?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To systematically summarize the findings from research studies examining the effects of whole-body hyperthermia (WBH) interventions on mood and symptoms of depression.
Methods: Systematic literature search of online and offline databases (e.g., Pubmed, Web of Knowledge, Cochrane, academic libraries). Risk of bias assessment and secondary analysis of effect sizes.
Study selection: Clinical studies with a pre/post-intervention design and outcome measures for mood and depression as accepted in the S-3 guidelines (Association of Scientific Medical Societies in Germany).
Data extraction: Study characteristics and outcomes (means and standard deviations) from participants receiving at least one WBH intervention.
Results: A total of 7 studies and 148 subjects with a mean age of 46 years (36–56 years) were identified. Three out of seven studies utilized hot baths and 4/7 infrared heating. Study duration ranged from 1 to 6 weeks with one or multiple interventions and an average treatment time of 66.37 min (42.55–140). Risk of bias analysis revealed small sample biases and lack of control groups in 3/7 studies. About 21 study end-points were extracted with 19 resulting in effects sizes (Cohen’s d) of 0.8 or greater. Target temperatures between 38 °C and 39 °C and slower increase in core body temperature during the intervention resulted in larger treatment effects.
Conclusion: WBH is a promising alternative treatment for depression with low risk for adverse reactions and side effects but still lacking sufficient evidence for general recommendations for clinical practice. However, as all other interventions have failed, the studies to date can provide a framework for clinical application.
Background
Depression has become a serious population health issues over the last decade with a significant medical, social and economic burden. In the United States for example, 10.3% of physician office visits indicated depression on the medical records in 2014 with more than 40,000 people committing suicide [Citation1]. According to the WHO, depressive disorders will surpass coronary artery disease as leading causes of debilitating illnesses by 2030 [Citation2]. New and effective strategies to address this continuously rising health issue are needed. The S-3 guidelines from the Association of Scientific Medical Societies in Germany (AWMF) [Citation3] recommend pharmacological and behavioral interventions for mild to moderate depression and electroconvulsive therapy (ECT) as the standard treatment for more severe and chronic symptomology. About 20–30% of patients receiving common, pharmacological treatments, do not respond to the intervention or develop side effects [Citation4,Citation5]. Furthermore, a treatment resistant depression has been associated with significant alterations of cognitive functions and even suicidal attempts [Citation5,Citation6]. This might reflect a need for effective alternative treatments or interventions to complement common clinical practice. Historically and prior to pharmacological discoveries, depressive symptoms were commonly treated with hyperthermia interventions. In fact, evidence for the use of hyperthermia dates back as far as the times of Galen of Pergamon (129–198 C.E.) who reportedly treated melancholia successfully by bathing his patients in hot tubs and massaging their skin [Citation7]. Over the last years, whole-body hyperthermia (WBH) interventions have received renewed attention and several studies have examined its effects on mental health and the potential clinical application. This includes case reports, open-label studies and also controlled and blinded trials. In 2016, we published the results of a randomized, double blind and sham-condition controlled study and provided the first scientific evidence for the anti-depressant effect of a single WBH session in a population diagnosed with depression. The published findings resulted in significant discussion in the scientific communities, mostly due to the small sample size and the relevance for clinical practice [Citation8–10]. While this study was the first to study the effects of whole-body heating on depression in a controlled environment, earlier data are available from quasi-experimental and observational reports as well as from another controlled study published in 2017. The present work systematically summarizes the findings from all studies to date and aims to provide a validation of study findings, an assessment of its value as well as recommendations for clinical practice and future research.
Methods
An electronic search of publications related to our objective, whole-body heating and depression, has been conducted in Pubmed, Web of Knowledge, clinicaltrials.gov and the Cochrane library. The literature search included the years 1950–2018. English and German keywords were used, such as whole-body hyperthermia, heat therapy, hot tube, mood, depression, depressive disorder, depressive affect, mental status, Ganzkörperhyperthermie, Überwärmungsbad, GkHT, GHT, Depression, depressiver Affekt and mentaler oder depressiver Status. A manual search of the libraries of the University of Freiburg (Germany) and the University of Graz (Austria) was conducted supplementary to the electronic search. Reference lists from relevant publications were scanned as well as authors contacted to provide additional information. Only studies with human subjects and in English or German language were included.
