Abstract
Background: Thermal ablation is a minimally invasive technique that is gradually acknowledged as an effective alternative to surgery to treat thyroid nodules. Two main techniques have been described: radiofrequency (RFA) and laser ablation.
Objective: To evaluate the safety and efficacy of the two main techniques (RFA and laser ablation) for the treatment of benign thyroid nodules.
Patients: This bicentric retrospective study included 166 consecutive patients, who received clinical, biological and ultrasound evaluations for thyroid nodules, from October 2013 to November 2017.
Methods: One of the two techniques was proposed if a nodule was proven to be benign after fine needle aspiration cytology or micro-biopsy. Adverse events and outcomes (symptoms, nodule reduction) were assessed at 6 weeks and 6, 12, and 18 months after treatment.
Results: One hundred and eighty-nine nodules (mean size 17.5 ± 16.9 mL, 86.1% palpable) were treated by RFA (n = 108 (57.1%)) or laser ablation (n = 81 (42.9%)) in 166 patients (80.1% women, mean age 51.7 years). Two cases of transient recurrent laryngeal nerve palsy, one hematoma, and two successfully drained abscesses (5/166 = 3%) were observed. Clinical symptoms improved significantly in the two groups (anterior cervical discomfort –83.6%, esthetic complaints –84.9% and dysphagia –86.4%). Nodule volume (mL) decreased significantly (baseline vs. 18 months) from 20.4 ± 18.6 to 5.8 ± 6.6 (−75%) in the RFA, and from 13.6 ± 13.3 to 3.4 ± 4.1 (−83.9%) in the laser ablation groups.
Conclusions: Transient but potentially serious adverse events were reported in 3% of patients. A significant volumetric reduction was achieved with both techniques, regardless of nodule’s characteristics, at 18 months.
Introduction
Thyroid nodules (TNs), one of the most common endocrine manifestations [Citation1,Citation2], are clinically detected in 4% to 7% of the general population. Autopsy series [Citation3] and ultrasound imaging show that up to 30% to 70% of TNs occur at a mean age of 50 years, particularly in the Asian population [Citation1,Citation4]. It is usually admitted that about 5% of nodules are malignant. Despite guidelines for TN management [Citation5–9], surgery still seems excessively performed for the treatment of TN, which poses a health care challenge [Citation10]. In fact, in 2016, 45 000 thyroidectomies (0.07% of the population) were performed in France and only 7000 new thyroid cancers (mainly papillary thyroid carcinoma (PTC) with good prognosis) [Citation10,Citation11] were diagnosed [Citation12]. The latest French national data reports that 10 000 unnecessary thyroidectomies are performed each year in patients with presumably benign nodules [Citation13]. In another study, more than half thyroid nodules were found to be benign after surgery [Citation14], meaning that there is an over-surgery in industrialized countries for thyroid nodules [Citation15,Citation16].
Surgical management of benign TNs can be complicated by laryngeal palsy, infection, compressive hematoma, hypocalcemia, and lifelong hormonal substitutive therapy, even after a bare lobectomy [Citation17]. As a result, cheaper and less invasive treatments are greatly needed. Thermal ablation (TA) could be proposed as a first-line treatment for some patients, as already recommended by the Korean Health Authorities (2012), the American Association of Clinical Endocrinologists, the American College of Endocrinology and the Italian Society of Endocrinology (Associazione Medici Endocrinologi) [Citation5,Citation18,Citation19].
Thermal ablation is already used in clinical practice for primary and metastatic malignant liver, breast, renal, bone and lung tumors [Citation20–23] but is not yet regularly used for TNs, despite their validation in Korean and Italian studies [Citation24–28]. Radiofrequency ablation (RFA), and laser ablation have already been studied separately for the treatment of benign TNs [Citation29–34] and in many retrospective and some prospective comparative studies with a long-term follow-up. Currently, RFA and laser ablation are considered effective for treating medium-large solid or cystic nodules (>10 ml) [Citation35–38] with similar results in compared studies [Citation35]. HIFU indications are still being debated. It is mainly performed to treat smaller nodules ( < 5 ml) that are inaccessible to other techniques or when patients refuse other minimally invasive techniques, but several studies have shown effective results [Citation33,Citation39–44].
Therefore, the aim of our study was to analyze the reproducibility of the safety and efficacy of the two main techniques RFA and laser ablation, outside an international reference center and to analyze the results according to nodule characteristics, in a large bicentric cohort.
Patients and methods
Study design
A longitudinal retrospective study was conducted in two centers (American Hospital of Paris and Sainte-Therese Polyclinic), where the two techniques were available, to compare the safety and efficacy of the techniques. Subgroups analysis were performed according to the function and the structure of the nodules, as well as according to the era of treatment.
