Abstract
Background: Interventional radiology, thanks to its low invasiveness and possibility to reduce the average time for the patients to come back to their normal activity, is becoming more and more promising and diffused in multiple fields. Employed without needles, MRgFUS is probably the less invasive techniques among the ones belonging to the field of interventional radiology.
Purpose: To evaluate safety and effectiveness of MRgFUS in the treatment of a rare and benign, though disabling, bone lesion: intra-articular osteoblastoma.
Materials and methods: A retrospective study was carried out on 6 patients (mean, 21 years) treated in the last 2 years with MRgFUS for symptomatic, histologically proved intra-articular osteoblastoma. The main inclusion criterion was the presence of a good acoustic window. The procedures consisted in MR-guided ablation, using high intensity ultrasound beams focused on the target lesion. Spinal anesthesia or peripheral nerve block was used. Clinical (based on pain and functional scales) and imaging follow-up studies were performed up to 1 year after treatment. Complications were recorded. Multiple linear regression and analysis of variance were used to assess correlations.
Results: All the procedures were technically successful; no complications were observed. Painful symptomatology decreased of 88% at 6 months and 98% at 12 months (p < 0.0001), and was associated to functional improvement (p = 0.002). MRI and CT controls showed disappearance of all signs of disease and bone inflammation with a marked tendency to bone healing.
Conclusion: This study shows the safety and effectiveness of MRgFUS in the treatment of intra-articular osteoblastoma with a good acoustic window.
Introduction
Osteoblastoma (OB) is a benign bone-forming tumor composed of woven bone spicules, and it accounts for approximately 1% of all bone tumors [Citation1]. OB and osteoid osteoma (OO) bear a striking histological resemblance to each other, and often they can only be differentiated by their dimensions (OO is usually characterized by a nidus measuring <1 cm) [Citation2]. OB is biologically more active than OO. Aggressive growth can occur [Citation2] and an associated tendency to grow in size is important for differential diagnosis and treatment. OB usually affects patients aged 10–30 years.
In up to one-third of the cases, OB occurs in the posterior elements of the spine [Citation1]. In the appendicular skeleton, they are particularly prevalent in the metaphysis of the femur and tibia, and only a low percentage of OBs occur at intra-articular sites [Citation3]. Intra-articular occurrences are unusual and diagnostically challenging [Citation4–6]. Symptoms are usually severe. Pain is the main symptom (the response to salicylates is lower than in OO) [Citation2] and the severe inflammation induced by the tumor leads to symptomatic synovitis with functional impairment of the affected joint. A treatment is mandatory as soon as possible.
The most common treatment options include surgery (now considered very invasive) [Citation7] and minimally invasive interventional radiology (IR). With regard to the latter, percutaneous ablation by needle (radiofrequency ablation) is an increasingly employed modality, particularly in the spine [Citation8]. It appears to be safe and effective. Due to intrinsic characteristics of ablation however (it usually entails a geometric volume of ablation), cartilage damage or synovial alterations can occur after treatment and this can result in precocious onset of osteoarthritis [Citation9]. Another IR technique known as magnetic resonance-guided focused ultrasound surgery (MRgFUS) is an evidently viable less invasive approach [Citation10–14]. MRgFUS is a needleless ablation technique that can be considered an advance with regard to reduction of invasiveness. Due to the lack of a needle there is no cutaneous incision. Ablation is achieved via the delivery of small amounts of energy that can accurately destroy only the lesion, avoiding the healthy tissue around it. The main limitation of MRgFUS is that it is constrained by the availability of an acoustic window (the possibility that an ultrasound beam can reach the lesion) (). The absence of an acoustic window completely negates the procedure.
Figure 1. Intra-articular osteoblastoma of the elbow (distal humerus). Transverse T2-weighted MRI obtained during treatment: the # indicates the transducer that generates the ultrasound; the dashed arrow is the US pathway between the transducer and the lesion (dashed line) that is free from structures that could interfere with the progression of the US beam.