Study selection
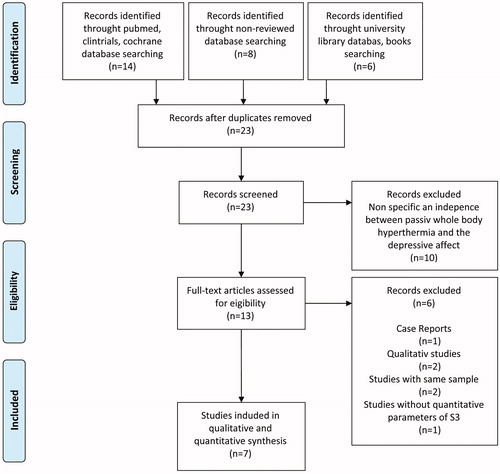
Selection of studies was conducted in four steps. First, we reviewed all publications matching our search criteria, independent of format, study and publication quality and evidence levels. Second, we excluded duplicates, single case-reports and qualitative studies since comparing the results to quantitative studies would not be appropriate. In a third step, we excluded previous reports if a study and its population was expanded and published consecutively at a later time. In this case we only included the most recent publication. Last, we selected all studies that utilized outcome measures for mood and depression that are accepted in the S-3 guidelines from the AWMF, such as Hamilton Depression Rating Scale (HAM-D), Patient Health Questionnaire (PHQ-D), Center for Epidemiologic Studies Depression Scale (CES-D), Beck Depression Inventory (BDI) or Allgemeine Depressionsskala (ADS) (German version of the CES-D). Please see for a PRISMA study flowchart. Overall, our literature search identified 13 studies [Citation11–23] for further review. After evaluating all studies with the aforementioned criteria, the seven studies [Citation14,Citation15,Citation18,Citation19,Citation21–23] outlined below were selected for further analysis and data extraction.
Schaper [Citation14] reported data from 20 patients (5 males, 15 females) receiving WBH interventions during an in-patient stay to treat their moderate uni- or bipolar symptoms. The average age of the study population was 41.95 (9.76) and included patients free of antidepressant medications as well as patients with previous or active pharmacological treatments protocols. All patients received a weekly passive WBH session in a bathtub. All sessions started with water temperatures of 37 °C and were increased by 1 °C every 10 min. Core body temperature was monitored throughout the entire session using sublingual thermometers. Prior to the intervention all patients received a subcutaneous injection of Primula/Onoprodon cp 1% (1 ml). The number of interventions received ranged from 4 to 13, depending on the length of the in-patient stay. Sessions were concluded once the sublingual temperature did not increase any further following increases in water temperature. The average session duration was 42.55 min (12.32) followed by a 60-min resting period wrapped in warm blankets. Core body temperature was measured sublingually and averaged at a maximum of 38.4 °C and an increase of 1.72 °C throughout the intervention. HAM-D interviews were conducted the day before the intervention and following every WBH session. Statistically significant reductions in depressive symptomology were reported following the second session and during the second week.
Gödl [Citation15] published the results from open-label, quasi-experimental observations on 10 patients (m/w = 4/6) with an average age of 41.67 (10.27). Only patients with diagnosed depressive disorder following The Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria from the American Psychiatric Association were included and patients with manic episodes or psychotic symptoms excluded. Eight patients were under active anti-depressive medication regimens and two reportedly did not take any psychopharmaceuticals. As part of a multimodal approach, study participants received a WBH treatment in a hot tub, starting at a water temperature of 37 °C and increasing by 1 °C every 10 min. Treatments were conducted once every week for 6–8 weeks. On average the maximum core body temperature (measured sublingual) was 39.3 °C with an average increase of 2.3 °C during an average bathing time of 46.7 (6.9) min. Following the hot bath, study participants rested for 1 h covered with a blanket to keep the warm. HAM-D interviews were conducted at the end of the multimodal treatment phase and 50% of study subjects (N = 5) were below the cutoff for depression. The focus of this study was on heart rate variability and baseline HAM-D values were unfortunately not obtained.
