Abstract
Background: Complete cytoreduction is acknowledged to be an effective way to achieve macroscopic tumor clearance for a variety of tumors confined to the peritoneal cavity. Recent trials have shown that surgery respecting anatomical planes results in excellent outcomes and even the chance of cure for some from what was once thought to be life-limiting disease.
Objective: To describe peritonectomy procedures in the current era.
Method: A thorough and systematic method for cytoreductive surgery aimed at complete surgical resection of peritoneal metastases (PMs) was described.
Results: The general principles of cytoreductive surgery were set out including preoperative preparation, patient positioning and incision. Strategies for assessing disease extent and planning surgical steps were outlined and established peritonectomy procedures such as Glisson’s capsulectomy, omentectomy, left and right diaphragmatic peritonectomy, lesser omentectomy, stripping of the omental bursa, and pelvic peritonectomy were described. Novel techniques such as anterior pancreatic peritonectomy, small bowel mesenteric peritonectomy and cardiophrenic lymph node dissection were explained, and illustrated with accompanying video.
Conclusion: Peritoneal metastases present a challenge to the surgeon which calls for a unique skill set if optimal outcomes are to be achieved. Attempts to standardize the surgical techniques described will allow further refinement as new technological advances occur.
Introduction
Involvement of the peritoneum from a primary peritoneal tumor or as a metastatic site most commonly from gastrointestinal or gynecological tumors presents a unique challenge [Citation1]. Medical and surgical oncologists often regard peritoneal metastases (PMs) as precluding curative treatment and when identified many patients develop bowel obstruction leading to death within 12 months of diagnosis [Citation2]. Development of aggressive locoregional therapy combining cytoreductive surgery (CRS) with intraperitoneal chemotherapy to eradicate both macroscopic and microscopic disease has transformed management of PMs, with long-term survival now a real possibility [Citation3–7].
Complete tumor removal combining peritonectomy procedures with multivisceral organ resection has consistently been shown to influence long-term survival [Citation8]. In 1995, Sugarbaker first described peritonectomy procedures [Citation9]. Since this first description, advances in technology have allowed refinement of surgical procedures such as Glisson’s capsulectomy of the liver [Citation10,Citation11], total mesenteric peritonectomy [Citation12,Citation13] and posterior lesser sac peritonectomy [Citation14].
This manuscript describes and illustrates peritonectomy procedures with updates on recent advances in cytoreductive surgery for PMs highlighting technical challenges and potential pitfalls.
Methods
General principles
The main goal of cytoreductive surgery is to ensure a complete macroscopic resection of the disease. With the exception of the falciform ligament and the greater and lesser omentum, disease-free peritoneum should not be removed. For peritoneal mesothelioma, a primary peritoneal malignancy, a complete parietal peritonectomy may be warranted and is under evaluation although not widely performed at present [Citation12]. Superficial lesions without organ involvement can be removed by peritonectomy whereas deeper invasion requires partial or complete resection of the involved organ depending on the circumstances. Greater omentum is commonly involved by tumor, and should be resected routinely in every cytoreductive surgery with curative intent. Several instruments are used for peritonectomy procedures, ball-tip electrosurgical hand piece, standard blade electrosurgical hand piece or bipolar scissor. When two colonic anastomoses or a low stapled colorectal anastomosis are performed, a defunctioning loop ileostomy may be indicated [Citation15]; although, a recent report by Sugarbaker reported that a double layered handsewn colorectal anastomosis was safe with no increase in fistula rate [Citation16].
Preoperative preparation
Optimization of enteral nutrition should be considered in all patients before surgery particularly those who are malnourished (albumin <30) to reduce morbidity and mortality [Citation17].
Bowel preparation can be helpful but may be avoided when limited CRS is planned (without extensive or multiple gastrointestinal resection). Potential stoma sites are marked bilaterally on the rectus abdominis ideally by someone experienced in the siting and management of stomas such as a Specialist Nurse. Although traditionally enhanced recovery is directed at reducing length of stay, some of its principles such as early mobilization are of great value. Pre-operative counseling by physiotherapists or exercise therapists can be beneficial in preparing patients with evidence suggesting prehabilitation reduces morbidity in other type of major surgery [Citation18,Citation19]. Pre-operative psychological assessment can also identify those patients who may need support in the immediate postoperative period when delirium and hallucinations can delay recovery as well as longer term after discharge.