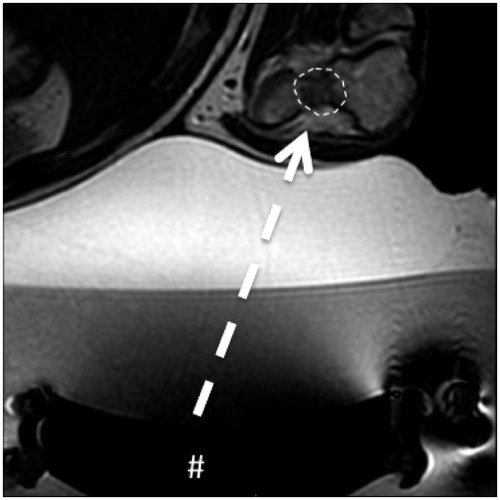
The purpose of the current study was to evaluate the efficacy of MRgFUS for the treatment of intra-articular osteoblastoma (IO) in terms of relief from symptomatology, and investigate imaging characteristics and technical considerations.
Materials and methods
Patients
The treatments and results thereof of 6 patients referred to us for severe pain and functional joint impairment with suspected diagnoses of OB were retrospectively analyzed (). Baseline evaluation consisted of perusal of MRI (3-T Signa unit, GE Healthcare, Milwaukee, WI) and CT (Aquilion One unit, Canon, Tokyo, Japan) scans of each patient. MRI included standard sequences (T1, T2, and short TI inversion recovery) on axial, coronal, and sagittal planes depending on the site of the lesion. CT examinations were limited to the areas of interest. Two radiologists with experience in the musculoskeletal field evaluated the images and a putative radiological diagnosis of IO was made. The radiologists also confirmed the feasibility of MRgFUS treatment due to the presence of a good acoustic window. During the same period of selection and treatment (2 years) further four patients with IO were not treated with MRgFUS due the lack of acoustic window (RFA was performed). Notably, all patients underwent CT-guided biopsy to confirm the diagnosis of IO prior to MRgFUS treatment. A baseline clinical evaluation also included the severity of pain symptoms and functional impairment as rated by the patient on separate visual analog scales (VASs) ranging from 0 to 10. Written informed consent to the treatment was obtained from patients on the day of treatment. The follow-up period was 12 months.
Table 1. Baseline characteristic of patient population.
Treatment
All patients were treated using a 3-T MRI unit with ExAblate 2100 (InSightec Ltd., Tirat Carmel, Israel) ( and ). The technical details of the technique used have been described previously [Citation10,Citation15]. Briefly, the process consists of a series of sequential administrations of doses of thermal energy measured in joules (J) in the form of an ultrasound beam, called “sonications,” focused at the site of the lesion. Each sonication lasts for a few seconds and can ablate few cubic millimeters of lesion. The treatment is considered complete and successful when the entire volume of the lesion has been ablated by multiple sonications. Each sonication was considered effective (and the tissue successfully destroyed), if a temperature of ≥60 °C was reached; otherwise the sonication was repeated until an effective temperature was achieved. It is possible to monitor the temperature reached in the lesion and surrounding tissue via specific MRI sequences [Citation10]. Briefly, proton resonance frequency (PRF) sequences are used: they are a subtraction technique that work measuring the temperature through measure of the phase changes (that are temperature-dependent) of the hydrogen bonds during the sonications. In the sequences, there is a subtraction of a pre-heated phase from a heating phase: so it is possible to calculate the phase change in tissue and therefore the change in temperature ().
Figure 2. Intra-articular osteoblatoma of the femoral neck. (a) Transverse T1-weighted fat-suppressed MRI with intra-articular administration of Gd-contrast: the lesion is indicated by the dashed line. (b) Transverse T2-weighted fat-suppressed MRI. Note the edema around the lesion (@) and the intra-articular reactive fluid (arrow) less evident that in the previous case as showed in Figure 1 and in this figure. (c) Transverse T2-weighted MRI obtained during treatment: the # indicates the transducer that generates the ultrasound; the dashed arrow is the US pathway between the transducer and the lesion (dashed line). (d) Transverse T2-weighted fat-suppressed MRI during the follow up: complete disappearance of the bone edema and of the reactive intra-articular fluid.