Hüppe [Citation19] studied the effects of a 45-min intervention in a WBH device (IRATHEM 1000) on mood in 36 patients diagnosed with lipophilic toxicants in a randomized controlled setting. The study population had an average age of 56.20 (6.20) and all participants were randomized to receiving either a 45-min session with a temperature increase of 1–1.5 °C rectal temperature (experimental group, 12 patients), a 45-min session with a temperature increase of less than 0.3 °C (control, 12 patients) or no intervention at all (12 patients). Sessions were conducted three times per week over a timeframe of 5 weeks. ADS scores (German equivalent to the CES-D) were obtained at baseline, week 1 and week 5 following the last intervention. Pre-intervention ADS scores ranged from 13.50 (6.88) in the control group, to 17.33 (9.78) in the experimental group and 18.17 (7.94) in the no intervention group. Subjects with scores of 23 and greater on the ADS scale are considered ‘depressed’ and it is to note that the baseline values in this study were below this clinical cutoff. However, results revealed a reduction in depressive symptomology in the experimental group from 17.33 (9.78) at baseline to 12.58 (10.53) at week 1 and 13.50 (9.04) at week 5 of the study. The control group did not show any changes at all.
Hanusch [Citation18] observed 17 patients (m/w = 5/12, age 46.65 (12.62)) also in a quasi-experimental study and moderate to severe uni- or bi-polar depression. Three out of fourteen patients were also treated with selective serotonin reuptake inhibitors (SSRI) at the time they received the WBH intervention. All subjects received a single hyperthermia session with IR-A light exposure in a Heckel HT-2000 device. The maximum core body temperature as measured with a rectal thermometer averaged at 38.4 °C with an increase of 1.2 °C during the intervention. Treatment duration on average was 46.2 min followed by a 60-min resting phase in the machine but without any further exposure to infrared lighting or any other heating devices. The treatment end-point was defined with a particular pattern in the increase in skin temperature rather than core body temperature. During the sessions, study participants were accompanied by clinical staff seated close to their head and providing cold washcloths on the forehead frequently. Hanusch found that the 14 patients who were currently free of psychopharmaceutic drugs experienced statistically significant reductions in depressive symptoms as measured with the ADS-L (German version of the CES-D) and during the first week and sixth week following the single session. Interestingly, the three patients who were under current SSRI treatment protocols did not experience any changes in depressive symptomology.
Romeyke [Citation21] studied the effects of whole-body heating in 103 patients (m/w = 2/102, average age 55.2 (10.0)) with fibromyalgia and also diagnosed with severe depression based on criteria from the American Psychiatric Association (DSM-V) and the International Statistical Classification of Disease and Related Health Problems. As part of this quasi-experimental observational study, patients were asked to complete a Patient Health Questionnaire (PHQ-D) with a focus on mental well-being and depression. Study participants received multiple WBH sessions in a Heckel HT-2000 device with Infrared-A lighting for the duration of 50 min and a target temperature of 38.5 °C. Following the intervention, subjects rested for 60 min while being covered with blankets. Core body temperature was measured using a rectal thermometer. When comparing the results to a control group that did not receive hyperthermia interventions, they did not find statistically significant differences between the groups (p = 0.055).