For female patients in age of childbearing, fertility preserving options should be discussed, based on tumor origin, and long-term expected outcomes. As there is no international consensus regarding management of the population of patient, a discussion between the patient, the surgical oncology team and the fertility team should address this point.
Position
The patient is positioned supine with the sacrum at the end of the abdominal portion of the table and legs are placed in the shark fin leg holder (Allen Medical, Acton, MA) to give complete access to the abdomen and perineum allowing stapled or hand sewn colorectal anastomosis or coloanal anastomosis. Proper positioning to avoid lower limb compartment syndrome or nerve compression is essential. Gel padding in the leg holder is positioned to avoid pressure points and intermittent pneumatic compression devices are used to reduce the risk of venous thrombosis. Warming blankets are placed over the arm, head and chest above the nipple line (Bair Hugger, 3M, Saint Paul, MN). Cooling blankets can be placed beneath the patient. Skin preparation is wide, from the upper chest to the perineum. Vaginal preparation is used in women. Bladder catheter and nasogastric tube are inserted routinely.
Incision and peritoneal cavity exploration
Exploration of the peritoneal cavity after the initial incision gives the surgeon an opportunity to plan the procedure (Video 1). The explorative phase starts with a midline laparotomy which can be extended superiorly to the xyphoid and inferiorly to the pubis if complete cytoreduction is deemed possible. Consideration needs to be given to excision of the umbilicus in patients with mucinous disease who have had prior laparotomy or laparoscopy. Port sites in the midline should also be resected. No resection should be considered before an exhaustive exploration. The goal of surgery is to achieve a complete resection of macroscopic disease safely minimizing the risks of postoperative complications and their subsequent impact on quality of life. A systematic exploration of the peritoneal cavity is needed before an accurate determination of the feasibility of complete cytoreduction can be made [Citation20]. It requires some dissection and a complete exploration of all the abdominal regions, including division of adhesions; which can be time consuming. It enables the thorough appreciation of disease extent which can be quantified using scoring systems such as the peritoneal cancer index (PCI) [Citation8]. What is required to achieve a complete cytoreduction must be balanced with the prognosis of the disease [Citation5,Citation21–25], the risk of postoperative morbidity and mortality [Citation26] and anticipated quality of life after surgery [Citation27].
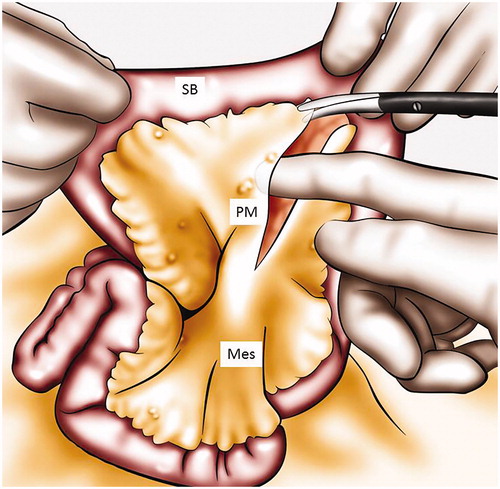
In some cases, an extensive and diffuse micronodular involvement of the small bowel, not detected on preoperative investigation will be found. A decision must then be made on whether to proceed with a tumor debulking procedure perhaps with colectomy and end ileostomy or to simply close the abdomen based on the risks of damaging small bowel causing enterocutaneous fistulation. A fixed cicatrized small bowel often precludes any further surgery. Two areas are well known to harbor tumor easily missed if not explored: the aorto caval groove which necessitates the opening of the pars flaccida and the posterior portion of the ligament of Treitz where deposits of mucinous tumors are often found ( and ).
Figure 1. Treitz ligament exploration. This figure illustrates how to explore the peritoneum behind the duodenojejunal flexure, by retracting the first jejunal loop. Panc: pancreas; IMV: inferior mesenteric vein; DJF: duodenojejunal flexure.
