?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Although acute thermal stress appears to be one of the most effective stressors that increase the intra- and extracellular concentrations of heat shock protein 72 (Hsp72), 17β-estradiol has been shown to inhibit heat-induced Hsp72 expression.
Materials and Methods: To determine whether severe whole-body hyperthermia (increase in rectal temperature up to 39.5 °C) induced by lower-body heating is a sufficient stimulus to modulate hormonal (17β-estradiol, progesterone, prolactin, epinephrine, and norepinephrine) and extracellular Hsp72 responses, we investigated young adult women (21 ± 1 yr).
Results and Conclusions: In the present study, we show that a severe whole-body hyperthermia (increase in rectal temperature of approximately 2.6 °C and heart rate of approximately 80 bpm from baseline) was sufficient to increase 17β-estradiol, progesterone, and prolactin and catecholamine norepinephrine concentration. Moreover, we show that the concentration of extracellular Hsp72 and catecholamine epinephrine were not affected by severe whole-body hyperthermia in young adult women. From the functional point of view, expression of ovarian hormones induced by passive heat stress may have therapeutic potential for young adult women in, for example, estrogen treatment and overall women’s health.
Introduction
It has become increasingly clear that reproductive hormones play an essential role in systematic homeostasis across a woman’s lifespan. Estrogen receptors are localized in several hypothalamic structures that are involved in temperature regulation [Citation1], and estrogen exposure in rat brain slice preparations has been shown to increase the firing rate of warm-sensitive neurons in the preoptic area of the anterior hypothalamus [Citation2]. This, in fact, promotes cutaneous vasodilation and sweating in humans and results in augmented heat dissipation and lower body temperature [Citation3]. Therefore, this effect of estradiol may decrease the risk of overheating by shifting the overall regulation of body temperature to lower levels and may decrease the risk of heat illness or heat stroke. Although the specific influences of progesterone on central neurons controlling body temperature are less clear, existing data suggest that estradiol and progesterone may have opposite effects on temperature regulation [Citation4]. Moreover, there is increasing evidence that estradiol mediates upregulation of progesterone receptors that are involved in temperature control. This suggests that progesterone does not predominate over estrogen with regard to thermoregulatory effector responses when the two are concomitantly increased [Citation4,Citation5].
To investigate the impact of these ovarian hormones on temperature regulation in humans, a variety of approaches have been used to alter estradiol and progesterone exposure, most notably (i) administration of oral contraceptives during different phases of the menstrual cycle [Citation6], (ii) use of ovarian hormone suppression combined with hormone administration [Citation7], and (iii) use of hormone therapy in postmenopausal women [Citation8]. However, the question remains whether passively induced severe whole-body hyperthermia (WBH) (defined as a rectal temperature (Tre) of ∼39.5 °C) in the absence of exercise (mechanical and/or metabolic) or other stresses (e.g., psychological) is a sufficient thermal stimulus to trigger expression of natural 17β-estradiol and progesterone, as these two are strongly involved in women’s thermoregulation control.
Extracellular (e) heat shock protein 72 (Hsp72) is a protective chaperone, and thermal stress appears to be one of the most effective stressors to increase eHsp72 concentrations [Citation9,Citation10]. In humans, catecholamine epinephrine rather than catecholamine norepinephrine mediates the release of Hsp72 to provide cytoprotection in response to heat stress [Citation11]. Intriguingly, however, 17β-estradiol inhibits hyperthermia-induced expression of Hsp72 [Citation12]. In addition to these hormonal and protective chaperone responses, it has been shown that increases in core temperature are the key stimulus for the release of the stress hormone prolactin [Citation13]. Estrogen stimulates the secretion of prolactin, and progesterone can, for example, partially inhibit the effect of estrogen on prolactin secretion [Citation14–16]. The extent to which severe WBH per se triggers a systematic cascade of these blood marker responses has not been studied in young adult women. Therefore, the purpose of our study was to determine whether severe WBH induced by lower-body heating in young adult women is a sufficient stimulus to modulate hormonal (17β-estradiol, progesterone, prolactin, epinephrine, and norepinephrine) and eHsp72 responses.