Janssen [Citation22] used a randomized, double-blind and sham-condition controlled study design in 34 patients with depression as assessed with HAM-D interviews and multiple interviewers. Seventeen study participants received an active WBH treatment and 17 a sham intervention in a Heckel HT-3000 device with water-filtered infrared-A heating. The average age of the 17 study participants who received the active intervention was 36.71 (15.20) with a female/male ratio of 12/5. Heating was applied until the rectal temperature reached 38.5 °C. Following the heating session, all subjects remained in the enclosed machine to rest for another 60 min without exposure to infrared light or any further heating. Two separate teams were trained to maintain a double-blind setting. Subjects were also accompanied by trained staff seated next to the study participant’s head. On average the maximum core body temperature was 38.85 °C with an increase of 1.91 °C in the active treatment group and 37.69 °C in participants receiving a sham treatment. Treatment time was 47 min on average (21–80 min) followed by a resting phase of 60 min. HAM-D values were obtained at baseline, 1, 2, 4 and 6 weeks after the one hyperthermia session. Throughout the study period, statistically significant improvements in mental condition, and reductions in depressive symptomology respectively, measured with HAM-D assessments occurred in the group receiving the active treatment when compared to the group receiving the sham session.
Naumann [Citation23] is the most recent WBH study and examined the effects of a hot tub intervention in 17 patients with depression. The study population was 47 years of age on average (11.9) and the ratio males/females was 3/14. Results were compared to a non-blinded control group of 19 subjects who received a sham treatment of green LED light exposure (10,000 Lux for 30 min). All 17 subjects received 2 weekly hyperthermia interventions in the hot tub over the course of 4 weeks. Study participants were either lying or sitting in a small pool-sized tub that was heated to 40.2 °C (0.3) for an average of 22.6 min (3.5). Participants received the intervention either individually or in a group setting. Following the intervention, subjects rested for 33.2 min (6.3) while wrapped in warm blankets. Core body temperature was measured using ear thermometers and temperature increased from 36.6 °C to 39.1 °C (average increase of 2.43 °C) followed by a decrease during the resting phase to 37.7 °C on average. HAM-D values were obtained at baseline, at 2 weeks and at 6 weeks. In the intervention group, effect sizes of 0.84 and 0.86 were reported for the week 2 and week 6 assessments, respectively. Statistically significant differences between the groups were reported for the week 2 measure but not at week 6.
In summary, three open label studies and four controlled studies were identified and selected for data extraction and subsequently included for further statistical analysis ().
Table 1. Overview of all studies included.
Data extraction
Means, standard deviations and number of participants were extracted from the intervention groups from all selected studies. To compare effect sizes across all studies, we calculated Cohen’s standardized mean change for the treatment group (δT) using the below formula [Citation24]:
Effect sizes > 0.80 were considered large, 0.50–0.80 moderate and 0.20–0.50 small, following Cohen’s recommendations. Additionally, 95% confidence intervals were calculated with the below formula :
Furthermore, study characteristics were extracted to inform an analysis of biases following the Cochrane libraries guidelines for randomized controlled trials and the recommendations for the analysis of non-controlled studies [Citation25]. Studies were either deemed as having low risk (++), potentially low risk (+), potentially high risk (-), high risk (- -) or unclear risk (/). Summary scores were created for each study analysis and described as percentages, with ≥20% as small risk, ≤50% moderate risk and >50% high risk of study bias ( and ).
Table 2. Risk of bias analysis for controlled studies.
Table 3. Risk of bias analysis for non-controlled studies.
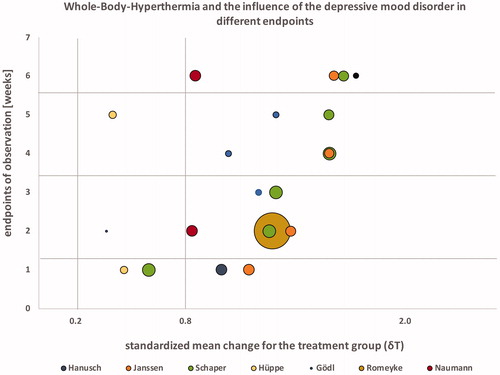
Since only four studies reported having a control group, we decided that the format of a common meta-analysis with forest plot visualization would be inappropriate and decided on a summary and comparison of the pre/post results from the intervention groups only (). In addition to extracting pre/post findings we analyzed the outcomes in association with their potential risk for bias at all study end-points and further conducted additional group comparisons based on temperature, treatment times and frequency of interventions. To visualize results we decided to utilize a GapMap with bubbles due to the diversity of the data. GapMaps are a common method in epidemiological research where data are often very heterogeneous.