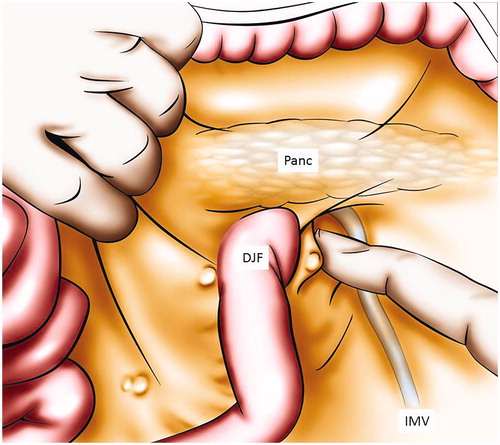
Figure 2. Inter cava caudate peritoneal recess exploration after incision of pars flaccida. This figure illustrates to explore the peritoneum between the vena cava and the caudate lobe by opening the pars flaccida. VC: vena cava; I: liver segment I; LL: liver left lobe; PF: pars flaccida.
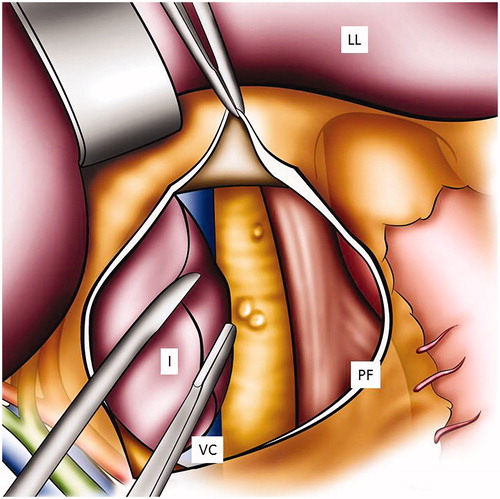
To get sufficient exposure to the upper abdomen, the xyphoid can be excised. A self-retaining retractor facilitates the quality of exposure and its use should be encouraged.
In the left upper quadrant, assessment of resectability is focused on the diaphragm, spleen, splenic hilum and tail of the pancreas. Invasion of the spleen or splenic hilum would necessitate a splenectomy with increased morbidity [Citation28]. Great care is needed to avoid traumatizing the tail of the pancreas. Injury may result in post-operative pancreatitis or pancreatic fistula which can result in a prolonged hospital stay and reliance on long-term parenteral nutrition. If the tail of the pancreas is invaded, distal spleno-pancreatectomy is needed, increasing morbidity [Citation29].
In the right upper quadrant, the diaphragm, liver, liver pedicle, hepatic veins and vena cava all require assessment. The hepatic pedicle is palpated to rule out invasion deeper than the peritoneal lining, which would preclude a complete cytoreduction. The liver is also examined to look for the presence of unresectable liver metastases that may have been missed pre-operatively or developed subsequent to the most recent imaging. Finally, invasion of the great vessels, principally the retro-hepatic segment of the vena cava or hepatic vein that would again prevent complete tumor removal.
In the pelvis, resectability depends upon invasion of iliac or femoral vessels, nerve roots and ureters. Invasion of the femoral nerve, spinal root, great vessels or bladder at the ureteric junction would be considered a relative contraindication to the surgery. Bone invasion must warrant special considerations preoperatively as complete resection necessitates multiple expert teams and requires forward planning. Tumor invasion of the bladder at the ureteric junction may require radical cystectomy, which should also be planned pre-operatively since this procedure carries significant morbidity and may not be appropriate depending on the tumor type.