Methods
Participants
Twenty participants were assessed for eligibility. Subjects with conditions (e.g., neurological pathology and/or cardiovascular pathology) that could worsen by exposure to hot water were excluded from this study. The criteria for inclusion were (a) female sex, (b) age 18–24 yr, (c) no excessive sport activities (i.e., less than three times per week), (d) regular menstrual cycle, (e) nonuse of oral contraceptives during the previous six months, (f) no involvement in any temperature-manipulation program or extreme temperature exposure for equal or more than three months, and (g) no medications that could affect natural thermoregulation. In total, 14 young apparently healthy women met the inclusion criteria and agreed to participate in this study. Participants’ physical characteristics are presented in . Written informed consent was obtained from all subjects after being fully informed about the investigation and possible related risks and discomforts. All procedures were approved by the Human Research Ethics Committee and were conducted according to the guidelines of the Declaration of Helsinki. Subjects were in self-reported good health, which was confirmed by medical history and physical examination. Women were studied during the follicular phase, five to seven days counted from the beginning of the onset of menses, at the lowest circulating 17β-estradiol and progesterone concentrations of the menstrual cycle.
Table 1. Baseline characteristics of study participants.
Experimental design
The experiment was performed at environment temperature 23 °C and 55% relative humidity. To control for circadian fluctuations in blood marker levels and body temperature, all experiments began at 07:30. Subjects were instructed to sleep for > 8 h the night before the experiment and to refrain from alcohol, heavy exercise, and caffeine for at least 24 h before the experiment. To avoid an effect of diet-induced thermogenesis, the subjects fasted for 12 h before the start of the experiment until its end. To standardize the state of hydration and the feeling of thirst, subjects were allowed to drink noncarbonated water as desired until 60 min before the body mass measurement. The participants’ anthropometric parameters were assessed at their arrival at the laboratory, and then, they were asked to rest in a semirecumbent posture for 10 min dressed in a T-shirt, swimming shorts, and socks. After this rest (before hot water immersion), control measurements of the skin temperature (Tsk) and Tre were made, and venous blood samples were collected and stored for later analysis. Then, the participants began the water immersion warming protocol. The water bath temperature was approximately 44 °C, and the participants were immersed to the waistline. The immersion continued until the Tre increased to 39.5 °C, and the exposure time required to achieve this Tre was recorded [Citation17]. Ratings of heat perception, heart rate (HR), and Tre were recorded every 5 min throughout the warming procedure. Before leaving the bath, blood samples were taken. Within 1 min after leaving the bath, the participants were towel-dried, and Tsk and Tre were measured.
Preliminary measurements
The subject’s weight (Wt) (in kg), fat-free mass (FFM) (in kg), and body fat (BF) (in %) were measured using a TBF-300 body composition scale (Tanita, UK Ltd., West Drayton, UK). Height (in cm) was assessed at their arrival at the laboratory. The subject’s body surface area (BSA) (in m2) was estimated using the following best-fit equation for women:
BSA = 0.01474 × weight0.47 × height0.55 [Citation18]. The body mass index (BMI) (in kg m−2) and the BSA/Wt ratio (in %) were calculated. Skinfold thickness (in mm) was measured using a skinfold caliper (SH5020; Saehan, Masan, Korea) at 10 sites: chin, subscapular, pectoral, suprailium, midaxillary, abdomen, triceps, anterior thigh, medial collateral ligament, and medial calf, and the mean skinfold thickness was calculated [Citation19].
Temperature and heart rate measurement
Tre was measured throughout the experimental trial using a thermocouple rectal probe (Ellab, Hvidovre, Denmark) with an accuracy of ±0.01 °C. The probe was inserted to a depth of 12 cm past the anal sphincter by the subject. Tsk was measured before and at the end of the water immersion. Tsk was measured using thermistors (DM852; Ellab) that were taped at three sites: calf (i.e., the needle insertion site), back, and forearm with an accuracy of ±0.01 °C. HR was measured throughout the heating protocol with an HR monitor (RCX5; Polar Electro, Kempele, Finland). HR was recorded before (at rest) and at the end of lower-body hot water immersion.
Physiological strain index
The physiological strain index (PSI) was measured as described by Moran et al. [Citation20] and the following formula was used to normalize PSI:
PSI measurements were taken before (Tre0 and HR0) and at the end (Tret and HRt) of passive warming. Tre and HR were assigned the same weight constant of 5. The index ranged from 0 to 10: 1–2 (no/little heat strain), 3–4 (low heat strain), 5–6 (moderate heat strain), 7–8 (high heat strain), and 9–10 (very high heat strain). The limits were within the following values: 36.5 ≤ Tre ≤ end Tre of 39.5 °C and 60 ≤ HR ≤ 180 beats·min−1.
Measurement of perception
Subjective heat perception for the whole body was measured as described by Brazaitis et al. [Citation21]. Briefly, the rating of thermal sensation ranged from 5 (neutral) to 9 (very hot). Thermal comfort sensation ranged from 1 (comfortable) to 5 (extremely uncomfortable).
