Abstract
A growing body of evidence is being published regarding the safety and efficacy of minimally invasive image-guided ablation techniques. While clinical applications of these techniques are increasing, international societies have started to publish treatment guidelines and to make efforts to standardize both terminology and reporting criteria for image-guided thyroid ablations. Laser ablation and radiofrequency ablation (RFA) are among the most common ablation techniques either for benign and malignant thyroid nodules. Unlike laser ablation and RFA in the treatment of benign thyroid nodules, where safety and efficacy have been widely demonstrated, evidence regarding local tumor control of thyroid malignancies is still limited. However, preliminary results are encouraging and image-guided thermal ablation techniques can be considered a valid alternative to surgery for the treatment of benign thyroid nodules and recurrent thyroid cancers. This review evaluates the basic concept of RFA and laser ablations, their techniques, clinical outcomes, and complications based on the suggestions of several society guidelines. Multidisciplinary collaboration remains critical to identify patients which may benefit from minimally invasive image-guided thermal ablations, especially if surgery or radioiodine therapy are not feasible options.
Introduction
Although most thyroid nodules are benign and treatment is unnecessary, some of them require treatment because of cosmetic reasons or subjective symptoms [Citation1,Citation2]. Surgery has been the traditional treatment option for benign and malignant thyroid tumors; however, it has several drawbacks, such as scars, the need for general anesthesia, and the risk of inducing hypothyroidism [Citation3]. To overcome these drawbacks, minimally invasive image-guided ablation techniques such as ethanol ablation (EA), laser ablation, and radiofrequency ablation (RFA) have been developed [Citation3–5]. A growing body of evidence is being published regarding their safety and efficacy. While clinical applications of these techniques are increasing, ablation guidelines have been published from Korean and Italian groups [Citation3,Citation6,Citation7], and a greater awareness has raised regarding standardization of terminology and reporting criteria for image-guided thyroid ablations [Citation8].
This review evaluates two widely used thermal ablation methods, laser ablation and RFA. Based on the suggestions of several society guidelines and currently available evidences, we focus on basic concepts of percutaneous thyroid ablations, techniques, clinical outcomes, and complications.
Basic concept and techniques
Laser ablation
Laser (an acronym for ‘light amplification by stimulated emission of radiation’) is a highly coherent, collimated and monochromatic energy that can be precisely delivered into small targets from a primary source or through optical fibers that allows a great variability in length and shape of applicators [Citation9]. Common primary sources are laser diode or neodymium–yttrium aluminum garnet, which produce optical wavelengths of 820 nm or 1064 nm. When laser light hits the target, a local increase of temperature occurs, causing permanent damages such as coagulative necrosis (46–100 °C) and tissue carbonization/vaporization (100–110 °C). The grade and rapidity of tissue damage depend on many factors, including the amount of energy released, the application time, the vascularization and the water content of the tissue [Citation10]. Laser ablation in the neck is usually performed under ultrasound (US) guidance, which allows a real-time monitoring of the procedure.
