Abstract
Purpose: Vascular-rich myomas are resistant to treatment involving transcervical microwave myolysis. To overcome cooling by blood perfusion, we injected dilute vasopressin solution into the space between the myometrium and the surface of the vascular-rich myomas.
Material and Methods: Seven outpatients [age (mean ± SD age), 44.9 ± 3.9 years] with a single symptomatic vascular-rich submucosal myoma measuring 4.2–9.2 cm (6.5 ± 2.5 cm) underwent transcervical microwave myolysis and microwave endometrial ablation. Before microwave irradiation, dilute vasopressin solution was injected into the space between the myometrium and the surface of the vascular-rich myoma. We assessed the changes in the volumes of the vascular-rich myomas and blood hemoglobin levels before and 3 and 6 months after treatment. In addition, improvements in menorrhagia and satisfaction after the operation were assessed using visual analog scales.
Results: Submyometrial injection of dilute vasopressin effectively reduced the abundant blood flow. The vascular-rich myomas were necrotized and shrank significantly by 69.0% at 3 months and 72.4% at 6 months after the operation (p < .05). Blood hemoglobin levels significantly increased at 3 months (p < .01). In addition, the visual analog scale results indicated that menorrhagia improved subjectively and the patients were satisfied with the results of the operation.
Conclusions: Vasopressin injection before transcervical microwave myolysis leads to extended necrosis of vascular-rich submucosal myomas.
Introduction
Uterine myomas are common pelvic tumors in women aged 30–49 years [Citation1,Citation2]. Successful microwave myolysis using ultrasound guidance has been reported [Citation3–5]. However, transcervical microwave myolysis (TCMM) for vascular-rich myomas has been reported to induce restricted tissue necrosis around the microwave applicator tip [Citation3]. Injection of a dilute vasopressin solution into the adjacent myometrium during laparoscopic or hysteroscopic myomectomy has been shown to reduce blood loss and facilitate resection [Citation6,Citation7]. Submyometrial vasopressin injection should effectively decrease blood flow that carries heat away from the microwave irradiated myoma tissue, with temperature in the marginal area maintained by conduction from the heat-generating central area around a microwave applicator tip.
In the present report, we examined whether TCMM, after dilute vasopressin is injected into the space between the myometrium and surface of the myoma, leads to extended necrosis of vascular-rich myomas.
Cases
In 2018, 35 outpatients with menorrhagia caused by submucosal myomas, who did not wish to preserve childbearing potential but who hoped to avoid hysterectomy, were preoperatively examined using magnetic resonance imaging (MRI) and ultrasonography including color Doppler flow mapping. Patients with intramural, small-sized submucosal or multiple myomas, adenomyosis, or histologically-diagnosed typical leiomyomas were excluded. Of the 35 outpatients, 7 (age: 44.9 ± 3.9 years) with a single symptomatic vascular-rich myoma were enrolled in this preliminary study. Preoperative diffusion-weighted magnetic resonance imaging (DW-MRI) of the myomas in the 7 patients revealed high signal intensity that indicated cellular leiomyomas or leiomyosarcomas [Citation8,Citation9]. To exclude leiomyosarcoma, histological diagnosis was performed using transcervical needle biopsy before treatment. Four patients were histologically diagnosed as cellular leiomyomas. Three patients refused needle biopsy, although gadolinium-enhanced T1-weighted MRI revealed that contrast medium had stronger signal intensity enhancement in the myoma than in the myometrium. According to the MRI findings, all patients had a single submucosal myoma >4 cm in size. The possibility of endometrial malignancy was excluded based on the findings of MRI, transvaginal ultrasonography, hysteroscopy and endometrial biopsy performed before surgery.
This study obtained approval from the institutional review board, and was planned and carried out according to the Helsinki declaration. Injecting dilute vasopressin solution during surgical treatment of the uterus has been commonly utilized. Therefore, the risk to patients in this preliminary study was thought to be minimal, because the protocol using dilute vasopressin was in compliance with the Helsinki declaration. Written informed consent was obtained from every patient prior to participation.