Table 4. Pre-post treatment effect sizes (Cohen’s d) with 95% confidence intervals, effect sizes >0.80 were considered large, 0.50–0.80 moderate and 0.20–0.50 small.
Results
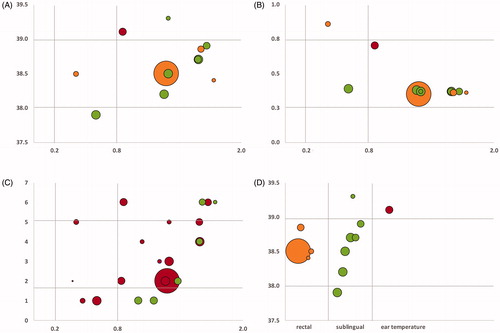
Seven studies on the effects of WBH on depression have been identified and included in this analysis. Four studies were controlled clinical trials. Overall, we were able to review data from 148 (10–55) patients with an average age of 46 (36–56) who received one or more treatment sessions. The majority of studies included patients currently taking psychopharmaceutic medications. Hanusch [Citation16–18] controlled for antidepressant medication use in his analysis and Janssen [Citation22] excluded patients who were actively treated with prescription drugs for depressive symptomology. Three studies provided whole-body heating in a hot bath (one in a hot tub and two in a hyperthermia bath), two utilized infrared-A heating chambers (Heckel HT-2000) and two water-filtered infrared-A heating chambers (one Heckel HT-3000 and one IRATHERM). In five studies, participants received multiple WBH sessions and in two studies only a single treatment was given. The average session duration was 66.37 min (42.55–140.00). Core body temperature was measured with various methods and four studies utilized rectal thermometers, two studies measured temperature sublingual and one study used an ear thermometer. Increases in measured temperature ranged from 1.0 °C to 2.43 °C during the sessions. No reliable average of core body temperature could be calculated due to the various methods used to measure temperature. Temperature measured sublingually seems to vary significantly and temperature measured in participants ears seems to be generally higher when compared to rectal measures of temperature (). Study participants were followed for a maximum of 6 weeks and at minimum for 1 week. Two studies (Gödl and Naumann) had variable treatment duration, two studies (Schaper and Romeyke) had variable frequency of treatments and two studies (Schaper and Gödl) had variable duration for the observation of patients. Some studies had variable study end-points since they observed patients during an in-patient stay and participation concluded with the end of the hospital stay. Six out of seven studies found statistically significant reductions in depressive symptomology somewhere between 1 and 6 weeks post-intervention. Romeyke [Citation21] did not report significant findings when measuring depressive symptomology using the PHQ-D assessment. Overall 21 study end-points were provided from the studies with significant anti-depressant effects reported for 19; only 2 end-points (Hüppe) did not reach statistical significance. All studies were analyzed for existing biases and grouped for their risk level. As expected, open-label and non-controlled studies showed increased risk for bias when compared to controlled clinical studies. Three out of seven studies were grouped as high risk for bias, two with a moderate risk and two as low risk for bias (see ). After calculating effect sizes, 17 study end-points revealed Cohen’s d values of 0.8 or higher and 4 study end-points had Cohen’s d effect sizes of less than 0.8 (). A positive trend was observed over the course of 6 weeks with increasing effect sizes (see GapMap visualization). Eighteen out of 21 study end-points showed a progressive increase in effects sizes, 2 out of 21 increased constantly and 1/21 appears to decrease in effect size. Sample sizes ranged from 4 to 55 and therefore provided reduced power and a small sample biases in select studies. Effect sizes were independent of sample size however when comparing studies with large sample sizes (Romeyke) with smaller ones (Schaper, Hanusch, Gödl and Janssen). Also, effect sizes seem to be independent of total number of WBH sessions and frequency as no differences can be reported between a single session (Janssen and Hanusch) and multiple sessions (Schaper, Gödl and Romeyke) (). Antidepressant effects further occurred in both participants currently treated with psychopharmarceutical drugs and without. Only one study [Citation16–18] controlled for use of antidepressant medication. The standard mean difference was calculated for the four controlled trials (N = 99 in the intervention group and N = 85 in the control group) using RevMan 5.3 and resulted in −0.59 [−0.97, −0.21] with an effect size of z = 3.05 (p = 0.002) for the first observations, suggesting that the active treatment was more effective in patients with depressive symptomology.