Before ablation, a comprehensive assessment of the target lesion to treat is performed with US or contrast-enhanced US (CEUS). The size and shape of the nodule, along with the spatial relations with adjacent organs needs to be carefully evaluated to avoid partial treatments or injury to surrounding organs. Local anesthesia is generally used, with or without conscious sedation depending on patient anxiety and operator preference. According to the size and shape of the nodule, one or more 21 gauge needles are inserted under real-time US guidance in the deepest portion of each nodule. Up to four needles can be inserted simultaneously. A distance of 1 cm should be ideally maintained between inserted needles [Citation4]. Then, a 300 µm diameter plane-cut quartz optical fiber is inserted and advanced up to the introducer needle tip. The introducer needles are then pulled back so as to expose the tip of each fiber at least 5 mm in direct contact with the target tissue [Citation11]. Common protocols are based on a mean power of 3–5 W, for a total energy delivery of 1200–1800 J for every illumination. After the first illumination, the fiber(s) can be withdrawn by 1/1.5 cm and a subsequent illumination can be performed (pull-back technique) until the whole target has been illuminated. Tissue ablation can be monitored in real time following the enlargement of iper-echogenicity due to gas formation during treatment. For benign nodules, the aim of the treatment is to achieve a volume reduction of at least >50% [Citation6,Citation12], and a variable amount of viable nodular tissue is generally maintained in the periphery of the nodule, also in order to reduce the risk of possible complications. Conversely, for malignant nodules, complete tissue destruction needs to be achieved. Thus, laser ablation for malignant nodules requires a careful assessment of the surrounding structure and an idoneous localization of the nodule, ideally 5 mm far from the thyroid capsule. Hydrodissection can be performed to displace other structures close to the tumor [Citation10]. After the ablation, CEUS can be performed to better identify the actual extent of the ablation area, which can be generally overestimated by the gas formed during ablation; a further ablation can be performed during the same session in case CEUS demonstrate untreated areas [Citation13,Citation14]. Laser ablation is generally performed as an outpatient procedure that does not require particular medication apart corticosteroids or analgesics in the rare case of post-ablation pain.
Radiofrequency ablation
RFA uses an alternating electric current oscillating between 200 and 1200 kHz [Citation15]. The radiofrequency electrode acts as a cathode. The ions of targeting tumors adjacent to the electrode tip vibrate rapidly in response to these alternating currents. This vibrating friction energy is transformed into heat, while energy deposition drops exponentially away from the tip. Tissue interaction with the temperature induced by radiofrequency is similar to that of laser [Citation15].
The revised 2017 thyroid RFA guidelines by Korean Society of Thyroid Radiology suggest several standard techniques [Citation3]. For benign thyroid nodules, perithyroidal lidocaine injection is recommended to control pain. The trans-isthmic approach and moving-shot technique are essential for thyroid lesions. This technique is useful to minimize complications and marginal nodule regrowth. Recently, a novel technique, named ‘vascular ablation technique’ has been reported [Citation16]. Two different vascular ablation techniques are suggested: artery-first ablation and marginal venous ablation (venous staining). These techniques have the potential to enhance treatment efficacy and reduce the risk of regrowth. For recurrent thyroid cancers, guidelines recommend careful evaluation of critical structures before ablation, hydrodissection to reduce the risk of thermal damage to surrounding critical structures, and the moving-shot technique.
Among possible complications, nerve damages are the most serious and feared one during RFA [Citation16]. Particularly, voice change induced by thermal damage of recurrent laryngeal nerve is the most common complication related to nerve damage. To lower the risk of damage, some technical measures have been suggested. Recently, cold 5% dextrose solution injection was introduced to treat nerve damage during ablation [Citation17]. If symptoms of nerve damage occur during RFA, such as voice change, palpitations, Horner syndrome, shoulder movement problems, or paresthesia, ablation should be immediately stopped and a cold 5% dextrose solution is injected directly into the space in which the nerves were located. In most patients, the cold fluids can treat effectively the thermal damage of nerves [Citation17].
Most operators performing thyroid RFA use a thyroid-dedicated straight-type internally cooled electrode with short shaft (7 cm) and small diameter (18–19 gauge) [Citation16]. In small recurrent thyroid cancers or parathyroid lesions, guidelines recommend 19 gauge electrode tip (i.e. 3.8 mm or 5 mm active tips). A recently introduced thyroid-dedicated bipolar electrode has been introduced for pregnant women and for patients carrying electrical devices (i.e. pacemaker) [Citation16].
