival rate did not change significantly after omitting any study (Supplemental Table 2).
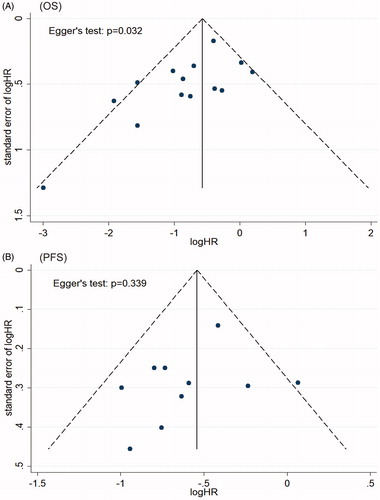
The asymmetrical funnel plot indicated publication bias in studies about OS, which was confirmed by Egger’s test (p = 0.032) (). Trim-and-fill method recalculated the pooled risk of OS and the result (HR = 0.62, 95% CI 0.44–0.89) was consistent with our primary result (), which indicated that the association of HIPEC with OS was not affected by publication bias. For studies about PFS, the funnel plot was symmetrical and Egger’s test also indicated no publication bias (p = 0.339) ().
4. Discussion
As we know, peritoneal carcinomatosis represents the advanced stage in the evolution of EOC, which has been considered as the main cause of recurrence [Citation1,Citation40,Citation44]. Although IP chemotherapy is associated with improved survival of advanced EOC [Citation6–8], it is not widely adopted as standard care due to concerns of excessive toxicity, chemotherapy catheter, abdominal pain and a worse quality of life [Citation7,Citation22]. HIPEC differs distinctly from postoperative IP chemotherapy in that it is a single treatment of intraoperative chemotherapy at the time of cytoreductive surgery. Thus, it may eliminate some of the defects associated with traditional IP therapy [Citation2]. But HIPEC has still not been advocated as a standard treatment for clinical practice, despite it was considered as an alternative method of IP [Citation2].
In present study, we found HIPEC can significantly improve the OS and PFS of EOC. Nevertheless, there existed heterogeneity which may be attributed to different inclusion criteria and study methods used. Subgroup analysis according to study design showed the heterogeneity was eliminated in RCTs with similar positive results. Unfortunately, there are only two RCTs about HIPEC and EOC up to date, one for primary EOC and the other for recurrent EOC. Due to the varied follow-up duration of EOC in different studies, further analysis about each year rate of OS and PFS was conducted. Which still suggested HIPEC could bring significantly survival benefit. Sensitivity analyses of OS, PFS and each year survival rate suggested the results did not change significantly. Consistent with previous studies [Citation1,Citation33], we also found the administration of HIPEC is safe, with limited and less morbidity and mortality compared with no HIPEC group in the majority of included studies.
In primary EOC patients, we found that HIPEC improved OS, PFS and each year survival rate (2–5 year of OS and each year of PFS). These results are consistent with previous meta-analysis of HIPEC [Citation2] and further suggest that the incorporation of HIPEC may result in better prognosis of primary EOC [Citation6]. Since the OS of all included studies about primary EOC were longer than 1 year (mean or median OS ranged from 13.4 to 72 months), it is a logical result that the 1-year OS showed no significant difference between both groups.
Although randomized trials support the use of HIPEC in colorectal cancer [Citation17,Citation20,Citation45,Citation46], most previous evidence of a beneficial effect from HIPEC in primary EOC has been limited to single-group trials or retrospective cohorts [Citation9,Citation10,Citation29,Citation35,Citation39,Citation42]. Until recently, van Driel et al. [Citation1] reported the first RCT about primary EOC and HIPEC. They randomized 245 stage III patients with complete or optimal interval CRS after three cycles of neoadjuvant chemotherapy (NAC) because of too extensive disease. The addition of HIPEC resulted in longer PFS (14.2 vs 10.7 months) and OS (45.7 vs 33.9 months) than no HIPEC group and did not result in higher rates of side effects. This study provided the evidence of HIPEC’s survival benefit in advanced EOC after NAC. Although the survival of no HIPEC group in this trial was shorter than that in the Gynecologic Oncology Group–172 study, which could attribute to the different inclusion criteria since the latter included only the patients eligible for primary cytoreduction [Citation6]. Additional trials are still needed to determine the optimal time for HIPEC administration and whether HIPEC is also effective after primary cytoreductive surgery.