Patients
All consecutive adult patients treated between October 2013 and November 2017 were included according to the criteria mentioned in . Clinical and biological data were collected from medical files and were analyzed anonymously (Supplemental Table).
Table 1. Inclusion and exclusion criteria in patients with thyroid nodules.
All patients gave their written informed consent before inclusion in the study protocol. Surgery was always proposed as a first-line treatment, and all patients treated with TA had rejected surgery, including a robot-assisted axillary surgical technique. Complex cases were systematically discussed in dedicated multi-disciplinary meetings. The best treatment option was chosen based on the available guidelines according to the nodule initial size, structure and location, the operator’s experience and the patient’s final decision.
This study followed the tenants of the Declaration of Helsinki and was approved by the institutional review board of each participating center (Ethics committee of the American Hospital of Paris).
Assessment before procedure
In each center, the diagnosis (clinical, biological, ultrasound, fine needle aspiration cytology (FNAC) and/or core needle biopsy (CNB)) and therapeutic procedure were conducted by the same operator. Both physicians were experienced thyroidologists who had been trained in Korean and Italian medical expert centers before starting thermal ablation in their own centers.
Thyroid ultrasound imaging was performed with a high frequency linear ultrasound transducer (ESAOTE MyLab Twice, LA435). Nodules were classified according to the Thyroid Image Reporting and Data System (TI-RADS) classification [Citation45]. Nodule volume was measured as follows: V (ellipsoid volume formula) = length × width × depth × 0.524. Nodule structure was reported as solid (≤10% of fluid component), predominantly solid (11–50% of fluid component), predominantly cystic (51–90% of fluid component), or cystic (>90% of fluid component) [Citation46]. Nodule vascularization was classified by color flow Doppler (pulse repetition frequency (PRF) 1 kHz) as type 1: no vascularization; type 2: perinodular vascularization; type 3: combined intranodular and perinodular vascularization; or type 4: intranodular vascularization). The vagal nerve was identified as the first “danger zone”, and the recurrent laryngeal nerve region constituted a second “danger zone”. All imaging data were recorded on a map-drawing.
All patients had two distinct FNAC procedures (27 G needle) performed at different time points; these were classified as Bethesda category II [Citation47] by an experienced pathologist in each center. If the results of the FNAC were uncertain, a CNB (using Bard® Mission® Disposable Core Biopsy Instrument, 20 G × 20 cm) was performed.
When nodules were associated with low TSH levels, I123 thyroid nuclear imaging was performed (Siemens Symbia® gamma camera) as well as non-contrast cervical CT-scans (GE Healthcare DISCOVERY CT750 HD System) for large compressive nodules with indistinct ultrasound limits.
Procedures
The procedure was conducted in an operating room. Patients were placed in supine position, received intravenous hydration and were monitored. The posterior extension of the neck was determined by the nodule localization. Local analgesia was provided with a skin patch, and a pericapsular anesthesia with injection of lidocaine/ropivacaine. Conscious sedation was performed using an intravenous administration of remifentanil or midazolam ± propofol. At the end of the procedure, a final intravenous injection of 0.5 mg/kg of dexamethasone was given to reduce local symptoms.
A pressure dressing and an ice bag were applied to the skin after thermal ablation to reduce inflammation and pain. Two hours after the procedure, another thyroid ultrasound examination was performed to rule out early complications (hematoma). Patients were discharged on the same day with a pain killer prescription when the anesthesiologist authorized it.
Radiofrequency ablation
For thermal ablation, we used a STARmed VIVA RF Generator and an 18-07s07F electrode cooled by a 0.9% sodium chloride solution bag at 4°. The electrode was inserted using a transisthmic approach starting from the deepest part of the nodule, and a moving-shot thermal ablation technique was performed (successive treatment of small parts of the nodule). We delivered 35 to 55 Watts of power.
Laser ablation
Needle-guided tracking was performed prior to the procedure, and patients wore safety glasses. One or two 21 G needles were inserted based on the largest nodule axis, and allowing the installation of one or two laser fibers (EchoLaser, ELESTA). The fiber was initially positioned as deeply as possible and gradually pulled back. The energy delivered ranged from 1100 to 5600 joules.
Outcomes
Adverse events
Adverse events, recorded at each clinical and ultrasound evaluation during follow-up, were classified into one of the three groups according to the guidelines criteria from the Society of Interventional Radiology (SIR) [Citation48]: group 1 (major complication), including Horner’s syndrome, recurrent laryngeal nerve palsy, compressive hematoma and sub-cutaneous abscess; group 2 (minor complication) consisting of transient dysphonia, non-compressive hematoma, and nodule rupture requiring conservative treatment; and group 3 (side effects), including pain during or following the procedure, thyroiditis, thyroid dysfunction and skin burn.