Benign thyroid nodule: outcomes and complications
Laser ablation
Laser ablation has been one of the first methods used to perform image-guided ablation of benign thyroid nodules and is nowadays considered among the recommended techniques for the treatment of benign nodules [Citation18]. A randomized clinical study comparing laser ablation and levothyroxine therapy reported a significant nodule volume shrinkage after 1 year following laser ablation with a negligible impact on thyroid function (TSH and FT4) [Citation19]. Furthermore, laser ablation proved superior than TSH suppression therapy regarding compression symptoms and volume reduction rate. Also in the long-term follow-up, reduction of tumor volume proved stable over time after laser ablation, with no need to reschedule additional ablative sessions. Conversely, serial thermal ablations are indicated for the treatment of large nodule (>20 mm) [Citation20,Citation21]. Moreover, laser ablation possibly reduces the invasiveness of the procedure due to its smaller energy applicators [Citation10,Citation22]. A recent meta-analysis of 19 papers and 2137 patients confirmed laser ablation to be safe and effective in reducing benign thyroid nodules volume, improving thyroid function and esthetic and ameliorating symptoms of compression, especially for hypervascular nodules [Citation23]. For the treatment of benign thyroid nodule, outcomes of laser ablation seem comparable to RFA, as also shown by a propensity score analysis [Citation10,Citation24].
In 2003, Døssing et al. [Citation25] reported the first case of a successful US-guided laser ablation of an autonomous thyroid nodule. Further studies confirmed that preliminary experience [Citation26–29]. Reduction in tumor volume was achieved in a large series of 82 patients with toxic nodular goiter-related hyperthyroidism, especially in those patients with nodules with a volume ≤15 ml. Being all patients pretreated with methimazole, all patients with an ablated goiter ≤5 ml could suspend medical therapy. For larger goiters, methimazole discontinuation rate after the procedure depended on the baseline goiter volume [Citation30]. Laser ablation has been compared to 131I radioiodine therapy for the treatment of hyperfunctioning benign nodules [Citation23,Citation26,Citation28,Citation30]. Despite a similar reduction in nodule volume, normalization of serum TSH was superior in the radioiodine groups, suggesting laser ablation to be indicated only in the risk of radioiodine-induced hypothyroidism or in pregnant women. Finally, combining laser ablation with 131I therapy seems to induce a faster resolution of systemic and local symptoms than 131I as single therapy. This combined approach also allows to reduce the therapeutic dose of 131I administration [Citation31,Citation32]. Two cases of laser ablation of a benign thyroid nodule are illustrated in and .
Figure 1. (a) US representation of a benign thyroid nodule. (b) Two laser applicators have been introduced within the nodule, with a distance of ∼5 mm between the needle tips. (c) Gas bubbles in the nodule are the immediate effect of thermal ablation. (d) Immediate post-procedural CEUS shows lack of contrast enhancement in the central part of the nodule. (e) Six-month follow-up shows volumetric reduction of the ablated nodule. (f) CEUS of the ablated nodule shows shrinkage and low vascularization of the inner core of the nodule, due to cicatricial effects.
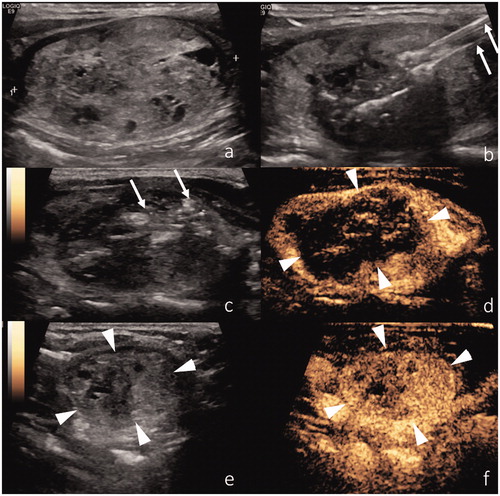
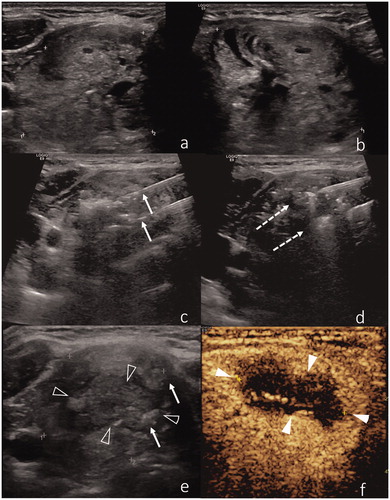
Figure 2. (a,b) US representation of a benign thyroid nodule. (b) Two laser applicators have been introduced within the nodule, with a distance of ∼5 mm between the needle tips (arrows). (c,d) Two needles are introduced in the nodule and gently moved during the procedure according to the pull-back technique (dotted arrows). (e) Air bubbles (arrows) within the ablated nodule (black arrowheads). (f) Post-procedural CEUS confirms lack of contrast enhancement inside the ablated nodule (white arrowheads).