Figure 2. Relationship between risk of bias (%) and effect sizes from all studies; the bubble size = sample size (n) of studies experimental groups.
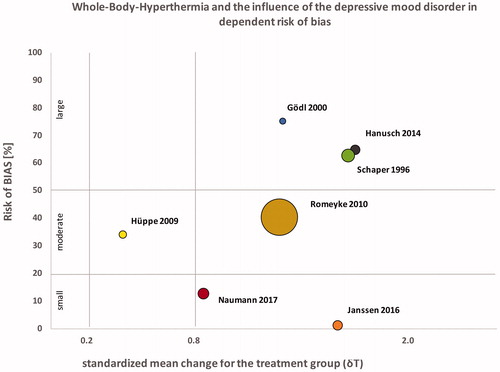
Figure 3. (A) Relationship between core body temperature during the intervention (y-axis) and effect sizes (x-axis) in °Celsius as grouped by measuring method (orange = rectal temperature, green = sublingual temperature, red = ear temperature). (B) Relationship between the ratio of highest core body temperature and time to reach the temperature (y-axis) and effect size (x-axis), grouped by intervention type (orange = infrared heating with IRATHERM device or Heckel device, green = intervention in a bath tub, red = hot tub). (C) Relationship between study end-point (y-axis) and effect size (x-axis) grouped by intervention frequency (green = single session, red = multiple sessions). (D) Relationship between the different methods of measuring core body temperature (x-axis) and core body temperature (y-axis).
Adverse events
Five out of seven studies reported adverse reactions during or following a hyperthermia session. Those events were either documented in medical records (Naumann, Schaper and Hanusch) or collected with standardized assessment such as the no observed adverse effect level and the patient rated inventory of side effects (Hüppe and Janssen). Adverse reactions during the sessions included restlessness, agitation, tachycardia, emotional reactions such as anger, sadness or feelings of incompetence and were reportedly of minor nature. Following the hyperthermia intervention reactions such as headaches, insomnia, vertigo, nausea, numbness in extremities, ringing in ears and reduced interest in sexual activities occurred also reportedly of short or very short duration and complete reversion. Both studies utilizing standardized assessments did not find differences in adverse reactions in comparison to the control group.
Limitations
The heterogeneous selection of studies, primarily in the study end-points, intervention type, study population and focus of the research study as well as measures applied is a limitation to this project. Due to the lack of control groups in 3/7 studies and missing information on responding and non-responding study participants, calculating an appropriate risk ratio and necessary number to treat was not possible. Another limitation is the lack of separation between medicated and non-medicated study participants. The work conducted by Hanusch and Nauman provides some preliminary evidence however.