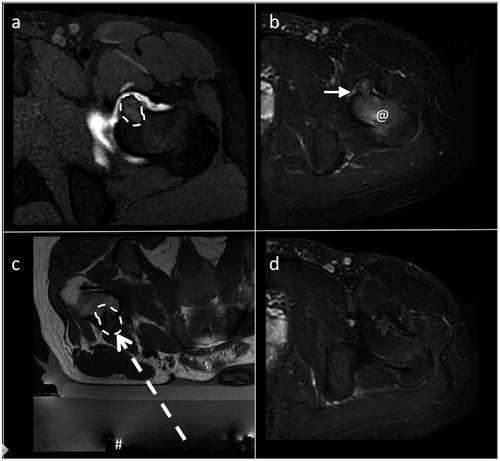
Table 2. Timing of the treatment.
Different anesthesiologic approaches were used depending on the site of the lesion. Patients with lesions located in the upper arms were administered US-guided peripheral nerve block, and spinal anesthesia was administered in patients with lesions affecting the hip joint. After the procedure pain was palliated with morphine, and gastro-protective and anti-emetic drugs were administered via elastomer for a total of 8 h. Additional cortisone therapy was administered during the following week. After the treatment, patients spent the night in the ward.
Follow-up analysis
Pretreatment, the dimensions of each lesion were calculated by measuring its maximum diameter, area, and volume. These data were acquired via the lesion management tool incorporated into the Carestream Health System (Rochester, NY) (Vue PACS, version u.11.3.2.4051). With regard to treatments, we analyzed the number of sonications performed, the energy delivered, the duration of the procedure, and the temperature reached during each sonication (all expressed as medians and means ± SD).
We investigated correlations between lesion size and treatment characteristics (number of sonications, energy delivered, duration of the procedure, and temperature reached).
Clinical and imaging (MRI and CT) examinations were performed prior the treatment and 6 and 12 months thereafter. Changes in functional impairment after treatment were investigated after dividing the patients into two groups based on the period of time between the onset of symptoms and the treatment. Three patients treated within 1 year of the onset of symptoms were assigned to group A, and three patients treated more than 1 year after the onset of symptoms were assigned to group B.
Using the imaging scans, the presence of bone edema and synovitis was assessed on a scale of 0–3, where 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. Bone remodeling was also investigated by using a region of interest on the lesion to measure the bone density determined via CT in Hounsfield units (HU) before treatment and 1 year thereafter (). The values were normalized using healthy bone located as close as possible to the lesion. Correlations between bone remodeling, patient characteristics (age and body mass index), and lesion parameters (dimensions and CT-determined density) were assessed in order to investigate the possibility of generating a prediction pattern pertaining to bone remodeling. Furthermore, using the above-mentioned sequences – particularly T1 sequences and CT – we investigated the health of the subchondral bone (and cartilage profile, when appropriate) around the treated lesions, and the morphology of the bone segment treated with regard to “restitutio ad integrum.”
Figure 3. Intra-articular osteoblastoma of the elbow (distal humerus), the same lesion of . (a) and (b) Transverse T2-weighted fat-suppressed MRI and (c) and (d) axial CT: the lesion is indicated by a dashed line in (a) and by an arrow in (c). In (b) and (d), note the disappearance of the lesion (bone healing), of the bone edema, and of the reactive intra-articular fluid.
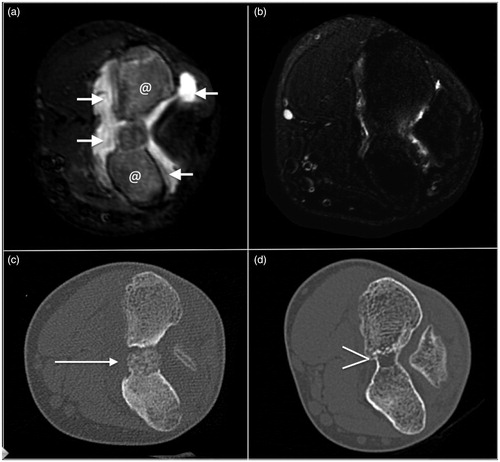
Statistical analysis
Multiple linear regression and analysis of variance were used to assess correlations. The t-test was used to identify significant differences in results during the follow-up period. An alpha error of 5% was used as the threshold of significance. All statistical analyses were performed using the XL STAT software package (StataCorp, College Station, TX).