There existed some negative results from previous studies of primary EOC, which might be potentially affected by the heterogeneity of study design, such as the different chemotherapy regimen, disease stage or histology and primary approach. Mendivil et al. [Citation30] compared the survival rates of advanced primary EOC who were treated with or without consolidation HIPEC. They did not discern any HIPEC-related OS advantage, although a significant PFS advantage was validated. However, the patients in HIPEC group were recruited between 2012 and 2015, while the patients in control group came from 2008 to 2014. Thus, the mean follow-up duration in HIPEC group was shorter than that in no HIPEC group, which might cause the similar OS in both groups. And Wu et al. [Citation29] reported no significant PFS advantage in HIPEC groups. But the complete remission rate in both groups were quite low (14.58% in control and 8.33% in HIPEC), which might result in the poor prognosis in both groups and affect the data analysis.
For recurrent EOC patients, our results suggested that HIPEC increased OS, PFS and each year survival rate (each year of OS and 3–5 year of PFS). These results of OS were consistent with previous meta-analysis of HIPEC [Citation2], while the results of PFS were concordant with previous study which found significant differences of the 2-, 4- and 5-year PFS between groups with and without HIPEC [Citation33]. As we know, the standard treatment of recurrent EOC is systemic chemotherapy and which usually results in median OS of lower than 30 months [Citation2]. While previous study had shown that CRS may improve prognosis for recurrent patients with resectable disease [Citation47]. In highly selected patients who received CRS, OS would range from 41 to 60 months and median PFS would reach 30.3 months [Citation47,Citation48]. The median survival in recurrent EOC without macroscopic residue was significantly longer than those with macroscopic residue (45.2 vs 19.7, HR = 3.71, p < 0.001) [Citation49]. The adoption of CRS would significantly change the survival of recurrent EOC, which could be the important reason for that the 1- and 2-year PFS showed no significant difference between groups with and without HIPEC. Thus, the affection of CRS shouldn’t be ignored. Further prospective studies stratified by resectable disease or not are needed for the recurrent EOC.
Baiocchi et al. [Citation31] concluded that the addition of HIPEC to CRS in patients with recurrent platinum-sensitive EOC does not improve survival after they analyzed a series of 79 patients with platinum sensitive recurrent EOC. But regarding the different extent of disease or surgery status of the included patients, selection bias couldn’t be ruled out. Spiliotis et al. [Citation32] carried out the first RCT about CRS and HIPEC in recurrent EOC. They randomized 120 recurrent EOC patients who received CRS followed by HIPEC or not. The results suggested HIPEC plus CRS showed survival benefit of recurrent EOC over CRS alone. Interestingly, similar survival was observed both in platinum sensitive and resistant disease of HIPEC group, which is not the case in the non-HIPEC group. The author attributed this phenomenon partly to the hyperthermia which made the tumor cell more sensitive by different molecular mechanism such as heat-shock proteins and epigenetic alterations [Citation50,Citation51]. This study also supported that complete CRS was associated with longer survival. However, the randomization process and primary end points were not clearly described in this RCT [Citation1,Citation52]. Further randomized studies about recurrent EOC are needed, due to lacking information of PFS, median follow-up and postoperative first-line treatment in this RCT.
Although numerous studies related HIPEC were carried out, most studies demonstrated substantial heterogeneity [Citation53]. There are some limitations existing in present meta-analysis. First, the inclusion criteria and HIPEC drug regimens for EOC are varied. Better microscopic residual disease conditions after CRS would contribute to improved prognosis [Citation3]. The extent of disease status and CRS are important prognostic factors for EOC and not consistent in different included studies, which also should be fully evaluated [Citation54]. No standardized HIPEC protocols existed, which would increase the difficulty of efficacy evaluation. Second, no robust quantitative measure of the morbidity related to HIPEC was established which would be critical for future use in clinical trial. Third, the potential publication bias of included studies was unavoidable due to insufficient RCTs data up to date.