RFA ablation
RFA reported excellent outcomes and low complication rates. An initial large population single-center study with 236 patients reported 84.8% volume reduction at 1-year follow-up [Citation33]. Lim et al. reported 90% volume reduction rate at and 94% at 1- and 4-year follow-up, with a 5.6% recurrence rate. Moreover, patients’ symptoms and cosmetic issues were improved after RFA [Citation34]. A recent large multicenter prospective study performed by trained radiologists from multiple institutions described a mean volume reduction at 12 months of 80.3%, increasing up to 95.3% after 5 years. Interestingly, also symptoms and cosmetic scores showed significant improvements [Citation35].
When compared with surgery, RFA showed similar efficacy, with the benefit of lower complication rates [Citation36,Citation37]. Similar to laser ablation, several papers discussed the role of RFA for the treatment of autonomously functioning thyroid nodules. Cesareo et al. [Citation38] confirmed that RFA is effective in achieving normalization of TSH levels in patients with small autonomously functioning nodule (≤12 ml), whose volume shrinked up to 84% at 2-year follow-up compared to 68% in nodules >12 ml. These results confirmed the greater efficacy of thermal ablation in small nodules, as also reported with laser technique [Citation30]. Bernardi et al. [Citation39] and Baek et al. [Citation40] described as a single RFA restored euthyroidism in 45–50% of patients with autonomously functioning benign thyroid nodules, with mean a volume reduction of 75% after 1 year from the procedure. A recent meta-analysis reported normalization of TSH occurring in 57% patients and volume reduction up to 79% at 1-year follow-up, with a direct correlation between baseline nodule volume and normalization of TSH [Citation38], as also observed after laser ablation [Citation30]. A case of thyroid RFA is shown in .
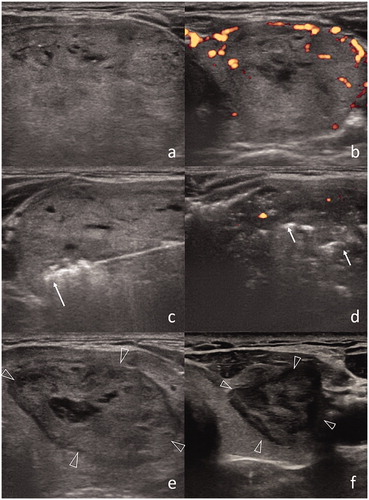
Figure 3. (a) US representation of a benign thyroid nodule (6.4 × 4.0 × 2.8 cm) in the left thyroid lobe. (b) Prominent vascularization is confirmed at Doppler US. (c) RF applicator with 1 cm size active tip is introduced within the nodule (arrow). (d) Post-procedural US shows decreased vascularization in nodule and air bubbles as hyperechoic areas (arrows). (e) After 1 month, the size of nodule is decreased (2.2 × 3.7 × 4.5 cm). (f) Further dimensional reduction after 1 year (1.3 × 1.4 × 2.4 cm).