Results
In all 6 patients (mean age 21.2 ± 8.3 years), after the diagnosis was confirmed histologically they were treated successfully from a technical perspective. With regard to lesion sites, there were three in the distal humerus, one in the humeral head, and two in the acetabular bone. No periprocedural or delayed complications were recorded.
Treatment variability
The treated lesions had a mean maximum diameter of 12.7 ± 4.7 mm and the median was 13 mm. The mean lesion area was 107.17 ± 62.87 mm2 and the median was 109.35 mm2. The mean lesion volume was 1043.02 ± 820.49 mm3 and the median was 969.10 mm3. The mean number of sonications performed during the treatments was 10.6 ± 5.0 and the median number was 9. The mean energy delivered during each sonication was 940 ± 579.05 J and the median was 694 J. The mean duration of each sonication was 32.8 ± 11.5 s and the median was 26 s. The mean temperature reached within the lesions during the treatments was 63.71 ± 14.83 °C and the median was 60 °C. When a temperature lesser then 56 °C was obtained, the sonication was repeated increasing just the energy to reach a temperature of at least 60 °C.
In multiple linear regression analysis, variability of the number of sonications and the duration of the procedure were associated with an adjusted R2 value of 0.457, which was not statistically significant. In the analysis of variance, lesion characteristic and treatment variables (with regards for the duration of the procedure in terms of number of sonications) were not significantly correlated ().
Table 3. ANOVA analysis between lesions size and treatment variable.
Image analysis
All patients exhibited severe bone edema before treatment (mean 3 ± 0, median 3), associated with moderate-to-severe synovitis (mean 2.33 ± 0.82, median 2.50). Significant reduction of bone edema was observed at the 6-month follow-up timepoint, with a mean score of 0.83 ± 0.75 (median 1) (p = 0.001). In most cases there was complete resolution after 1 year, with a mean score of 0.17 ± 0.40 (median 0) (p < 0.0001). Synovitis was significantly improved after 6 months (mean score 1.33 ± 0.82, median 1) (p = 0.033), up to a mean score of 0.833 ± 1.17 (median 0.5) (p = 0.021) after 12 months ( and ).
One patient had periostitis pretreatment that had disappeared completely by the 1-year follow-up timepoint.
With regard to bone remodeling, the treated lesions had a mean bone density of 239.06 ± 131.32 HU (median 239.33), with a normalized mean value of 0.77 ± 0.42 (median 0.7). After 1 year, the same lesions had a mean bone density of 558.17 ± 210.13 HU (median 541.52), with a normalized mean value of 1.96 ± 1.30 (median 1.7). The mean bone densification was 51 ± 27% (median 47%). The increase in lesion HU values was significant in all cases (p = 0.037) (.
Figure 4. Sagittal reformatting of CT of the cases of the previous figures before (a) and (c) and 1 year after treatment (b) and (d). Note the complete disappearance and ossification of both the lesions with the restitutio ad integrum of the bone segments.
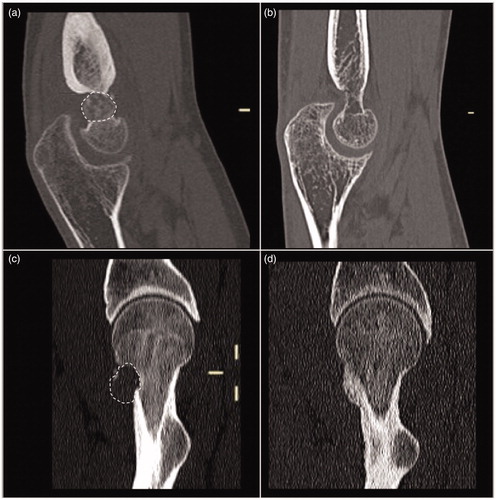
In the analysis of variance, no factors (age, body mass index, lesion dimensions, or CT-determined density) were significantly correlated with bone remodeling (p = 0.119, 0.776, 0.755, and 0.526, respectively). Multiple linear regression analysis yielded respective adjusted R2 values of −0.551 and 0.326, and none of the factors analyzed were significantly correlated in the type III sum of the square test.