Hopefully, there are 14 ongoing RCTs assessing the impact of HIPEC in primary and recurrent EOC (). In all these RCTs, HIPEC will be administrated at the end of CRS. Platinum and/or paclitaxel are the most common used drugs for HIPEC, except one study using cisplatin and doxorubicin. Except for the efficacy of HIPEC, quality of life and/or morbidity will also be evaluated in most studies. Among seven trials of primary EOC, HIPEC following NAC and CRS will be assessed in four trials, while HIPEC following primary CRS will be assessed in another three trials. There are three trials of platinum-sensitive recurrent EOC, one of which will assess the pharmacokinetics of HIPEC. The remaining four trails include both primary and recurrent EOC, one of which will assess the pharmacodynamics of cisplatin. Obviously, these studies will provide more precise and useful information about HIPEC and help us to determine the benefit and utility of HIPEC in EOC.
Table 3. The 14 ongoing randomized controlled HIPEC trials.
In conclusion, this meta-analysis first provided the pooled results about the relationship between HIPEC and OS/PFS of EOC patients. Our study suggested that HIPEC would improve OS and PFS in both primary and recurrent EOC patients. However, no significant increased 1- and 2-year of PFS was reached in recurrent EOC treated with HIPEC. Future well-designed and stratified studies are still recommended to investigate the survival benefit of HIPEC in EOC, especially for recurrent disease.
Supplemental Material
Download PDF (418.7 KB)Supplemental Material
Download PDF (105.9 KB)Acknowledgements
We would like to thank the corresponding author who replied to our request for data. We would also like to thank our colleagues Associate Professor Yi Shen (Department of Epidemiology and Health Statistics, Zhejiang University School of Medicine) for his careful guidance of statistics analyses.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240.
- Huo YR, Richards A, Liauw W, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:1578–1589.
- NCCN. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018:MS3-9. Available from: https://www.nccn.org/.
- Denny L, Quinn M. FIGO cancer report 2015. Int J Gynaecol Obstet. 2015;131:S111.
- Markman M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev Anticancer Ther. 2001;1:142–148.
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43.
- Wright AA, Cronin A, Milne DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. JCO. 2015;33:2841–2847.
- Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. JCO. 2015;33:1460–1466.
- van Driel WJ, Lok CA, Verwaal V, et al. The role of hyperthermic intraperitoneal intraoperative chemotherapy in ovarian cancer. Curr Treat Options Oncol. 2015;16:14.
- Cowan RA, O’Cearbhaill RE, Zivanovic O, et al. Current status and future prospects of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) clinical trials in ovarian cancer. Int J Hyperthermia. 2017;33:548–553.
- Bhatt A, Glehen O. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (hipec) in ovarian cancer: a review. Indian J Surg Oncol. 2016;7:188–197.
- Los G, Sminia P, Wondergem J, et al. Optimisation of intraperitoneal cisplatin therapy with regional hyperthermia in rats. Eur J Cancer. 1991;27:472–477.
- Los G, van Vugt MJ, den Engelse L, et al. Effects of temperature on the interaction of cisplatin and carboplatin with cellular DNA. Biochem Pharmacol. 1993;46:1229–1237.
- Los G, van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer. 1994;69:235–241.
- Hettinga JV, Konings AW, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia – a review. Int J Hyperthermia. 1997;13:439–457.
- Reed E, Parker RJ, Gill I, et al. Platinum-DNA adduct in leukocyte DNA of a cohort of 49 patients with 24 different types of malignancies. Cancer Res. 1993;53:3694–3699.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. JCO. 2003;21:3737–3743.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. JCO. 2012;30:2449–2456.
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:1686–1693.
- Esquivel J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: survival outcomes and patient selection. J Gastrointest Oncol. 2016;7:72–78.
- Seretis C, Shariff U, Raju T, et al. Proceedings of the 9th International Congress on Peritoneal Surface Malignancies, October 9th–11th 2014, Amsterdam. J Buon. 2015;20:346–347.
- Cascales PA, Gil J, Galindo PJ, et al. Heterogeneity in patients and methods. A problem for hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 2011;158:361–362.
- Chiva LM, Gonzalez-Martin A. A critical appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of advanced and recurrent ovarian cancer. Gynecol Oncol. 2015;136:130–135.
- du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009;115:1234–1244.
- Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol. 2012;124:10–14.
- Harter P, Beutel B, Alesina PF, et al. Prognostic and predictive value of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score in surgery for recurrent ovarian cancer. Gynecol Oncol. 2014;132:537–541.