Complications
Complications of laser and RFA are similar in terms of incidence and type [Citation10]. They are rare and include transient dysphonia, local pain, hematoma, fever, skin infections and mild burns. In a large retrospective series of thyroid laser ablations, major complications occurred in 0.5% of cases (8 patients) and consisted of transient vocal cord palsy, which completely recovered after 3 months. Minor complications were reported in 0.5% (9 patients) of cases, including subcapsular or perithyroidal hematoma and mild skin burn. Mild pain was described as a side effect during the treatment (10.6%) [Citation21]. A large meta-analysis evaluating 2421 patients with either benign and recurrent tumors treated with RFA demonstrated overall complication rate to be 2.4–3.3%, with a 1.4% major complication rate [Citation16]. Only six patients (0.2%) had persistent complication, including permanent voice change, necessity for lobectomy due to abscess formation and permanent hypothyroidism. Large population single-center study [Citation41] showed that the complication rate was higher in recurrent tumors than benign ones (8.6% vs. 3.3%). Voice change was most common and serious complication, caused either by lidocaine injection, nerve compression due to hematoma, local inflammation or perineural fibrosis [Citation42]; however, permanent damage (dysphonia or vocal cord palsy) is extremely rare [Citation17], and most patients recovered without special treatment within 3 months [Citation43]. Despite the rarity of permanent voice changes, a higher risk exists for patients with recurrent thyroid cancer [Citation41]. Other reported complications are exceedingly rare and include Horner syndrome, brachial plexus nerve injury, transient thyrotoxicosis or hypothyroidism [Citation43]. The application of standard techniques, a deep knowledge of the anatomy of the neck and prompt management of nerve damage is required to minimize complications.
Malignant thyroid nodule: outcomes and complications
Laser ablation
Laser ablation can be applied in the treatment of primary thyroid tumors or in recurrent tumors that are not suitable for surgery [Citation4,Citation29]. Moreover, laser ablation has been also proposed as alternative to active surveillance or surgery in the treatment of papillary microcarcinoma [Citation4,Citation44–48]. Even though evidences regarding the outcome of laser ablation are still limited compared to the benign pathology, good clinical results have been reported for tumor debulking and temporary improvement of mass-related symptoms [Citation29]. Up to 10 years ago, the body literature of laser ablation for the treatment of malignant thyroid nodules was substantially limited to case reports and small case series [Citation9,Citation47,Citation48]. In the meanwhile, its feasibility and efficacy have been demonstrated in ex-vivo models and in larger clinical series [Citation49]. Zhang et al. [Citation50] described clinical outcomes of 64 cases of papillary thyroid microcarcinomas treated with laser ablation, reporting only two incomplete ablations that were detected by CEUS performed immediately after the procedure. US-guided fine-needle aspiration at 1, 6, and 12 months after treatment did not show residual tumors, with no regrowth of treated lesions detected during a mean follow-up of 26 months. Successful tumor control in metastatic lymph node in the neck from papillary thyroid carcinomas have been reported by Papini et al. in a small series of five patients who experienced a mean volume reduction was 64% and 88% at 6 and 12 months, respectively. Moreover, no regrowth along with normalization of serum Tireoglobulin on LT4 was registered [Citation51]. Similarly, Mauri et al. [Citation13] described a larger series of patients experiencing successful local tumor control in 73% of cases at 6 months and 71.4% of cases at 12 months. All patients had previously undergone surgery and radioiodine ablation, and were at high risk of re-intervention, with a negative radioiodine scan. Authors obtained technical success in all cases with no major complications and reported a local tumor control in 86.9% of cases over a follow-up of 30 months and no residual disease on imaging in 79.1% of cases. Local control was achieved in 71.4% of cases at 1 year. Laser ablation has been used successfully for the treatment of local recurrence of papillary cancer in a cohort of 21 patients, which underwent 18 single ablative sessions and 3 repeated treatments due to viable tumor areas detected at CEUS [Citation52]. A recent paper by Ji et al. reported successful outcome in 37 patients diagnosed with papillary microcarcinoma. After a gradual volume increase due to tissue edema within the first 3 months, at 24-month follow-up tumor volume decrease from a mean of 5.5 mm to 1.1 mm, 32.4% primary lesions completely disappeared, and 64.9% remained as cicatricial hyperplasia. Only one patient had cervical lymph node and underwent open surgery [Citation53]. These results are very similar to the outcomes described by Zhou et al. few years before [Citation54], thus confirming percutaneous laser ablation to be safe and effective for the treatment of single papillary thyroid microcarcinoma.