Symptoms and nodule size reduction
All patients had a physical examination and an ultrasound nodule size evaluation at baseline and at 6 weeks, and then at 6, 12 and 18 months. Symptoms (anterior cervical discomfort or esthetic complaint, dysphonia and dysphagia) were each evaluated on a binary scale. The nodule volume reduction rate in percentage was calculated as follows: (final nodule volume (mL)/initial nodule volume (mL) × 100.
Statistical analysis
Continuous variables are expressed as means (standard deviation) and categorical variables are expressed as numbers (percentage). Normality of distributions was assessed using histograms and the Shapiro-Wilk test. The main patient characteristics were compared between the two main study groups (RFA vs. laser ablation) using the Student’s t-test for continuous variables and the Chi-Square test for categorical variables. Nodule characteristics were compared between the two main study groups using linear mixed models for continuous variables, and generalized linear models for categorical variables (binary, ordinal or multinomial logistic models).
Changes in nodule volume over 18 months (calculated as a percentage of volume change from baseline) were compared between the two study groups (RFA vs. laser ablation) using a linear mixed model for repeated measures (an unstructured covariance pattern model to account for the correlation between repeated measures within the same nodule); in this model, time, the study group and interaction time*study group were introduced as fixed effects and patients as a random effect (to account for the multiple nodules per patient). Post-hoc comparisons at each follow-up were done using linear contrast. Normality and homoscedasticity of the residuals were checked graphically. Comparisons of volume changes were further adjusted for prespecified factors (age, baseline nodule volume, nodule structure, TI-RADS category and nodule vascularization). We finally performed subgroups analysis to evaluate the influence of the time, the number of adverse events, of volume regained, the treatment duration and the total delivered energy in autonomous and non-autonomous nodule and in each type of nodule structure between eras (2013 and 2014 vs. 2015, 2016 and 2017). Changes in nodule volume over 18 months were compared between eras in each subgroup of nodule (‘autonomous nodule’ and ‘structure’) using the same linear mixed model that above. Statistical testing was conducted using a two-tailed alpha level of 0.05. Data were analyzed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Whole cohort and group characteristics
One hundred and sixty-six adult patients (mean age 51.7 ± 12.5 years old, 80.1% women) with a total of 189 TNs were enrolled and treated (). A detailed cohort description is shown in and .
Table 2. Baseline characteristics of the whole cohort.
Table 3. Description and comparison of patient characteristics and nodules according to intervention group.
The follow-up extended to 18 months. The number of nodules (n) at each visit is shown in . The mean nodule volume was 17.5 ± 16.9 ml and was significantly higher in the RFA group compared with the laser ablation group (p < .05). Most nodules were classified as TI-RADS category 3 (54.5%) and had type 1 vascularization (30.2%). Twenty-six patients (13.8%) had an autonomous nodule, and 13 patients (7.8%) had symptomatic hyperthyroidism with a low TSH level < 0.4 µIU/mL. The mean delivered energy was 6.8 ± 5.6 kJ, which was significantly higher in the RFA group (7.8 ± 5.8 kJ) compared with the laser ablation group (2.6 ± 1.0 kJ). The mean treatment duration was 16.8 ± 10.4 min and was significantly higher in the RFA group (18.7 ± 10.6) than in the laser ablation (8.9 ± 4.3) group (p < .05).
Table 4. Comparison of volume reduction over time and between radiofrequency and laser ablation.
Safety
Five major adverse events (3%), 25 minor adverse events (15.1%) and 101 side effects (60.8%) occurred during follow-up ().
Table 5. Comparison of the safety and efficacy in the two groups at the last post-thermal ablation evaluation.
Although the difference was not significant, we observed a higher rate of transient recurrent laryngeal nerve palsy (2%) and nodule rupture with conservative treatment (2%) in the RFA group compared with the laser ablation group (0%). In both patients with transient recurrent laryngeal nerve palsy, symptoms completely regressed after 1 and 3 months, respectively. Two subcutaneous abscesses and one compressive hematoma occurred within the first 48 h in the laser ablation group. They were surgically drained without further complications.
Peri- and post-procedural pains were the main side effects, concerning 92 out of 166 patients (55.4%). The pain did not exceed 4/10 on a visual analog scale (VAS), did not last for more than a week and was successfully treated with category 1 or 2 painkillers. Pain complaints were comparable in the RFA and laser ablation groups (57.6% vs. 53%). Seven postprocedure cases of transient dysphonia (4.2%) were also observed in the whole cohort, but a systematic postprocedural laryngoscopy did not identify any vocal cord dysfunction. Hyperthyroidism (low TSH level) after thermal ablation occurred in three cases (1.8%), including one patient who developed Grave’s disease (TRAb negative before the procedure, becoming detectable) without orbitopathy, in the first month after the procedure and who required antithyroid therapy. No hyperthyroidism recurred during the clinical and biological follow-up. No new cases of hypothyroidism or hypoparathyroidism were observed during the 18 months of follow-up.