Conclusion
Using a somewhat unconventional and innovative but scientifically sound approach, we were able to combine and compare the findings from a diverse set of seven studies examining the effects of WBH on depression and mental health. Our analysis revealed large effects sizes in 6/7 studies, independent of sample size, total number and frequency of treatment sessions as well as study quality. The data reported by Hüppe [Citation19] were the only to result in a small effect size (). This might be due to their study population failing to reach the clinical cutoff for depression at baseline on the ADS scale and it is questionable if the study participants were suffering from depression and rather had dysthymic disorders which are defined as episodic mood changes over 2 or more years but lacking the overall number of symptoms required for the diagnosis of clinical depression [Citation3]. According to the ADS scale, patients scoring between 16 and 23 are considered dysthymic. Three out of seven studies were assessed as having small sample biases and lack of control groups. Small sample sizes are not uncommon in clinical research and results can still be impactful for clinical guidelines. The German guidelines for the treatment of depression for example include ECT as a treatment with high evidence (level A). Assuming that larger scale studies using ECT have been conducted, the guidelines use two systematic reviews that summarized studies with small sample sizes. The evidence at hand suggests that WBH is starting to become a promising alternative treatment for depression. The results from the seven studies to date can be used to guide its clinical application and inform future research. It seems that a slow increase in ambient temperature and a target core body temperature of 38 °C–39 °C provides the largest effects (). Interestingly, this might be a potential explanation for sauna studies failing to provide similar results since the ambient temperature in a sauna is set at the beginning of the intervention. Future research should focus on examining the underlying biological and psychological mechanisms of action and also the broader clinical applicability in for example non-responders to pharmaceutical interventions or as a complementary treatment to achieve cumulative effects. For example, one mechanism through which WBH treatments might improve mood is by activating temperature-sensitive ion channels in the skin. Those have been shown to signal directly to brainstem serotonergic neurons when activated during warmer temperatures [Citation26]. We would also recommend future research studies to include study end-points at week 1 to capture acute effects, during 6–12 weeks to be comparable to other interventions where this is a common follow-up duration and further re-assess participants after 6 months to study whether WBH can result in, or contribute to, a complete remission of symptoms. For clinical application a single WBH intervention with a target core body temperature of 38.5 ± 0.5 °C, measured rectally, appears to be a valid recommendation, especially if achieved with a slow increase in ambient temperature and followed by a resting period of 60 min. If symptoms relapse we would recommend an additional intervention. Based on the findings summarized above and the evidence to date we are convinced that WBH has the potential to become a promising alternative treatment for depression but there is not sufficient evidence yet to recommend it for general application and to provide guidelines for clinical practice. Further controlled and larger trials and meta-analytic work is necessary. However, if physicians decide to offer WBH as a last treatment option for patients if all other interventions have failed, we recommend to apply a mild to moderate treatment with core body temperatures of 38.5 °C–40.5 °C since this range has been proven most effective based on the evidence summarized in this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rui P, Hing E, Okeyode T. National Ambulatory Medical Care Survey: 2014 State and National Summary Tables 2014; [cited 2018 Jun 17]. Available from: https://www.cdc.gov/nchs/ahcd/ahcd_products.htm
- WHO. Depression and other common mental disorders: global health estimates. Geneva (Switzerland): World Health Organization; 2017.
- Schneider F, Härter M, Schorr S. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depressionen. Berlin Heidelberg (Germany): Springer-Verlag; 2015. (Deutschen Gesellschaft für Psychiatrie und Psychotherapie, Psychosomatik und Nervenheilkunde).
- McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affective Disord. 2014;156:1–7.
- Caraci F, Calabrese F, Molteni R, et al. International Union of Basic and Clinical Pharmacology CIV. The neurobiology of treatment-resistant depression: from antidepressant classifications to novel pharmacological targets. Pharmacol Rev. 2018;70:475–504.
- Gaynes B. Assessing the risk factors for difficult-to-treat depression and treatment-resistant depression. J Clin Psychiatr. 2016;77:4–8.
- Schaper LC. Wiederholte Hyperthermiebehandlung durch Überwärmungsbäder bei Patienten mit depressiven Störungen: Effekte auf die Produktion von Interleukin-6 sowie auf die mittlere Körpertemperatur und den psychopathologischen Befund. Freiburg (Germany): Hochsch.-Verlag; 1995.