Digital glissonectomy
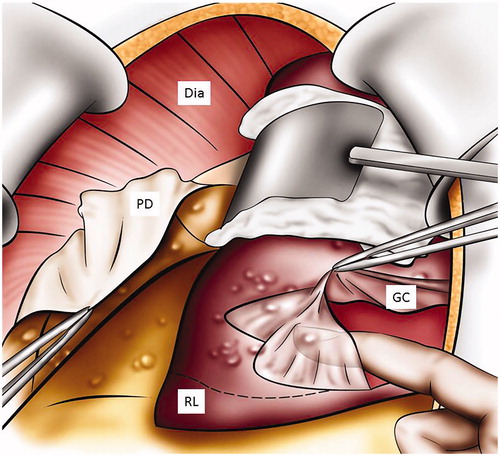
Glissonectomy starts 1–2 cm away from the disease, within normal peritoneum. The capsule is incised and stripped long enough to ensure a complete resection of the disease; this must also be large enough to allow at least one finger to fit between liver parenchyma and Glisson’s capsule to allow for efficient blunt dissection (). The critical part of this peritonectomy is to find the plane between the liver parenchyma and Glisson’s capsule. The dissection is performed using forceps to lift Glisson’s capsule, blunt scissors and bipolar forceps to separate the capsule from the parenchyma, in an avascular plane. Once the plane between the liver parenchyma and the peritoneum is found and divided, the capsule is then stripped with digital dissection and extended beyond the diseased peritoneum. Bipolar forceps and scissors can be used when the capsule is more adherent. The stripping is an easy dissection. If resistance is encountered in freeing the capsule, it is usually due to false dissection into or tumor involvement of the parenchyma. If the tumor from Glisson’s capsule invades the parenchyma, a formal liver resection may be needed. The blunt digital dissection is made easier by wetting the gloves which helps separate the liver parenchyma from the capsule. Once all diseased capsule is released from liver parenchyma, the specimen can be removed and sent to pathology. The liver is packed using a large sponge, and this is left in place for the remainder of the procedure. When required, the glissonectomy is best performed at the beginning of the surgical procedure to allow time for hemostasis and identification of bile leaks [Citation10,Citation11].
Figure 3. Digital glissonectomy to remove liver surface involvement. This figure illustrates how to expose the peritoneum of the diaphragm and the liver right lobe. A digital peritonectomy of the Glisson capsule is performed. Dia: diaphragm; RL: liver right lobe; GC: Glisson’s capsule; PD: peritonectomy of the diaphragm.
Pitfalls
Major bleeding can occur if the hepatic packing is not performed adequately. Packing of the liver will usually control bleeding if left for long enough. If a bile leak is seen, effort should be made to find the origin and suture it.
An alternative to digital glissonectomy is electroevaporation of Glisson’s capsule using high powered ball tip diathermy on the surface of the liver using the cut setting. This works well for mucinous tumors such as pseudomyxoma peritonei although does create large volumes of surgical smoke for which effective smoke evacuation is required. The advantage is less blood loss and a layer of tissue necrosis that leaves the surface of the liver devoid of tumor cells. The main drawback is the absence of pathological analysis, that could confirmed the completeness of cytoreduction.
Omentectomy
Once resectability has been confirmed during initial inspection of the peritoneal cavity, two unfolded sponges are placed behind the spleen to reduce traction on it and minimize capsular trauma to the inferior pole. This maneuver also facilitates splenic flexure mobilization. The omentum is elevated and separated from the transverse colon. Omentum and transverse colon are divided using electrosurgery and the dissection can be continued beneath the peritoneum that covers the transverse mesocolon in order to expose the lower border of the pancreas. The right part of the omentum is divided totally from the hepatic flexure of the right colon. In the absence of macroscopic involvement of the gastroepiploic arcade or stomach, vessels can be preserved and branches of the gastroepiploic arcade to the omentum are ligated. Evers et al. evaluated preservation of the right gastroepiploic artery and did not find any reduction in postoperative gastroparesis [Citation30]. If involved, branches of the gastroepiploic arcade to the greater curvature of the stomach are ligated in continuity and then divided. This can be done with vessel sealing device. Short gastric vessels should be preserved if gastroepiploic arcade is resected. Care should be taken to protect the greater curve of the stomach, as thermal spread can occur with vessel sealing devices resulting in delayed perforation of the stomach. Dissection of the most lateral part of the omentum is started superficially. Every encountered vessel is controlled individually. When the separation of the omentum and splenic hilum/pancreatic tail is completed, infarction of the lower pole of the spleen can be seen, and can usually be ignored.
Pitfalls
It is important to resect the entire omentum including both lateral portions at the splenic and hepatic flexure. Care should be taken during splenic mobilization, as tears in the splenic capsule are easily made but difficult to control and often end in a splenectomy to achieve hemostasis which can add to morbidity particularly if the tail of the pancreas is damaged inadvertently. This can result in a pancreatic fistula that may prolong hospital stay. Caution should be exercised to preserve the left gastric artery if possible as if damaged a total gastrectomy may be required [Citation28].