No signs of subchondral damage were recorded in the tissues surrounding the lesions treated. The subchondral bone exhibited a regular intensity signal on T1 sequences without modification compared with the pretreatment examinations. Moreover, in cases where the lesion treated was very close to cartilage, no alterations in the cartilage were detected during follow-up. In the qualitative assessment of bone morphology, we detected progressive restitutio ad integrum of the bone segment treated in all patients, and at the 1-year follow-up timepoint this process was almost complete in 4 of the 6 cases.
Clinical results
Before treatment all patients had disabling conditions involving severe pain (mean VAS score 8.83 ± 0.75, median 9) and significant functional impairment (mean VAS score 8.33 ± 1.75, median 9). After treatment all patients exhibited significant improvements in pain, with means of 1.00 ± 0.89 (median 1) after 6 months and 0.16 ± 0.41 (median 0) after 1 year. In all cases, the decrease in VAS score was statistically significant (p < 0.0001).
Functional impairment was significantly improved after 6 months (mean VAS score 4.00 ± 2.53, median 5, p = 0.008) and after 1 year (mean VAS score 2.17 ± 2.32, median 1.5, p = 0.002). With regard to the period of time from the onset of symptoms to treatment, group A exhibited a pretreatment mean functional impairment score of 7.33 ± 2.08 (median 8), and this was significantly reduced at 6 months (mean 2 ± 2, median 2, p < 0.0001) and it was further reduced at 1 year (mean 0.33 ± 0.57, median 0). In group B, the reductions were not significant, with the pretreatment mean of 9.33 ± 0.58 (median 9) dropping to 6.00 ± 0.00 (median 6) after 6 months and to 4.00 ± 1.73 (median 5) after 1 year (respective p values = 0.205 and 0.144). Functional impairment VAS scores were not significantly correlated with pain VAS scores (p = 0.135).
Discussion
In this retrospective study, we describe the clinical and imaging outcomes in the only series of histologically confirmed IO cases treated with MRgFUS reported in the literature.
The most diffuse surgical approaches for this pathology include arthroscopy and open techniques such as excisional biopsy, intra-regional curettage, and en bloc excision, which require prolonged hospitalization, and recovery times. The main complications are a risk of avascular necrosis, precocious onset of osteoarthritis, and synovial adhesion [Citation16–18]. One case of coxa magna occurring after an excisional biopsy of an intra-articular OO has also been described [Citation19]. Because of their lower invasiveness, IR techniques, and in particular, percutaneous ablation by needle under CT guidance, are currently considered the first-line treatment for benign bone lesions such as OO and OB. Nevertheless, damage to the cartilage of the intra-articular structures (probably related to thermal ablation rather than needles), infections, skin burns, bleeding, nerve injury, tendonitis, thrombosis, and pathological fractures have been described [Citation9,Citation16,Citation20].
MRgFUS is a viable less invasive approach, well-established for definitive or palliative treatment of primitive or secondary bone lesions [Citation10,Citation12,Citation15,Citation21,Citation22].
All patients were suffering prior to treatment, with severe pain, impaired movement, and clumsiness. In some cases the nonspecific nature of the articular pain and impairment caused a delay in the diagnosis of OB of up to 2 years. Even though MRI and CT examinations were suggestive of OB (with signs of severe inflammation), in order to acquire histological diagnoses all patients underwent CT-guided biopsy, which confirmed the diagnosis in all cases. MRgFUS was proposed to all patients as a first-line treatment, due to the lesion sites and accessibility.