Clinical efficacy and volume reduction
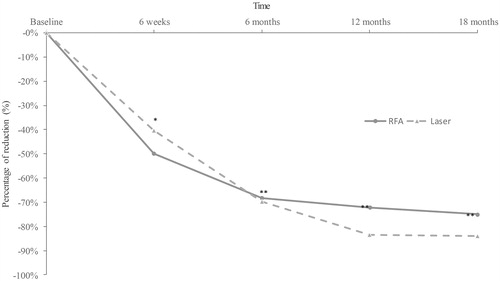
All results are shown in and . The nodule volumes decreased significantly in the two groups (−75% in the RFA and −84% in the laser ablation groups). Anterior cervical discomfort, esthetic discomfort and dysphagia were significantly reduced over the 18-month follow-up period.
Reduction percentages differed significantly when RFA was compared with laser ablation treatment at 6 weeks (p < .0001), but not at 6 months (p = .87), 12 months (p = .51) or 18 months (p = .71) ().
Figure 2. Median nodule volume reduction over time, in the two interventional groups. Percentage decrease significantly differed when comparing RFA to laser ablation treatment at 6 weeks (p < .0001). *p value significance < .05; ** Not statistically significant.
Five nodules (4.6%) in the RFA group and eleven (13.9%) in the laser ablation group recurred in the first 6 months, one of which was contemporaneous with hormone treatment for infertility. Three patients (1.8%) ultimately had surgery (total thyroidectomy or lobectomy) due to the persistence or recurrence of anterior cervical discomfort.
Subgroups analysis
Autonomously functioning nodule (AFN vs. non-AFN group)
Twenty-six patients (13.8%) had hot nodules on thyroid scintigraphy. Ten patients out of the 13 (76.9%) who underwent TA for hyperthyroidism had regressive hyperthyroidism symptoms, a significant reduction in nodule volume (p < .05) and normalization of TSH levels. We did not find any difference regarding the adverse events (12 adverse events in AFN vs. 83 in non-AFN, p = .51), the number of nodule regrowth (2 nodule regrowth in AFN vs. 14 in non-AFN, p = .83), the delivered energy (7.02 ± 5.89 kJ for AFN vs. 4.70 ± 3.0 kJ for non-AFN, p = .17) and the duration of the procedure (12.33 ± 5.27 min for AFN vs. 17.49 ± 10.82 min for non-AFN, p = .07). In AFN group, the percentage of volume reduction did not differ between RFA and LA, whereas in non-AFN group, percentage of volume reduction was significantly higher with RFA than LA (p < .0001). All the analysis were adjusted on pre-defined factors.
Nodule structure
In the whole cohort, we treated 95 (47.5%) solid nodules (70 in RFA group (64.8%) vs. 25 in laser ablation group (30.9%), p = .025), 34 (17%) cystic nodules (15 in RFA group (13.9%) vs. 19 in laser ablation group (23.5%), p = .77) and 63 (33.3%) mixed nodules (23 in RFA group (21.3%) vs. 40 in laser ablation group (49.4%), p = .65) (). We did not find any difference concerning the adverse events between RFA and laser ablation, regarding the three nodule structures: ORsolid≠cystic = 1.009, IC 95% [0.483–2.109], p = .38; ORcystic≠mixed = 0.557, IC 95% [0.217–1.431], p = .47; ORsolid≠mixed = 1.280, IC 95% [0.657–2.494], p = .42. We found a significantly higher number of volume regrowth in the ‘solid nodule’ group than in the ‘mixed nodule’ group: ORsolid≠mixed = 21.840, IC 95% (2.584–184.564), p = .0038, regardless of the techniques used. We did not find any difference concerning the delivered energy, regardless of the nodule structure and the techniques used. Finally, the percentage of volume reduction of solid nodules did not differ between RFA and laser ablation. Regarding cystic and mixed nodules, RFA was more efficient than laser ablation, p < .0001 and p = .0003, respectively.
Period-effect
A higher volume reduction at 6 and 12 months (p < .05) and a lower incidence of adverse events (p < .05) was observed for nodules treated in 2016 and 2017 compared with those treated in 2013 and 2014. The duration of the procedure was longer (p < .001), the volume regrowth (OR = 16.540, IC 95% [1.840–148.645], p = .012) and the delivered energy was lower (p < .001) at the beginning of the study than for more recently treated nodules (2013, 2014 vs. 2015, 2016, 2017).