RFA ablation
RFA achieved excellent results for treating recurrent thyroid cancers [Citation55]. Current thyroid RFA guideline suggested two treatment strategies, being cure or palliative treatment [Citation3]. Cure is defined as treatment of all visible tumors on US. In terms of outcomes, a meta-analysis found that recurrent tumors were completely disappeared in 69% patients, with a 71.6% serum thyroglobulin reduction rate [Citation55]. Recent long-term follow-up study (mean follow-up, 80 months) reported 91.3% completely ablated tumors that completely disappeared during follow-up, together with a significant decrease of serum thyroglobulin level; however, four new recurrent tumors and two distant metastases were detected during the follow-up period [Citation56]. Meta-analysis comparing RFA and EA demonstrated RFA to be superior in terms of efficacy and to require fewer treatment sessions, even if EA showed lower complication rates [Citation55]. Several studies compared the efficacy and safety of RFA with those of surgery using a propensity score analysis [Citation57,Citation58]. The results showed that recurrence-free survival was similar in both groups, but RFA showed low complication rates than surgery. RFA also achieved good results in primary thyroid cancers, especially microcarcinoma (T1N0M0) [Citation59]. Zhang et al. [Citation60] reported a prospective study for treating microcarcinoma using RFA. The mean volume tumor reduction ratio was 96% at 1 year and complete tumor disappearance was 10.2%. No local tumor recurrence or metastasis to lymph nodes in the neck was observed during the short follow-up period (7.8 months). Kim et al. [Citation61] reported in a 4-year follow-up study mean volume reduction rate of 98.5% and complete disappearance rate of 66.7%, with no local tumor recurrence or lymph nodes metastases in the neck. RFA was tolerated in all patients with primary thyroid carcinoma without major complications or procedure-related deaths [Citation62].
Conclusion
Image-guided thermal ablations such as laser ablation and RFA are well-established minimally invasive procedures that can be considered valid alternatives to surgery in the treatment of benign thyroid nodules. In patients with thyroid malignancies, evidences are still limited but results in terms of local tumor control are promising. Multidisciplinary collaboration is critical to identify thermal ablation as a treatment option in patient where surgery and radioiodine therapy are not feasible. To maximize clinical outcomes and minimize complications, operators should be properly trained, master the anatomy of the neck and apply standard techniques recommended by current guidelines.
Financial activities not related to the present article
B.J.H.: patent holder of unidirectional ablation electrode; Consultant for STARmed and RF medical. G.M. Consultant for Elesta SrL.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–395.
- Kim J-H, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19:632–655.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38:25–36.
- Hahn SY, Shin JH, Na DG, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean society of thyroid radiology. Korean J Radiol. 2019;20:609–620.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:1–7.
- Kim J-H, Baek JH, Lim HK, et al. Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography. 2019;38:125–134.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618.
- Papini E, Bizzarri G, Pacella CM. Percutaneous laser ablation of benign and malignant thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2008;15:434–439.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;33:295–299.
- Pescatori LC, Torcia P, Nicosia L, et al. Which needle in the treatment of thyroid nodules? Gland Surg. 2018;7:111–116.
- Achille G, Zizzi S, Di Stasio E, et al. Ultrasound-guided percutaneous laser ablation in treating symptomatic solid benign thyroid nodules: our experience in 45 patients. Head Neck. 2016;38:677–682.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98:E1203–E1207.