Blood analysis
To evaluate catecholamines, sex hormones, and eHsp72, venous blood samples were obtained before and immediately after passive lower-body warming. Venous blood samples for epinephrine and norepinephrine measurements were collected in vacuum tubes using EDTA as an anticoagulant (EDTA-K3, 3 ml), mixed gently by inverting 8–10 times, and kept at 2–8 °C until centrifugation. Blood samples were centrifuged at 1200 × g for 15 min within 30 min of blood collection. Plasma samples were separated from the red cells as soon as possible after centrifugation (maximum lapse of time: 10–15 min) and kept at –80 °C until analysis. Norepinephrine and epinephrine concentrations were measured using a CatCombi enzyme-linked immunosorbent assay (ELISA) kit (Gemini Analyzer; Stratec Biomedical GmbH, Birkenfeld, Germany).
Blood samples for prolactin, eHsp72, 17β-estradiol, and progesterone determination were collected by venipuncture in vacuum tubes for serum, containing a gel separator (5 ml). Blood samples were allowed to clot for 30 min and sera were separated by centrifugation (1200 × g, 15 min.) at 4 °C. The serum samples were aliquoted and stored at –80 °C until analysis. An automated enzyme immunoassay analyzer AIA-2000 (Tosoh Corporation, Tokyo, Japan) was used for prolactin, 17β-estradiol, and progesterone analysis.
Serum eHsp72 was measured using a commercially available high-sensitivity sandwich ELISA kit (Assay Designs EKS-715; Ann Arbor, MI). Briefly, samples and standards were added to wells coated with a mouse monoclonal antibody. eHsp72 was captured by this antibody and detected by adding a rabbit polyclonal antibody. Both antibodies are specific for inducible eHsp72 and do not react with other members of the HSP70 family such as Hsc73, Grp78, Dnak, or Hsp71. A horseradish peroxidase-conjugated antibody was used for detection and color development was accomplished by the addition of tetramethylbenzidine substrate and stopped with an acid stop solution. The optical density of the samples was read at 450 nm using a Gemini Analyzer (Stratec Biomedical GmbH), and they were compared to a standard curve generated from known concentrations of recombinant Hsp72 ranging from 0.1 to 12.5 ng·mL−1 (r2 = 0.989). The sensitivity of the assay was 0.09 ng·mL−1 as described by the manufacturer.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics software (v. 22; IBM Corp., Armonk, NY). Data are presented as means ± SEM, and p ≤ .05 was considered to be significant. Differences in blood marker concentrations, body temperature, and HR before and after lower-body heating were assessed via one-way repeated-measures ANOVA using Tukey’s adjustment for within-subject factors. Calculations of observed power (OP), expressed as percentage, were performed, and the partial eta squared (ηp2) was estimated as a measure of severe WBH effect size.
Results
Effects of lower-body warming on thermal strain
By using a passive lower-body warming technique in the present study, the time to warm the body from Tre 36.91 ± 0.05 °C before warming to 39.5 °C at the end of the warming was 54.64 ± 4.23 min. In addition to the Tre change, Tsk increased from a resting 31.72 ± 0.22 °C to 36.59 ± 0.21 °C (p < .001; ηp2 = 0.47; OP = 100%) at the end of the warming, and the HR increased from 70.14 ± 2.50 at resting to 148.07 ± 5.11 bpm (p < .001; ηp2 = 0.51; OP = 100%) at the end of the warming. Passive lower-body warming resulted in a PSI of 8.51 ± 0.25, which corresponds to “high heat strain”. At the end of the lower-body warming, the subjects felt “hot” (8.00 ± 0.10 points) and “uncomfortable” (3.33 ± 0.21 points).
Effects of severe WBH on blood markers
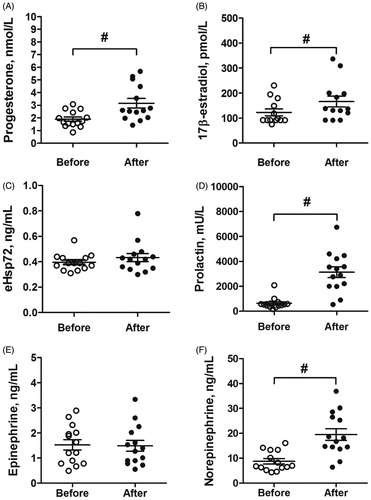
Passive lower-body warming that resulted in severe WBH increased the concentrations of circulating progesterone (p < .05; ηp2 = 0.36; OP = 96%; ), 17β-estradiol (p < .05; ηp2 = 0.32; OP = 91%; ), prolactin (p < .001; ηp2 = 0.66; OP = 100%; ), and norepinephrine (p < .001; ηp2 = 0.52; OP = 100%; ). However, there was no effect of severe WBH on eHsp72 () and epinephrine () concentrations (p > .05).