Discussion
The purpose of our study was to assess the safety and efficacy of the two main techniques used for thermal ablation of benign thyroid nodules, over a period of 18 months ( and ). We found 3% of – all regressive – major adverse events and around 15% of minor adverse events related to thermal ablation. These two techniques showed similar efficacy at 18 months on volume reduction of benign TNs reaching 75 to 84%, regardless of the initial size of the nodule, the technique used and the center. It is one of the first and largest study to assess these techniques in a European country outside of Asian and Italian centers, which first investigated these procedures.
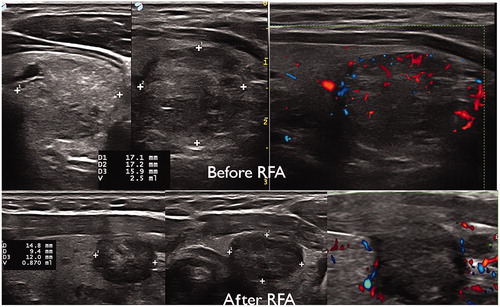
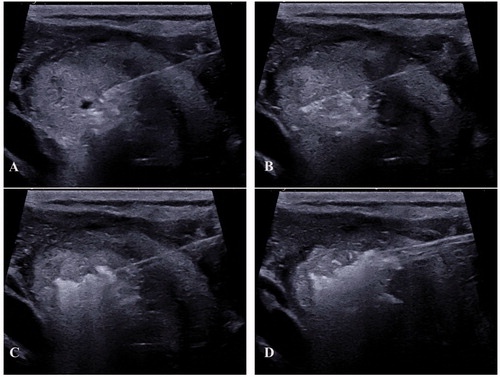
Figure 4. ‘pTA-UsNodule’: post-Thermal-Ablation Ultrasound Nodule. The electrode is introduced slowly in the nodule (A) after a peri-capsular anesthesia with lidocaine. The electrode is located one centimeter before the limits of the nodule (to avoid carotid and vagal nerve contact = ‘danger zone’) (B). The micro bubbles correspond to gas due to carbonization of the tissue (hyper-echoic aspect) (C). The electrode was slowly removed until the anterior limit (see bubbles around the nodule) (D).
Our study has, however, some limitations: we used a retrospective non randomized approach; therefore, inherent selection bias was unavoidable even with adjustment on pre-defined factors.
Nevertheless, patient recruitment, procedure and the follow-up were carried out in a standardized manner with very few missing data up till 12 months and an acceptable level of “loss to follow-up” patients at 18 months (RFA: 15.7% and laser ablation: 22.2%).
Adverse events were observed in 3% of cases which is not completely negligible but lower than with surgery and all regressive. Of note is the fact that 70% of them were observed in the first era. This underlines the need for formalized practical training and the identification of the “danger zone” to choose the most appropriate technique. These serious adverse events were regressive, in contrast with surgery, which is complicated by definitive recurrent laryngeal nerve palsy in 1 to 6% of cases [Citation49], voice changes in 5 to 25% of cases [Citation50] and hypoparathyroidism in 1 to 12% of cases [Citation51,Citation52]. Although the efficacy was similar in reducing symptoms, a comparative study (surgery vs. RFA) [Citation53] showed that the frequency of complications was higher with surgery for the treatment of nodular goiters (6% vs. 1%). In our cohort, no permanent adverse events were reported at 18 months. Previous data have shown that 30 to 44% of post-lobectomy patients develop hypothyroidism and then require lifelong L-thyroxine replacement therapy [Citation54–56]. In our cohort, no cases of hypothyroidism or hypoparathyroidism were reported. We observed 3 cases (1.7%) of hyperthyroidism, as reported in previous studies [Citation36,Citation57] but none reported Grave’s disease. Thus, the involvement of the TA procedure on the occurrence of the Grave’s disease observed in our cohort is unclear. In any case, this illustrates the importance of the endocrine follow-up.
Concerning side effects, the pain felt during and after the procedure never exceeded 4/10 (mild pain) on a visual analog scale; it was controlled by level 1 analgesics, and did not persist over time, regardless of the techniques used. Over half of the pain cases were caused by muscle contractures related to hyperextension of the neck during the procedure, which could be improved by better patient positioning [Citation58].