The main contraindication for MRgFUS is the absence of an acoustic window [Citation15,Citation23], which is defined as a linear pathway from the skin to the lesion that is followed by the ultrasound beam and must be free from everything that can reflect or deviate the ultrasounds (i.e., bone, air, metallic devices, among others). All patients in the present series had a good acoustic window, which as well as being a requirement for the treatment is also a positive prognostic factor because it enables the use of a moderate level of energy, thus reducing the risks of articular inflammation, tendonitis, fat edema, and skin burns that are the most common adverse events associated with this type of treatment described in literature [Citation24]. During the planning of treatment of pseudoinflammatory benign bone lesions, including OO, the presence of an exuberant periosteal reaction (induced by the inflammatory lesion) around the lesion is sometimes detected.
Due to the thickness and density of the periosteal reaction it may necessitate the use of increased levels of energy to allow conductive heating to reach the nidus rather than direct heating. This thickening may contraindicate the treatment entirely because it makes it impossible to achieve an effective temperature in the nidus. An increase in energy is essentially created by increasing the ultrasound power, increasing the time of the sonication or increasing both together. An increase in local bone surface heating due to higher energy levels however, may cause thermal injury in the surrounding tissue. Due to the lack of periosteum in intra-articular lesions intense periosteal activity is not common and accordingly these types of lesions are usually suitable for treatment. In the present series, no periosteal reactions were detected.
The most informative results of the present study are those pertaining to safety. No complications were recorded. In our opinion, this was mainly due to very accurate control during the delivery of energy, in terms of both release location and the amount of energy released. MRI revealed a very detailed depiction of the anatomy of the region of interest, facilitating planning of the best pathway for the therapeutic ultrasound beam that avoided sensitive structures. Magnetic resonance thermometry enables evaluation of the temperature reached in the area of interest in real time. Thus, it is possible to use only the amount of energy needed to ablate the pathological tissue, and avoid damaging the surrounding structures. It is also advantageous that the system works via the release of single doses of energy (called sonications). The overall ablation is generated by the sum of all the sonications. Even though a single sonication can cause damage, the procedure is relatively controllable and safe because the total amount of energy needed to complete the treatment is split into multiple smaller amounts of energy. The absence of signs of chondral or subchondral damage in the present series confirms the very minimally invasive nature of MRgFUS. It suggests that the thermal energy, once that the operator has checked that the US beam is correctly focused on the target using thermometry and MR imaging, can be only applied precisely to the pathological tissue. Indeed, it is possible to draw a sharp border between the tissue to be ablated and the tissue to be protected.
From a clinical perspective, very good results were obtained in the current series with respect to pain relief. Because the lesion itself is responsible for pain, reduction in bone pain is the first biological indicator of the success of the procedure. All patients exhibited a significant decrease in VAS score after 6 months, and complete resolution after 1 year (p values < 0.0001). The results pertaining to functional impairment differ slightly. Unfortunately, even where ablation removes the acute stimulus (resulting in pain relief), this may not be sufficient to resolve chronic alterations such as synovial thickening and calcification that result from a long period of severe inflammation. In the subgroup analysis in the present series, there was a significant resolution of functional impairment in patients in which the diagnosis and treatment were performed shortly after symptoms onset (group A). In contrast, due to chronic alterations, patients in which diagnosis was delayed only exhibited moderate functional improvement. The lack of a significant correlation between VAS-determined pain change and VAS-determined functional impairment changes suggests that they may be independent of each other, and, therefore, pain resolution does not correspond to complete physical restitutio ad integrum. In these patients (group B), physical rehabilitation is probably also necessary.
In the imaging analysis, we observed significant reductions in bone edema and synovitis, with complete resolution. These findings confirm the effectiveness of the treatment, and in particular, the complete ablation of the lesion (i.e., the source of inflammatory stimuli). Another observation pertaining to bone remodeling was a tendency to achieve restitutio ad integrum in terms of both bone densification and bone morphology (. In the analysis of bone densification, Student’s t-test yielded a significant increase in lesion density, indicating the presence of significant bone remodeling after the treatment. Ossification was not significantly correlated with patient characteristics or lesion characteristics, suggesting that bone remodeling was only a consequence of the procedure. This attests to the high capacity of MRgFUS treatment to induce complete destruction of the pathological tissue that results in bone densification [Citation25].