Discussion
In the present study, we investigated whether severe WBH induced by lower-body warming in young adult women who were studied during their follicular phase (i.e., at the lowest circulating 17β-estradiol and progesterone concentrations) of the menstrual cycle is a sufficient stimulus to modulate hormonal (17β-estradiol, progesterone, prolactin, epinephrine, and norepinephrine) and eHsp72 responses. We increased the whole-body temperature up to severe hyperthermia (Tre increase from normal ≥1.5 °C) [Citation22] in the absence of any exercise. Considering that at the peak of Tre and HR (at the end of the heating) the participants did not rate their perception as maximally hot and uncomfortable may indicate that whole-body heat stress induced by passive lower-body warming was well tolerated by young, nonheat-acclimated women. To our knowledge, only one study has investigated the effect of passive whole-body warming on eHsp72, prolactin, and catecholamine concentrations in six young adult women who were compared with seven young adult men [Citation10]. Although that study reported that an overall low heat dose (0.8 °C increase in Tre) was associated with an increase in circulating eHsp72, prolactin, and norepinephrine, it failed to show this explicitly in female subjects. To date, however, despite current knowledge of sex-specific mechanisms of human thermoregulation to heat stress [Citation3–5,Citation23,Citation24], most thermal research has been conducted in men; thus, our general understanding of the eHsp72 and hormonal responses to severe WBH is based on data obtained from male subjects.
Existing evidence indicates that circulating eHsp72 is highly stress-inducible and normally responds to hyperthermia, exercise, oxidative stress, osmotic stress, infection or inflammation, protein damage, and many other stimuli in a systemic, dose-dependent manner [Citation25–29]. In response to heat stress, eHsp72 expression is upregulated via a specific exocytotic pathway by the neuroendocrine hormone epinephrine in humans [Citation11], or by norepinephrine in animals [Citation25]. It may be released, for example, from hepatosplanchnic organs [Citation25]. Functionally, eHsp72 can bind to cells, penetrate the plasma membrane, and protect cells from cytotoxicity [Citation30,Citation31] by improving cell survival in the face of a broad array of cellular stressors [Citation32,Citation33]. Intriguingly, however, in the present study, we showed that eHsp72 and epinephrine were not affected by severe WBH in young adult women. This result might be partly explained by the heat-induced increase in serum 17β-estradiol expression. As shown, estrogen protects a variety of tissues from structural damage by interacting with Hsp [Citation34]. Existing evidence indicates that 17β-estradiol inhibits heat-induced Hsp72 expression at the transcriptional level by suppressing the activation of heat shock factor-1 [Citation12], and this may be mediated through its indirect antioxidant properties by stabilizing cellular membranes [Citation35].
Research data also suggest that estradiol and progesterone may have opposite effects on temperature regulation [Citation4]. Here, we observed that both of the most studied ovarian hormones (17β-estradiol and progesterone) were increased by severe WBH in young adult women. As estradiol mediates the upregulation of progesterone receptors involved in the control of temperature and increases the firing rate of warm-sensitive neurons in the preoptic area of the anterior hypothalamus [Citation2], this may suggest that progesterone does not predominate over estrogen in thermoregulatory effector responses when the two are concomitantly increased [Citation4,Citation5]. In addition to those hormonal thermoregulatory responses, estrogen has also been shown to stimulate the secretion of prolactin, an indirect marker of central serotonergic and dopaminergic activity in the brain that is implicated in the thermoregulatory control of body temperature, changes in behavior, motor control, motivation, and immune system regulation [Citation36,Citation37]. Thus, consistent with previous research [Citation10,Citation38], our results show a fivefold increase in severe WBH-induced prolactin response in young adult women. Taken together, the findings of our study suggest that severe WBH per se is a sufficient stimulus to induce the expression of ovarian hormones and that this may have therapeutic potential for young adult women in, for example, estrogen treatment and overall women’s health.
Conclusion
To our knowledge, this study is the first to show in young adult women that severe whole-body hyperthermia (increase in Tre of approximately 2.6 °C and increase in HR of approximately 80 bpm from baseline) is sufficient to increase 17β-estradiol, progesterone, and prolactin, and catecholamine norepinephrine concentrations. In addition, our data show that the concentration of extracellular Hsp72 and catecholamine epinephrine were not affected by severe whole-body hyperthermia in young adult women.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227.
- Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–632.
- Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86:22–28.
- Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol. 2000;88:1643–1649.
- Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women. Compr Physiol. 2014;4:793–804.
- Charkoudian N, Johnson JM. Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol. 1997;83:2012–2018.
- Stachenfeld NS, Taylor HS. Sex hormone effects on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. J Appl Physiol. 2009;107:864–872.
- Prior JC, Hitchcock CL. Progesterone for hot flush and night sweat treatment–effectiveness for severe vasomotor symptoms and lack of withdrawal rebound. Gynecol Endocrinol. 2012;28:7–11.
- Whitham M, Laing SJ, Jackson A, et al. Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol. 2007;103:1251–1256.
- Iguchi M, Littmann AE, Chang S-H, et al. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train. 2012;47:184–190.
- Whitham M, Walker GJ, Bishop NC. Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol. 2006;101:1222–1227.
- Shinohara T, Takahashi N, Ooie T, et al. Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. J Mol Cell Cardiol. 2004;37:1053–1061.
- Low D, Purvis A, Reilly T, et al. The prolactin responses to active and passive heating in man. Exp Physiol. 2005;90:909–917.
- Chen CL, Meites J. Effects of thyroxine and thiouracil on hypothalamic PIF and pituitary prolactin levels. Proc Soc Exp Biol Med. 1969;131:576–578.
- Elbourne KB, Keisler D, McMurray RW. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus. 1998;7:420–427.
- Soaje M, Deis RP. Participation of the opioid system in the regulation of prolactin secretion in androgenized rats: effect of ovarian steroids. Eur J Pharmacol. 1999;371:43–49.
- Brazaitis M, Paulauskas H, Eimantas N, et al. Motor performance is preserved in healthy aged adults following severe whole-body hyperthermia. Int J Hyperthermia. 2019;36:65–74.
- Tikuisis P, Meunier P, Jubenville CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. 2001;85:264–271.
- McArdle WD, Toner MM, Magel JR, et al. Thermal responses of men and women during cold-water immersion: influence of exercise intensity. Europ J Appl Physiol. 1992;65:265–270.
- Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:R129–134.
- Brazaitis M, Paulauskas H, Eimantas N, et al. Heat transfer and loss by whole-body hyperthermia during severe lower-body heating are impaired in healthy older men. Exp Gerontol. 2017;96:12–18.
- Bain AR, Nybo L, Ainslie PN. Cerebral vascular control and metabolism in heat stress. Compr Physiol. 2015;5:1345–1380.
- Kenney WL. A review of comparative responses of men and women to heat stress. Environ Res. 1985;37:1–11.
- Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci. 2016;196:75–80.
- Fleshner M, Campisi J, Amiri L, et al. Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–1152.
- Chen T, Cao X. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. Eur J Immunol. 2010;40:1541–1544.
- Lanneau D, Wettstein G, Bonniaud P, et al. Heat shock proteins: cell protection through protein triage. ScientificWorldJ. 2010;10:1543–1552.
- Young JC. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300.
- Lee E-H, Muñoz CX, McDermott BP, et al. Extracellular and cellular Hsp72 differ as biomarkers in acute exercise/environmental stress and recovery. Scand J Med Sci Sports. 2017;27:66–74.
- Guzhova IV, Arnholdt AC, Darieva ZA, et al. Effects of exogenous stress protein 70 on the functional properties of human promonocytes through binding to cell surface and internalization. Cell Stress Chaper. 1998;3:67–77.
- Novoselova TV, Margulis BA, Novoselov SS, et al. Treatment with extracellular HSP70/HSC70 protein can reduce polyglutamine toxicity and aggregation. J Neurochem. 2005;94:597–606.
- Buchner J. The biology of heat shock proteins and molecular chaperones: edited by R.I. Morimoto, A. Tisslères and C. Georgopoulos, Cold Spring Harbor Laboratory Press, 1994. $97.00 (vii + 610 pages) ISBN 0 87969 427 0. Trends Biochem Sci. 1994;19:559.
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579.
- Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40:41–58.
- Paroo Z, Dipchand ES, Noble EG. Estrogen attenuates postexercise HSP70 expression in skeletal muscle. Am J Physiol, Cell Physiol. 2002;282:C245–251.
- Freeman ME, Kanyicska B, Lerant A, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631.
- Bridge MW, Weller AS, Rayson M, et al. Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol. 2003;89:451–459.
- Pranskunas A, Pranskuniene Z, Milieskaite E, et al. Effects of whole body heat stress on sublingual microcirculation in healthy humans. Eur J Appl Physiol. 2015;115:157–165.