Concerning efficacy, the results are concordant with other - all retrospective - series of literature, that have included 100 to 1500 patients followed until 5 years, with safety and efficacy analysis [Citation23,Citation34,Citation38,Citation47]. These results mean that the technique is reproducible from a country to another, if it is performed in standardized conditions. The reduction of clinical symptoms and nodule volume was early and significant in the two groups; however none of the two techniques showed superiority over another, except at six weeks when radiofrequency had better results than laser. This result could be explain by the distinct characteristic of RFA which is performed with an electrode using the moving-shot approach, and the energy is applied all over the nodule (rapidly alternating electric current → vibration movement of the tissue’s bipolar molecules → transmit between adjacent molecules → frictional energy loss → deposit in the biological tissues → hyperthermia → “coagulation” necrosis). In contrast, the energy is generally applied on a well-delimited area of tissue with laser ablation. The volume reduction was sustained in the mid-long term (18 months) with the two techniques. Recurrence occurred less often with RFA than with laser ablation. It is noteworthy that the volume reduction was less significant and the incidence of adverse events was higher at the beginning of our work compared to more recent procedures. This could be explained by shorter procedure duration despite higher delivered energy.
Concerning indications, our study included a subgroup analysis according to the function and the structure of the nodules. The results show that the two techniques have similar efficacy for the treatment of autonomously functioning nodules but for cold nodule, RFA seem more efficient than laser ablation, confirming the data from literature [Citation31,Citation59]. Besides, a recent retrospective study that compared a single-session of RFA with radioiodine therapy did not show any differences concerning nodule volume reduction, but emphasized that RFA was effective in all patients with no case of post-treatment clinical hypothyroidism, and with no radiation exposure [Citation60]. We highlighted a significant number of volume regrowth in solid nodule group than in mixed nodule group, regardless of the techniques used. We observed that for solid nodule, there was no difference between RFA and laser ablation concerning the percentage of volume reduction, but for cystic and mixed nodules, RFA seemed more efficient than laser ablation. This indicates that a nodule expert cartography must be carried out in the first place, with all the explorations performed in a standardized way to target precisely the indications and the technique to be used, and to provide clear information to the patient on the benefits and risks of the treatment. Indeed and in contrast with TA, surgery has the advantage of definitively treating benign nodules and allows a complete pathological study. Although we observed recurrence more frequently with laser ablation than RFA (about 11% vs. 5%), ending in surgery for three patients, the recurrences (regrowth) were not related to misrecognized cancers. These techniques may open up perspectives for the treatment of primary or locally recurrent thyroid carcinomas, particularly in non-operable patients [Citation29,Citation61].
Although it was not the aim of this study, the comparison of the cost-effectiveness of thermal ablation and surgery is of interest. Indeed, the cost of the consumable for the radiofrequency ablation ranges from 550 to 1,040 euros ($585 to $1,180), approximately 350 euros ($395) for one laser fiber, with at least two fibers per procedure. The cost of total thyroidectomy (International Classification of Diseases (ICD-10)), for the surgical procedure itself is 460 euros ($540), and then, in most of cases, it requires a lifelong L-thyroxine replacement therapy (about 240 euros ($267) per year for a mean dose of 75 µgr/day). The thermal ablation is performed in an outpatient setting and therefore does not demand any hospitalization as opposed to surgery, which requires at least one night of monitoring (about 1500 euros ($1750)) for simple post-operative care. Finally, the duration of sick leave is shorter after thermal ablation ranging from 1 to 7 days as opposed to 10 to 30 days in case of surgery (French National Authority for Health, www.has-sante.fr) [Citation62]. Therefore thermal ablation seems less expensive even if we consider follow-up duration (18 months), the necessity of several procedures in rare cases of large nodules, and ultimate possible need for surgery (1.8% of patients in this series). In addition, the surgical management of benign nodules is still debated, despite the development of robot-assisted surgery, which is particularly widespread in Asia compared with Europe and France [Citation63]. A socio-economic study comparing the cost of the two techniques and the different surgical methods would be of great interest, taking into account the average length of stay in hospitals (ALOS) and the work leave after thyroidectomy, which may vary a lot from one country to another in Europe.
Conclusion
This bicentric cohort study shows that TA of benign TNs was associated with an overall 3% frequency of transient serious adverse events, mainly due to a learning curve. The nodule volume decreased overall by two-thirds, regardless of the technique.
Long-term follow-up is necessary to determine the prognostic factors of regrowth. However, as a first-line alternative treatment, TA appears to be a reasonable option for more than 30% of nodules that are usually treated by surgery. It responds to the patients’ and physicians’ increasing demands to conserve thyroid function. Nevertheless, it should be performed in expert centers and should be carefully evaluated in case of doubtful or malignant nodules, for which surgery remains the gold standard treatment [Citation64].
Disclosure statement
Hervé MONPEYSSEN has previously consulted for THERACLION and STARMED (oral conference communication). Edouard GHANASSIA has previously consulted for ABLATECH. Other authors have nothing to disclose regarding this paper.