- Zhang L, Zhou W, Zhan W. Role of ultrasound in the assessment of percutaneous laser ablation of cervical metastatic lymph nodes from thyroid carcinoma. Acta Radiol. 2018;59:434–440.
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18:615–623.
- Chung SR, Baek JH, Choi YJ, et al. Management strategy for nerve damage during radiofrequency ablation of thyroid nodules. Int J Hyperthermia. 2019;36:204–210.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44:14–36.
- Papini E, Guglielmi R, Bizzarri G, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid. 2007;17:229–235.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99:3653–3659.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:11–22.
- Sui WF, Li JY, Fu JH. Percutaneous laser ablation for benign thyroid nodules: a meta-analysis. Oncotarget. 2017;8:83225–83236.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.
- Døssing H, Bennedbaek FN, Hegedüs L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid. 2003;13:885–888.
- Spiezia S, Vitale G, Di Somma C, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003;13:941–947.
- Døssing H, Bennedbaek FN, Bonnema SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157:95–100.
- Barbaro D, Orsini P, Lapi P, et al. Percutaneous laser ablation in the treatment of toxic and pretoxic nodular goiter. Endocr Pract. 2007;13:30–36.
- Valcavi R, Piana S, Bortolan GS, et al. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23:1578–1582.
- Gambelunghe G, Stefanetti E, Colella R, et al. A single session of laser ablation for toxic thyroid nodules: three-year follow-up results. Int J Hyperthermia. 2018;34:631–635.
- Negro R, Greco G. Quality of life and outcomes in patients with a large toxic adenoma undergoing laser ablation plus radioiodine vs. lobectomy. Int J Hyperthermia. 2019;36:191–195.
- Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab. 2014;99:E1283–E1286.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. Am J Neuroradiol. 2015;36:1321–1325.
- Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperthermia. 2018;34:617–623.
- Bernardi S, Stacul F, Michelli A, et al. 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine 2017;57:402–408.
- Baek JH, Moon W-J, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33:1971–1977.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342.
- Wang J-F, Wu T, Hu K-P, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med. 2017;130:1361–1370.
- Haser GC, Tuttle RM, Su HK, et al. Active surveillance for papillary thyroid microcarcinoma: new challenges and opportunities for the health care system. Endocr Pract. 2016;22:602–611.
- Iñiguez-Ariza NM, Brito JP. Management of low-risk papillary thyroid cancer. Endocrinol Metab. 2018;33:185–194.
- Mauri G, Sconfienza LM. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int J Hyperthermia. 2017;33:489–490.
- Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004;232:272–280.
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917–920.
- Lee J, Jung JH, Kim WW, et al. Ultrasound-guided laser ablation using multidirectional-firing fiber for papillary thyroid carcinoma: an ex vivo study with evaluation of tumor cell viability. Photomed Laser Surg. 2016;34:300–304.
- Zhang L, Zhou W, Zhan W, et al. Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg. 2018;42:2476–2484.
- Papini E, Bizzarri G, Bianchini A, et al. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab. 2013;98:E92–E97.
- Zhou W, Zhang L, Zhan W, et al. Percutaneous laser ablation for treatment of locally recurrent papillary thyroid carcinoma <15 mm. Clin Radiol. 2016;71:1233–1239.
- Ji L, Wu Q, Gu J, et al. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19:16.
- Zhou W, Jiang S, Zhan W, et al. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: preliminary results. Eur Radiol. 2017;27:2934–2940.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26:420–428.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;1–7. DOI:10.1007/s00330-019-06063-5.
- Kim J, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276:909–918.
- Choi Y, Jung SL, Bae J-S, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36:359–367.
- Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34:611–616.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26:1581–1587.
- Kim J-H, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroid carcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33:212–219.
- Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. Int J Hyperthermia. 2017;33:938–945.