- Dailymail. How warming yourself up could banish the blues: two hours in a heated chamber may help reset chemicals in the brain; 2016. Available from: http://www.dailymail.co.uk/health/article-3707308/How-warming-banish-blues-Two-hours-heated-chamber-help-reset-chemicals-brain.html
- Fried E. Hyperthermia as depression treatment; 2016. Available from: http://eiko-fried.com/hyperthermia-as-depression-treatment/
- Neuroskeptic. Hyperthermia as an Antidepressant? (update): Neuroskeptic; 2016. Available from: http://blogs.discovermagazine.com/neuroskeptic/2016/05/15/hyperthermia-as-antidepressant/#.WaBgAq3qhBw
- Lampert H. Heilung durch Überwärmung: Eine Anleitung f.d. prakt. Gebrauch. Hannover (Germany): Bruno-Wilkens Verlag; 1967.
- Bühring M. Klinik der Hyperthermie: Untersuchungen im Überwärmungsbad. Stuttgart (Germany): Hippokrates-Verlag; 1984.
- Koltyn KF, Robins HI, Schmitt CL, et al. Changes in mood state following whole-body hyperthermia. Int J Hyperthermia. 1992;8:305–307.
- Schaper LC. Wiederholte Hyperthermiebehandlung durch Überwärmungsbäder bei Patienten mit depressiven Störungen Effekte auf die Produktion von Interleukin-6 sowie auf die mittlere Körpertemperatur und den psychopathologischen Befund. Vol. Bd.3. Freiburg (Germany): Hochschulverlag GmbH; 1996. (Hochschulsammlung Medizin/Neurologie und Psychiatrie; Bd.3).
- Gödl R, Glied N. Veränderungen der autonomen Regulation durch Überwärmungsbadtherapie bei Patienten mit depressiven Störungen. Karl-Franzens-Universität Graz: Universitätsbibliothek; 2000. (Hochschulsammlung medizinische Dissertationen).
- Hanusch KU, Janssen CH. Die passive Ganzkörperhyperthermie in der Psychiatrie – Eine historische Analyse. Die Naturheilkunde. 2013;90:40–43.
- Hanusch KU, Janssen CH. Passive nicht-onkologische Ganzkörperhyperthermie in der physikalischen Medizin – Wirkmodell, Einflussparameter und Limitationen. Die Naturheilkunde. 2013;90:31–35.
- Hanusch KU, Janssen CH, Billheimer D, et al. Whole-body hyperthermia for the treatment of major depression: associations with thermoregulatory cooling [Open-Label Kohortenstudie im Quasi-Experiementellen pre-posttest Design]. AJP. 2013;170:802–804.
- Hüppe M, Müller JS, Wernze H, et al. Treatment of patients burdened with lipophilic toxicants: a randomized controlled trial. Act Nerv Super Rediviva. 2009;51:133–141.
- Janssen CH, Hanusch KU. Passive Ganzkörperhyperthermie bei Depression – Gezielte Übererwärmung für die innere Balance. Die Naturheilkunde. 2011;88:14–16.
- Romeyke T, Stummer H. Multi-modal pain therapy of fibromyalgia syndrome with integration of systemic whole-body hyperthermia – effects on pain intensity and mental state: a non-randomised controlled study. J Musculoskeletal Pain. 2014;22:341–355.
- Janssen CW, Lowry CA, Mehl MR, et al. Whole-body hyperthermia for the treatment of major depressive disorder: a randomized clinical trial. JAMA Psychiatr. 2016;73:789–795.
- Naumann J, Grebe J, Kaifel S, et al. Effects of hyperthermic baths on depression, sleep and heart rate variability in patients with depressive disorder: a randomized clinical pilot trial. BMC Complement Altern Med. 2017;17:172.
- Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386.
- Cochrane D. Bewertung des Biasrisikos (Risiko systematischer Fehler) in klinischen Studien: ein Manual für die Leitlinienerstellung.: Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften – Institut für Medizinisches Wissensmanagement; 2016. Available from: www.cochrane.de/de/rob-manual.
- Lowry CA, Lightman SL, Nutt DJ. That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J Psychopharmacol. 2009;23:392–400.