References
- Jiang H, Tian Y, Yan W, et al. The prevalence of thyroid nodules and an analysis of related lifestyle factors in beijing communities. Int J Environ Res Public Health. 2016;13:442.
- Panagiotou G, Komninou D, Anagnostis P, et al. Association between lifestyle and anthropometric parameters and thyroid nodule features. Endocrine. 2017;56:560–567.
- Stanicić J, Prpić M, Jukić T, et al. Thyroid nodularity-true epidemic or improved diagnostics. Acta Clin Croat. 2009;48:413–418.
- Moon JH, Hyun MK, Lee JY, et al. Prevalence of thyroid nodules and their associated clinical parameters: a large-scale, multicenter-based health checkup study. Korean J Intern Med. 2018;33:753–762.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules – 2016 Update: Appendix. Endocrine Practice. 2016;22:1–39.
- Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123:372–381.
- Haugen BR, Sawka AM, Alexander EK, et al. American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27:481–483.
- Wémeau J-L, Sadoul J-L, d’Herbomez M, French Socity of Endocrinology, et al. [Recommendations of the French Society of Endocrinology for the management of thyroid nodules]. Presse Med. 40:793–826.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376–382.
- Jegerlehner S, Bulliard J-L, Aujesky D, NICER Working Group, et al. Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study. PLoS One. 2017;12:e0179387.
- Al Dawish MA, Alwin Robert A, Thabet MA, et al. Thyroid nodule management: thyroid-stimulating hormone, ultrasound, and cytological classification system for predicting malignancy. Cancer Inform. 2018;17:1176935118765132.
- Incidence et mortalité projetées en 2017 du cancer de la thyroïde - Nombre de cas et taux standardisés monde, contribution et rang; 2019 [2019 Jan 30]. Available at: http://lesdonnees.e-cancer.fr/Fiches-Indicateurs/Localisation-Thyroide/incidence-et-mortalit-projetes-en-2017-de-l-ensemble-des-cancers-nombre-de-cas-et-taux-standardiss-monde#graphique.
- Mathonnet M, Cuerq A, Tresallet C, et al. What is the care pathway of patients who undergo thyroid surgery in France and its potential pitfalls? A national cohort. BMJ Open. 2017;7:e013589.
- Singh Ospina N, Maraka S, Espinosa de Ycaza AE, et al. Prognosis of patients with benign thyroid nodules: a population-based study. Endocrine. 2016;54:148–155.
- Durante C, Grani G, Lamartina L, et al. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319:914–924.
- Sakorafas GH, Peros G, Farley DR. Thyroid nodules: does the suspicion for malignancy really justify the increased thyroidectomy rates? Surg Oncol. 2006;15:43–55.
- Vaiman M, Nagibin A, Hagag P, et al. Hypothyroidism following partial thyroidectomy. Otolaryngol Head Neck Surg. 2008;138:98–100.
- Na DG, Lee JH, Jung SL, Korean Society of Radiology, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125.
- Kim J-H, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Bachar GN, Greif F, Mor E, et al. Radiofrequency ablation for the management of liver tumors. Isr Med Assoc J. 2003;5:496–500.
- Ito T, Oura S, Nagamine S, et al. Radiofrequency ablation of breast cancer: a retrospective study. Clin Breast Cancer. 2018;18:e495–e500.
- Peek MCL, Douek M. Ablative techniques for the treatment of benign and malignant breast tumours. J Ther Ultrasound. 2017;5:18.
- Zhao Q, Tian G, Chen F, et al. CT-guided percutaneous laser ablation of metastatic lung cancer: three cases report and literature review. Oncotarget. 2017;8:2187–2196.
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540.
- Cervelli R, Mazzeo S, De Napoli L, et al. Radiofrequency ablation in the treatment of benign thyroid nodules: an efficient and safe alternative to surgery. J Vasc Interv Radiol. 2017;28:1400–1408.
- Ugurlu MU, Uprak K, Akpinar IN, et al. Radiofrequency ablation of benign symptomatic thyroid nodules: prospective safety and efficacy study. World J Surg. 2015;39:961–968.
- Papini E, Gugliemi R, Pacella CM. Laser, radiofrequency, and ethanol ablation for the management of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2016;23:400–406.
- Sui WF, Li JY, Fu JH. Percutaneous laser ablation for benign thyroid nodules: a meta-analysis. Oncotarget. 2017;8:83225–83236.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:920–930.
- Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab. 2017;61:173–179.
- Bernardi S, Stacul F, Michelli A, et al. 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine. 2017;57:402–408.
- Geach T. Thyroid: laser ablation of thyroid nodules is rapid, safe and effective. Nat Rev Endocrinol. 2015;11:631.