All 7 patients were admitted at 10:00 am and underwent surgery at 12:00 pm. The patients were placed in the lithotomy position under continuous intravenous infusion of propofol and underwent TCMM after microwave endometrial ablation (MEA) was completed. MEA was performed with a Microtaze AZM 550 device (Alfresa Pharma Co., Osaka, Japan), which could generate microwaves at a frequency of 2.45 GHz, using a thin, curved microwave applicator (Sounding Applicator, Alfresa Pharma Co.). The TCMM procedure has been described in detail previously [Citation4]. Before inserting the microwave applicator, a dilute vasopressin solution (concentration: 0.8 U/ml; maximal dose: 40 U) was transcervically injected into the space between the myometrium and myoma using a 21-G percutaneous transhepatic cholangiography (PTC) needle 20 cm in length under transabdominal ultrasound guidance (). The needle was then replaced with a straight microwave applicator (4 mm in diameter). The duration of irradiation for myolysis was selected based on the size of the myoma [Citation10]. The vascular-rich myoma was continuously irradiated with microwaves at several irradiation sites at an output of 40 W at the microwave applicator tip, for a total of 175–1200 s. As objective indexes, we investigated the reduction of submucosal myoma volume and blood hemoglobin levels. The size of the submucosal myoma in 3 dimensions was measured using MRI or ultrasonography before, and at 3 and 6 months after the operation. In addition, the subjective improvement in menorrhagia and satisfaction with the operation were assessed using a visual analog scale (VAS: 0–10) 3 months after the operation. The preoperative VAS score for menorrhagia was 10, whereas that for amenorrhea was 0. The preoperative VAS score for satisfaction with the operation was also 0. Complete satisfaction after the operation was indicated by a score of 10. At 1 month after the operation, gadolinium-enhanced MRI was performed to detect de novo avascular areas indicating necrosis of intrauterine tissue. The observation period was 6 months. The data were written by the mean ± standard deviation. Paired t-tests were used to analyze changes in the myoma volume.
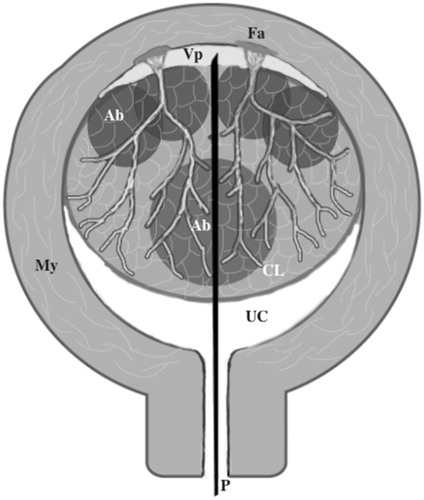
Figure 1. Transcervical injection of a dilute vasopressin solution into the space between the myometrium and surface of the leiomyoma through the leiomyoma using a PTC needle. My: myometrium; P: PTC needle; UC: uterine cavity; CL: cellular leiomyoma; Fa: feeding artery; Vp: vasopressin solution in the space between the myometrium and leiomyoma; Ab: ablation spot.