Peritonectomy of the left hemidiaphragm
The free edge of peritoneum at the midline surgical incision is a convenient place to begin this peritonectomy which is extended toward the diaphragm according to the extent of disease. This dissection occurs in a plane below the posterior rectus aponeurosis. Removal of the peritoneal layer is facilitated by application of traction on the peritoneum with an appropriate instrument or by hand. Mobilizing the splenic flexure aids the diaphragmatic peritonectomy allowing the left kidney to drop back. The peritoneum is released from the diaphragmatic muscle, the left adrenal gland and the superior half of the perirenal fat. On the medial portion of the dissection, the left triangular and coronary ligaments are divided and the left lobe of the liver is reflected medially. The dissection is finalized at the lateral edge of the spleen. If there is splenic invasion, the spleen can be resected ‘en bloc’ with the peritoneum of the left hemidiaphragm. In case of diaphragmatic invasion, muscle or tendinous resection may be needed. The tissue infiltrated by tumor should be resected, which usually requires an elliptical incision of a portion of the hemidiaphragm. Particular attention should be taken to prevent cells spilling into the thoracic cavity. If a portion of the diaphragm is resected, a stich placed on the peritoneal or pleural side will allow the pathologist to orientate the resected specimen. The diaphragm can be closed in a variety of ways with a continuous or interrupted non-absorbable large caliber suture before or after HIPEC depending on the degree of involvement or contamination of the chest cavity. If the diaphragm is left open during HIPEC the anesthetist needs to be aware so that they can monitor ventilation accordingly [Citation31]. Diaphragmatic resection can also be performed using a stapler which prevents opening of the thoracic cavity [Citation32]. If the defect in the diaphragm is large then a mesh can be used to aid closure. Chest drain insertion after diaphragmatic stripping allows drainage of pleural fluid postoperatively and can be useful after administration of HIPEC or if early postoperative intraperitoneal chemotherapy (EPIC) is planned. It is not necessary to place a wide bore chest drain although anything smaller than 12 French may become blocked with clot or debris.
Pitfalls
Splenectomy can increase morbidity and if possible electroevaporation of tumor from the splenic capsule with the high-powered ball tip diathermy may allow splenic preservation keeping in mind a small risk of delayed hemorrhage with this approach. The use of hemostatic agents such as a fibrin sealant patch on the surface of the spleen may avoid unnecessary splenectomy if bleeding is controlled.
Peritonectomy of the right hemidiaphragm
Peritoneum is stripped from beneath the right posterior rectus sheath to begin the peritonectomy in the right upper quadrant of the abdomen applying traction to the free edge in the midline. The first portion of peritoneum stripped from the posterior rectus sheath is tracked with clamp. As soon as enough space is created in the dissection plane the retractor blade can be used to provide adequate traction. Strong counter-traction on the peritoneum aids dissection at the interface of the peritoneum and the muscle or tendinous portion of the diaphragm, using blunt dissection combined with electrocoagulation. Coagulation is used to divide the blood vessel between diaphragm and peritoneum. The stripping of the tumor continues until the coronary and triangular ligaments are divided and the bare area of the liver is encountered. At that point, tumor on the superior surface of the liver can be electroevaporated until the liver surface is cleared according to Sugarbaker’s description [Citation9]. However, blunt dissection of the liver capsule (glissonectomy) is also an option and may provide a better pathological specimen. Falciform and round ligaments are resected. Division of the pons hepatis which is present in around 50% of patients is sometimes required to clear tumor at the base of the falciform ligament. At the base of the pons, the left hepatic artery or an early branch can be encountered and there is danger of damage to this if care is not taken to visualize it. Tumor from beneath the right hemidiaphragm, the right sub-hepatic space (Morrison) and the surface of the liver forms an envelope that has to be removed en bloc. Dissection is greatly facilitated if the tumor specimen is kept intact. Dissection continues laterally on the right to encounter the perirenal fat covering the right kidney. The right adrenal gland is identified and carefully preserved while tumor is stripped from the right subhepatic space. As the peritoneal reflection at the posterior aspect of the liver is divided, care must be taken not to traumatize the vena cava or to disrupt the caudate lobe vein that passes between the anterior surface of the vena cava and segment 1 of the liver. If the malignancy is invasive, tumor may be densely adherent to the tendinous central portion of the hemidiaphragm. In case of diaphragmatic invasion, the steps described previously can be followed.