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731.
- Vernaccini N, Stefano B, Bertozzi S, et al. Surgical cytoreduction and hyperthermic intraperitoneal chemotherapy in patients affected by recurrent or persistent peritoneal carcinomatosis form epithelial ovarian cancer with high peritoneal cancer index values: our single center experience. Euro J Surg Oncol. 2016;42:S215.
- Wu Y, Chen L, Zhao Y, et al. Efficacy and safety of hyperthermic intraoperative intraperitoneal chemotherapy with Paclitaxel and Cisplatin in the treatment of ovarian cancer. Anti-Tumor Pharmacy. 2016;6:291–295.
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: a 3 year experience. Cancer Chemother Pharmacol. 2017;80:405–410.
- Baiocchi G, Ferreira FO, Mantoan H, et al. Hyperthermic intraperitoneal chemotherapy after secondary cytoreduction in epithelial ovarian cancer: a single-center comparative analysis. Ann Surg Oncol. 2016;23:1294–1301.
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22:1570–1575.
- Cascales-Campos PA, Gil J, Feliciangeli E, et al. The role of hyperthermic intraperitoneal chemotherapy using paclitaxel in platinum-sensitive recurrent epithelial ovarian cancer patients with microscopic residual disease after cytoreduction. Ann Surg Oncol. 2015;22:987–993.
- Le Brun JF, Campion L, Berton-Rigaud D, et al. Survival benefit of hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer: a multi-institutional case control study. Ann Surg Oncol. 2014;21:3621–3627.
- Cascales-Campos PA, Gil J, Gil E, et al. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann Surg Oncol. 2014;21:2383–2389.
- Konigsrainer I, Horvath P, Struller F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in recurrent epithelial ovarian cancer with peritoneal metastases: a single centre experience. Langenbecks Arch Surg. 2014;399:589–594.
- Fagotti A, Costantini B, Petrillo M, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up. Gynecol Oncol. 2012;127:502–505.
- Warschkow R, Tarantino I, Lange J, et al. Does hyperthermic intraoperative chemotherapy lead to improved outcomes in patients with ovarian cancer? A single center cohort study in 111 consecutive patients. Patient Saf Surg. 2012;6:12.
- Kim JH, Lee JM, Ryu KS, et al. Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J Surg Oncol. 2010;101:149–155.
- Munoz-Casares FC, Rufian S, Rubio MJ, et al. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin Transl Oncol. 2009;11:753–759.
- Gori J, Castano R, Toziano M, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer. 2005;15:233–239.
- Ryu KS, Kim JH, Ko HS, et al. Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol. 2004;94:325–332.
- Safra T, Grisaru D, Inbar M, et al. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients- a case-control study. J Surg Oncol. 2014;110:661–665.
- Spiliotis J, Halkia E, de Bree E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy-current perspectives. Curr Oncol. 2016;23:e266–275.
- Chua TC, Esquivel J, Pelz JO, et al. Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol. 2013;107:566–573.
- Vanounou T, Garfinkle R. Evaluation of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin in the era of value-based medicine. Ann Surg Oncol. 2016;23:2556–2561.
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112:265–274.
- Munkarah AR, Coleman RL. Critical evaluation of secondary cytoreduction in recurrent ovarian cancer. Gynecol Oncol. 2004;95:273–280.
- Harter P, Du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–1710.
- Didelot C, Lanneau D, Brunet M, et al. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. CMC. 2007;14:2839–2847.
- Hegyi G, Szigeti GP, Szasz A. Hyperthermia versus oncothermia: cellular effects in complementary cancer therapy. Evid Based Complement Alternat Med. 2013;2013:672873.
- Harter P, Reuss A, Sehouli J, et al. Brief report about the role of hyperthermic intraperitoneal chemotherapy in a prospective randomized phase 3 study in recurrent ovarian cancer from Spiliotis et al. Int J Gynecol Cancer. 2017;27:246–247.
- Hotouras A, Desai D, Bhan C, et al. Heated intraperitoneal chemotherapy (HIPEC) for patients with recurrent ovarian cancer: a systematic literature review. Int J Gynecol Cancer. 2016;26:661–670.
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009;64:211–218.