The mean duration of surgery was 59.4 ± 22.4 min. The mean myoma volume showed reduction at 3 and 6 months after the operation (136.5 ± 98.7(before) vs. 42.4 ± 40.4(3 months) vs. 33.8 ± 39.4(6 months) cm3; p < .05). The myomas shrank by 69.0%±27.9% at 3 months and 76.2% ± 31.6% at 6 months (p < .05) after the operation. Hemoglobin levels significantly improved at 3 months as compared with levels before the operation (8.7 ± 2.0(before) vs. 11.4 ± 2.3(3 months) g/dl; p < .01). The menstrual bleeding volume decreased on a subjective scale to VAS score 1.4 ± 1.6 at 3 months after the operation. After TCMM and MEA, each patient felt that menorrhagia symptoms improved, with a postoperative VAS score <5. The mean VAS score for satisfaction after TCMM and MEA was 9.3 ± 1.5 (maximum score, 10) at 3 months after the operation (). demonstrates the effects of TCMM after injecting dilute vasopressin solution. Tissue necrosis caused by TCMM was depicted as a de novo avascular area on gadolinium-enhanced MRI 1 month after the operation. The de novo avascular area replaced the area of the submucosal myoma identified before the operation. Needle biopsy before the operation revealed that the myoma was pathologically a cellular leiomyoma (). After injection of a dilute vasopressin solution, the hypoechogenic area in the space between the myometrium and myoma increased on imaging (). Arterial blood flow in this space before and after the injection of a dilute vasopressin solution is shown in the cellular leiomyoma in . The pulsatility index of the submyometrial blood flow changed from 0.94 to 1.40, indicating an increase in arterial blood flow resistance in this space after the injection of a dilute vasopressin solution. In short, injecting vasopressin decreased arterial blood flow.
Figure 2. (a) T2-weighted magnetic resonance imaging (MRI) of a cellular leiomyoma (triangle) before transcervical microwave myolysis (TCMM). (b) Gadolinium-enhanced T1-weighted MRI of a cellular leiomyoma one month after TCMM & microwave endometrial ablation. The necrotic changes of the cellular leiomyoma are depicted as a de novo avascular area (triangle). The leiomyoma had shrunk from 5.6 cm to 3.4 cm in size. (c) Histological findings of a cellular leiomyoma on needle biopsy before TCMM. Stained with hematoxylin and eosin. Magnification ×200.
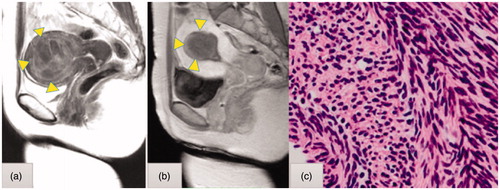
Figure 3. (a) Ultrasonic findings before injection of a dilute vasopressin solution (percutaneous transabdominal cholangiography [PTC] needle). (b) After injection of a dilute vasopressin solution into the space between the myometrium and surface of the leiomyoma using a 21-G PTC needle under transabdominal ultrasound guidance, a hypoechoic lesion into the myometrium was visualized (Δ dilute vasopressin solution).
![Figure 3. (a) Ultrasonic findings before injection of a dilute vasopressin solution (percutaneous transabdominal cholangiography [PTC] needle). (b) After injection of a dilute vasopressin solution into the space between the myometrium and surface of the leiomyoma using a 21-G PTC needle under transabdominal ultrasound guidance, a hypoechoic lesion into the myometrium was visualized (Δ dilute vasopressin solution).](/cms/asset/002ab1eb-d7f4-4628-9986-167f63c9a95a/ihyt_a_1612102_f0003_c.jpg)
Figure 4. Changes in velocity of blood flow before (1) and after (2) injecting a dilute vasopressin solution determined based on the pulsation of the space between the myometrium and surface of the leiomyoma. PI on blood flow in the space changed from 0.94 to 1.40.
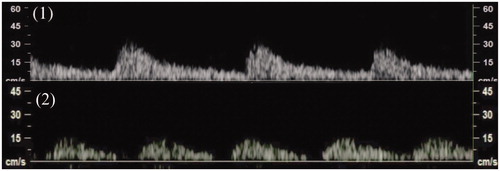
Table 1. Patients characteristics and results.
Discussion
Direct necrosis occurs at temperatures above 60 °C because most tissue proteins denature within a few seconds. In addition to direct tissue necrosis within the 60 °C isotherm, extended necrosis caused by heat conduction occurs in neighboring tissue depending on both temperature and duration when cooling by blood flow is negligible. However, vascular-rich tumors, such as hepatocellular carcinomas are resistant to microwave heating. Similar to the refrigerant cooling system of a car radiator, blood flow efficiently cools vascular-rich tissues. As an example of blood flow affecting necrotized tissue volume, microwave ablation treatment in hepatocellular carcinomas, using combined hepatic arterial embolization and temporary hepatic venous flow interruption, necrotizes significantly larger tumor volumes than those treated with hepatic arterial embolization alone [Citation11]. Similarly, interstitial microwave irradiation cannot develop large necrotized volumes in vascular-rich myomas.