Pitfalls
Careful hepatic mobilization must be performed to avoid hepatic vein laceration. The inferior phrenic veins can be followed to their sites of drainage as a landmark for identification of the hepatic veins if surrounded by tumor. If the hepatic veins are damaged during liver capsulectomy, then a Pringle maneuver can help reduce portal blood flow and facilitate suture of the hepatic veins to control bleeding. Resection of the falciform ligament is undertaken routinely. Care should be taken around the right adrenal vein when mobilizing the right liver as it can be avulsed with excessive traction resulting in rapid blood loss.
Lesser omentectomy/omental bursectomy
After division of the left triangular and coronary ligament, the left lobe of the liver is retracted medially to expose the lesser omentum. Starting at the medial border of the pars flaccida, the caudate lobe can be retracted with a finger or narrow deaver retractors. The peritoneum from the aorto-caval groove is then stripped either bluntly or with bipolar scissors (the anterior portion of the sub-hepatic vena cava). The peritoneum is dissected up to the right diaphragmatic crus. Care is taken to protect the right phrenic artery. Then, the peritoneum at the lesser curvature of the stomach is stripped from the vascular arcade. The dissection proceeds with caution to avoid traumatizing the right and left gastric arteries, the left gastric vein and the anterior vagal nerve.
Pitfalls
Particular attention should be given to avoid injury of vascular and biliary structures that will be dissected. Tumor is often not cleared from this area due to inadequate exploration.
Pelvic peritonectomy
The pelvic peritonectomy is started from above and is then developed laterally on both sides and then medially towards the pouch of Douglas which is addressed at the end of the procedure. Right and left colons are mobilized. All the bowel content is retracted superiorly using a wet abdominal sponge. Two unfolded sponges are used to protect the bowel from a flexible blade positioned in a ‘C’ form to create space in the pelvis. Right and left ureters are identified and preserved. Lateral incisions on uninvolved peritoneum are performed to delineate the surgical field commonly at the iliac vessels. The tumor-bearing peritoneum is stripped from the posterior surface of the lower abdominal incision, exposing the rectus muscle. In women, right and left ovarian vessels are ligated after clamp control at the lower pole of the kidney. After dissecting the peritoneum on the right and left side of the bladder, the urachus is localized and placed on strong traction using a Babcock clamp. The peritoneum without fatty tissues is stripped away from the surface of the bladder. If identification of the bladder is difficult, the bladder can be emptied to help identification, using the balloon of the bladder catheter as a landmark. If there is space in the pelvis then filling the bladder with water may also help to demonstrate anatomical planes. Broad traction on the entire anterior parietal peritoneum surface and frequent saline irrigation clears the point for tissue transection, which is precisely located between the bladder musculature and its adherent fatty tissue with peritoneum. For the male pelvis, the deferent canals and testicular vessels are identified and preserved. The inferior limit of dissection is the seminal vesicles. If the seminal vesicles are surrounded by fatty tissue, a clear deep margin on the pelvic peritoneum may be obtained. If tumor invades the seminal vesicles posteriorly and indents the base of the bladder anteriorly, resection of the seminal vesicles and a part of the prostate may be necessary [Citation33]. In women, the inferior limit of dissection is the cervix or proximal vagina. The round ligaments are divided as they enter the internal inguinal ring. The right and left ovarian vessels are ligated after clamp control at the lower pole of the kidney, after incising the peritoneum. The right and left ovarian vessels are divided only after identification of the ureters. Extraperitoneal ligation and division, after clamp control of the uterine vessels is performed just above the ureters and near the base of the bladder. The ureters are completely dissected and released from the cardinal ligaments of the uterus. The bladder is dissected away from the cervix/anterior aspect of the vagina. The cardinal and uterosacral ligaments are clamped and transected proximally to the uterus. When hysterectomy is indicated, the vagina is transected with electro surgery and the rectovaginal septum is exposed. An Allis clamp is placed on the posterior cut edge of the vagina for traction. The peritoneum from the pararectal fossa, posterior aspect of the vagina and middle third of the rectum are removed as a ‘cul-de-sacectomy’. Closure of the vaginal stump is carried out with sutures. For resection of the Pouch of Douglas, the peritoneal incision around the pelvis is connected to the peritoneal incision of the right and left paracolic sulci. Mobilization of the rectum in the total mesorectal excision (TME) plane aids pelvic peritonectomy including the Pouch of Douglas as involved peritoneum is lifted out of a narrow deep pelvis. The specimen is removed en bloc after separation from the rectal wall. A rectal resection is performed in case of rectal invasion. Serosal damage to the anterior wall of the rectum can be repaired with interrupted sutures and integrity of the rectum tested with rectal insufflation submerging the rectum under water. If an anterior resection is needed to clear tumor then a defunctioning loop ileostomy can help mitigate the consequences of an anastomotic leak from a colorectal anastomosis. Following a stapled colorectal anastomosis, a second layer of interrupted sutures to reinforce the anastomosis is an alternative to defunctioning [Citation16].