- Korkusuz H, Fehre N, Sennert M, et al. Volume reduction of benign thyroid nodules 3 months after a single treatment with high-intensity focused ultrasound (HIFU). J Ther Ultrasound. 2015;3:4.
- Tang X, Cui D, Chi J, et al. Evaluation of the safety and efficacy of radiofrequency ablation for treating benign thyroid nodules. J Cancer. 2017;8:754–760.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA. Int J Hyperthermia. 2017;33:295–299.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004;232:272–280.
- Papini E, Guglielmi R, Bizzarri G, et al. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract. 2004;10:276–283.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology. 2015;276:597–605.
- Korkusuz H, Sennert M, Fehre N, et al. Local thyroid tissue ablation by high-intensity focused ultrasound: effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia. 2014;30:480–485.
- Korkusuz H, Fehre N, Sennert M, et al. Early assessment of high-intensity focused ultrasound treatment of benign thyroid nodules by scintigraphic means. J Ther Ultrasound. 2014;2:18.
- Sennert M, Happel C, Korkusuz Y, et al. Further investigation on high-intensity focused ultrasound (HIFU) treatment for thyroid nodules: effectiveness related to baseline volumes. Acad Radiol. 2018;25:88–94.
- Lang BH, Wu ALH. The efficacy and safety of high-intensity focused ultrasound ablation of benign thyroid nodules. Ultrasonography. 2018;37:89–97.
- Lang BH, Wu ALH. 2017 High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules - a systematic review. J Ther Ultrasound. 5:11.
- Russ G, Bigorgne C, Royer B, et al. [The thyroid imaging reporting and data system (TIRADS) for ultrasound of the thyroid]. J Radiol. 2011;92:701–713.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618.
- Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165.
- Cardella JF, Kundu S, Miller DL, Society of Interventional Radiology, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Sajid T, Qamar Naqvi SR, Qamar Naqvi SS, et al. Recurrent laryngeal nerve injury in total versus subtotal thyroidectomy. J Ayub Med Coll Abbottabad. 2016;28:559–561.
- Borel F, Christou N, Marret O, et al. Long-term voice quality outcomes after total thyroidectomy: a prospective multicenter study. Surgery. 2018;163:796–800.
- Chen K-C, Iqbal U, Nguyen P-A, et al. The impact of different surgical procedures on hypoparathyroidism after thyroidectomy: a population-based study. Medicine (Baltimore). 2017;96:e8245.
- Marcinkowska M, Sniecikowska B, Zygmunt A, et al. Postoperative hypoparathyroidism in patients after total thyroidectomy - retrospective analysis. Neuro Endocrinol Lett. 2017;38:488–494.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Elmas F, Lauber F, Linder T, et al. [Hypothyreosis after Hemithyroidectomy - surprisingly frequent complication in aftercare]. Laryngorhinootologie. 2018;97:24–29.
- Chotigavanich C, Sureepong P, Ongard S, et al. Hypothyroidism after hemithyroidectomy: the incidence and risk factors. J Med Assoc Thai. 2016;99:77–83.
- Ahn D, Sohn JH, Jeon JH. Hypothyroidism following hemithyroidectomy: incidence, risk factors, and clinical characteristics. J Clin Endocrinol Metab. 2016;101:1429–1436.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Oddo S, Felix E, Mussap M, et al. Quality of life in patients treated with percutaneous laser ablation for non-functioning benign thyroid nodules: a prospective single-center study. Korean J Radiol. 2018;19:175–184.
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25:112–117.
- Cervelli R, Mazzeo S, Boni G, et al. Comparison between radioiodine therapy and single-session radiofrequency ablation of autonomously functioning thyroid nodules: a retrospective study. Clin Endocrinol (Oxf)). 2019;90:608–616.
- Yoo RE, Kim JH, Paeng JC, et al. Radiofrequency ablation for treatment of locally recurrent thyroid cancer presenting as a metastatic lymph node with dense macrocalcification: a case report and literature review. Medicine (Baltimore)). 2018;97:e0003.
- Haute Autorité de Santé - Avis de la HAS sur le référentiel concernant la durée d’arrêt de travail: saisine du 15 septembre 2010; 2018 [2018 OCT 3]. Available at https://www.has-sante.fr/portail/jcms/c_1069881/avis-de-la-has-sur-le-referentiel-concernant-la-duree-d-arret-de-travail-saisine-du-15-septembre-2010.
- Paek SH, Kang KH, Park SJ. Expanding indications of robotic thyroidectomy. Surg Endosc. 2018;32:3480–3485.
- Ma B, Wei W, Xu W, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: a retrospective case review and literature review. Thyroid. 2018;28:1134–1142.