Despite the finding that tissue necrosis is restricted in vascular-rich myomas without the use of vasopressin, the margin of the avascular area reached the border between the myoma and myometrium in the present cases. TCMM after injection of dilute vasopressin was as effective in vascular-rich myomas as in typical vascular-poor myomas.
We expected that injecting a dilute vasopressin solution into the space between the myometrium and myoma would decrease the blood flow in vascular-rich myomas, including cellular leiomyomas. In fact, this approach contracted the main feeding vessels and successfully reduced the blood flow in cellular leiomyomas. After preparative injection of vasopressin, interstitial microwave irradiation sufficiently elevated tissue temperature to induce large necrotic volumes in vascular-rich myomas.
We believe that needle biopsy before treatment of vascular-rich myomas is desirable to rule out uterine sarcomas, even though they are extremely rare. The histopathological results of this study suggest that vascular-rich myomas include cellular leiomyomas. The vascular-rich characteristics of cellular leiomyomas might be associated with the requirement for abundant blood flow for the maintenance of high cellular density.
Using TCMM and MEA for ambulatory surgery in an office setting, we were able to treat patients with menorrhagia caused by vascular-rich myomas including cellular myomas.
Recently, the SONATA system [Citation12] has been reported to effectively treat submucosal and intramural myomas. However, it remains unclear whether a vascular-rich myoma can be treated as a small or moderate-size typical myoma. On the other hand, it is expected that a dilute vasopressin injection will be helpful as pretreatment using the SONATA system.
Although injecting a dilute vasopressin solution before TCMM and MEA appears promising for the treatment of vascular-rich submucosal myomas, several limitations remain. A longer observation period and larger number of patients is needed to verify the usefulness.
Conclusion
Injecting a dilute vasopressin solution before TCMM and MEA is useful to overcome the cooling effect in vascular-rich myomas. This approach may be an alternative to hysterectomy for menorrhagia caused by vascular-rich submucosal myomas in perimenopausal women.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298.
- Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012;19:339–353.
- Kanaoka Y, Yoshida C, Fukuda T, et al. Transcervical microwave myolysis for uterine myomas assisted by transvaginal ultrasonic guidance. J Obstet Gynecol Res. 2009;35:145–151.
- Tsuda A, Kanaoka Y. Outpatient transcervical microwave myolysis assisted by transabdominal ultrasonic guidance for menorrhagia caused by submucosal myomas. Int J Hyperthermia. 2015;31:588–592.
- Zhang J, Feng L, Zhang B, et al. Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment–a clinical study. Int J Hyperthermia. 2011;27:510–516.
- Song T, Kim MK, Kim ML, et al. Use of vasopressin vs epinephrine to reduce haemorrhage during myomectomy: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015;195:177–181.
- Wong AS, Cheung EC, Leung KT, et al. Transcervical intralesional vasopressin injection in hysteroscopic myomectomy–description of a new technique. J Laparoendoscopic Adv Surg Tech. 2013;23:258–262.
- Tamai K, Koyama T, Saga T, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723–730.
- Sato K, Yuasa N, Fujita M, et al. Clinical application diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014;210:368.e1–368.e8.
- Kanaoka Y, Hirai H, Ishiko O. Microwave power and duration without extrauterine thermal damage in microwave endomyometrial ablation at 2.45 GHz. J Obstet Gynaecol Res. 2005;31:359–367.
- Ishida T, Murakami T, Shibata T, et al. Percutaneous microwave tumor coagulation for hepatocellular carcinomas with interruption of segmental hepatic blood flow. J Vasc Interv Radiol. 2002;13:185–191.
- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the sonata system. Curr Obstet Gynecol Rep. 2017;6:67–73.