Pitfalls
If the ureters are traumatized during ureterolysis then ureteric stenting is advisable. Ischemic necrosis of the ureters can occur particularly with the use of high voltage diathermy so care must be taken. As a result, a urethral catheter to drain the bladder is routinely advocated.
Novel peritonectomy procedures
Anterior pancreatic peritonectomy
The inferior border of the pancreatic peritoneum is dissected with bipolar scissors or other electrocoagulation device. The peritoneal layer is put on traction then stripped from the pancreas using mainly electrosurgery and blunt dissection progressing superiorly from the inferior border. Care must be taken to minimize trauma to the pancreas by maintaining constant traction on the peritoneum to keep the dissection plane in view. The superior border is transected at the upper limit of the pancreas avoiding the splenic vessels which run along the superior border. Bleeding from the surface of the pancreas can be controlled with diathermy or fibrin patch sealant. An abdominal drain should be left at the tail of the pancreas as pancreatic fistula is a possible complication.
Total mesenteric peritonectomy
Deraco et al. has described this technique for dealing with tumor on the mesenteric surface of the small bowel which is often seen in peritoneal mesothelioma [Citation12]. The dissection is started at the root of the small bowel mesentery with a peritoneal incision. The peritoneum is then slowly stripped from the mesenteric fat with a combination of blunt and electrosurgical dissection. The plane is totally avascular when the dissection is kept superficial. Gentle traction, to avoid vessels tearing, is applied on the peritoneum with an artery forceps and bowel is retracted in the opposite direction with sponge. Dissection can be continued bluntly with finger bipolar scissors or pencil diathermy as required lifting the peritoneum off the underlying mesenteric fat up to the mesenteric border of the small bowel and its serosal surface (). Both sides of the mesentery can be stripped in this way [Citation12,Citation13]. Particular attention should be paid to preserve the mesenteric vessels. Similar to other peritonectomies, the dissection is much easier when the specimen is kept intact.
Cardiophrenic lymph nodes dissection
The presence and the prognostic impact of lymph nodes in this area have not been well defined but there may be merit in their removal if detected on preoperative investigations [Citation34,Citation35]. The subxiphoid approach utilizes the upper portion of the laparotomy incision and leaves the diaphragm intact, entering the pleural space anterior to the diaphragm and pericardium. The dissection plane is just under the xyphoid and allows direct access to the cardiophrenic lymph nodes, which are put on traction with a Babcock clamp and dissected with an energy device (either monopolar or bipolar). The vascular pedicle is isolated and controlled with a small clip (Video 2).
Discussion
Cytoreductive surgery and HIPEC has evolved over the last 20 years. The learning curve for this surgery is steep but sharing technical tips from those with experience can shorten the time taken to achieve competence with minimal associated morbidity. These procedures, combined with HIPEC, can achieve cure in a significant proportion of carefully selected patients. The quality of surgery is influenced by operative decision making as well as technical skill. The combination of peritonectomy and visceral resection comes with high morbidity (about 40%) and significant mortality (around 4%) even in experienced hands [Citation36]. Centralization is key with high volume centers producing good outcomes often with at least two consultant surgeons involved in each case. The global learning curve should not be ignored by those establishing new peritoneal malignancy centers [Citation37]. As the indications for cytoreductive surgery and HIPEC broaden from rare large volume pseudomyxoma peritonei to more common limited colorectal PMs, it is important that surgeons treating these patients are aware of the challenges these procedures present. An update of surgical techniques and approaches provides a platform to refine cytoreductive surgery so that morbidity is minimized and the best oncological outcomes are achieved not only in survival but also in quality of life. Standardization of these techniques will help the surgical community to undertake these complex procedures safely and effectively but also to continue to improve them.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65:284–298.
- Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–1719.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743.
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581.
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with Pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
- Dagbert F, Passot G, Glehen O, et al. Glisson capsulectomy for extensive superficial liver involvement in peritoneal carcinomatosis (with video). J Vis Surg. 2015;152:332–333.
- Passot G, Kim BJ, Vaudoyer D, et al. Digital glissonectomy: a safe perihepatic peritonectomy. Ann Surg Oncol. 2016;23:3978–3985.
- Deraco M, Baratti D, Kusamura S, et al. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol. 2009;100:321–328.
- Cazauran JB, Lasseur A, Pasquer A, et al. Total mesenteric peritonectomy for peritoneal metastases (with video). Ann Surg Oncol. 2017;24:3988–3989.
- Deraco M, Glehen O, Helm CW, et al. An overview of peritonectomy, visceral resections, and perioperative chemotherapy for peritoneal surface malignancy. In: Sugarbaker PH, editor. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Woodbury (CT): Ciné-Med; 2013.
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68.
- Sugarbaker PH. Avoiding diverting ileostomy in patients requiring complete pelvic peritonectomy. Ann Surg Oncol. 2016;23:1481–1485.
- Banaste N, Rousset P, Mercier F, et al. Preoperative nutritional risk assessment in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for colorectal carcinomatosis. Int J Hyperthermia. 2018;34:589–594.
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6:CD012020.
- Snowdon D, Haines TP, Skinner EH. Preoperative intervention reduces postoperative pulmonary complications but not length of stay in cardiac surgical patients: a systematic review. J Physiother. 2014;60:66–77.
- Sugarbaker PH. It’s what the surgeon doesn't see that kills the patient. J Nippon Med Sch. 2000;67:5–8.
- Baumgartner JM, Tobin L, Heavey SF, et al. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;22:1716–1721.
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39:1435–1443.
- Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of Pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300–308.
- Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol. 2014;21:1147–1152.
- Mercier F, Passot G, Villeneuve L, et al. Peritoneal carcinomatosis of urachus origin treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): an international registry of 36 patients. Ann Surg Oncol. 2018;25:1094–1100.
- Passot G, Vaudoyer D, Villeneuve L, et al. A perioperative clinical pathway can dramatically reduce failure-to-rescue rates after cytoreductive surgery for peritoneal carcinomatosis: a retrospective study of 666 consecutive cytoreductions. Ann Surg. 2017;265:806–813.
- Passot G, Bakrin N, Roux AS, et al. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: a prospective study of 216 patients. Eur J Surg Oncol. 2014;40:529–535.
- Dagbert F, Thievenaz R, Decullier E, et al. Splenectomy increases postoperative complications following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23:1980–1985.
- Schwarz L, Votanopoulos K, Morris D, et al. Is the combination of distal pancreatectomy and cytoreductive surgery with HIPEC reasonable? Results of an International Multicenter Study. Ann Surg. 2016;263:369–375.
- Evers DJ, Smeenk RM, Bottenberg PD, et al. Effect of preservation of the right gastro-epiploic artery on delayed gastric emptying after cytoreductive surgery and HIPEC: a randomized clinical trial. Eur J Surg Oncol. 2011;37:162–167.
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228.
- Karoui M, Tayar C, Laurent A, et al. En bloc stapled diaphragmatic resection for local invasion during hepatectomy: a simple technique without opening the pleural cavity. Am J Surg. 2007;193:786–788.
- Sugarbaker PH. Peritoneal metastases invading the seminal vesicles: radiologic appearance and outcome of treatment. Eur J Surg Oncol. 2018;44:805–809.
- Elias D, Borget I, Farron M, et al. Prognostic significance of visible cardiophrenic angle lymph nodes in the presence of peritoneal metastases from colorectal cancers. Eur J Surg Oncol. 2013;39:1214–1218.
- Cowan RA, Tseng J, Murthy V, et al. Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol Oncol. 2017;147:262–266.
- Passot G, Vaudoyer D, Villeneuve L, et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113:796–803.
- Kusamura S, Gonzalez-Moreno S, Nizri E, et al. Learning curve, training program, and monitorization of surgical performance of peritoneal surface malignancies centers. Surg Oncol Clin N Am. 2018;27:507–517.