In the analysis of variance, the number of sonications was not significantly associated with the dimensions of the lesion expressed as area, volume, or longitudinal diameter. During the MRgFUS procedure, the system provides the specific number of sonications required to cover the entire lesion, thus, it could be assumed that the bigger the lesion the longer the treatment duration (interpreted as the sum of sonications). Instead, however, there was a lack of statistical significance in the present study. There are two probable reasons for this. The effect of each single sonication on the lesion has to be validated by the operator. To achieve technical success during the treatment, the main parameter to be considered is the temperature reached in the focal area during each sonication. This evaluation is conducted by the interventional radiologist, and thermal coagulation is obtained by maintaining a temperature > 56 °C for > 1 s. Via this real-time analysis each sonication is judged as effective or not. In the latter case, the sonication must be repeated, and accordingly the total number of sonications will be increased. The experience of the operator conducting the treatment is paramount. Another consideration is that we only compared lesions with small diameters and lesions confined within a well-defined range (12.7 ± 4.71 mm). The differences in the numbers of sonications used to ablate the entirety of the lesions may have been too small to yield statistically significant relationships between the number of sonications used and other parameters of the lesions.
This current study had some limitations. One was the low number of patients, and another was the lack of comparisons with other minimally invasive techniques. These two limitations are related, and they are due to the epidemiological rarity of the disease. Notably, however, to the best of our knowledge, this is the largest reported series of treated cases of IO. Moreover, the use of a single technique negated dispersal of the already small number of subjects into the multiple groups required for a comparative study. A multicenter study including different techniques of ablation (radiofrequency ablation in particular) may be useful to clarify the main role of interventional radiology in the management of this condition.
The results of the current study suggest that MRgFUS is a very safe and effective technique to treat IO. Its main strengths are a capacity to deliver energy in an extremely precise way, and the very accurate depiction of the pathological tissue and sensitive structures to be spared, due to the incorporation of MRI. The main drawback is the absolute requirement of an acoustic window to reach the lesion with the ultrasound beam.
Acknowledgments
The authors wish to thank Angela Martella for translating the manuscript. All procedures in this study were performed in accordance with the Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Fletcher CD, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. World Health Organization Classification of tumours. Lyon: Press IARC, 2002; p. 1–415.
- Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993;22:485–500.
- McLeod R, Dahlin D, Beabout JW. The spectrum of osteoblastoma. Ajr Am J Roentgenol. 1976;126:321–335.
- Abolghasemian M, Rezaie M, Behgoo A, et al. Exostosis-Like Intra-articular periosteal osteoblastoma: a rare case. Am J Orthoped. 2010; 39:E50–E53.
- Franceschi F, Marinozzi A, Papalia R, et al. Intra- and juxta-articular osteoid osteoma: a diagnostic challenge. Arch Orthop Trauma Surg. 2006; 126:660–667.
- Barca F, Leti Acciaro A, Spina V. Intra-articular osteoid osteoma of the lower extremity: diagnostic problems. Foot Ankle Int. 2002; 23:264–267.
- Angelini A, Varela-Osorio AF, Trovarelli G, et al. Osteoblastoma of the elbow: analysis of 13 patients and literature review. Eur J Orthop Surg Traumatol. 2017;6:1–9.
- Arrigoni F, Antonio B, Luigi Z, et al. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow- up. Int J Hyperthermia. 2017;0:1.
- Bosschaert PP, Deprez FC. Acetabular osteoid osteoma treated by percutaneous radiofrequency ablation: delayed articular cartilage damage. JBR-BTR. 2010;93:204–206.
- Napoli A, Mastantuono M, Cavallo Marincola B, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology. 2013;267:514–521.
- Geiger D, Napoli A, Conchiglia A, et al. MR-guided Focused Ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: a prospective multicenter evaluation. J Bone Joint Surg Am. 2014;96:743–751.
- Barile A, Arrigoni F, Zugaro L, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34:1–11.
- Rodrigues DB, Stauffer PR, Vrba D, et al. Focused ultrasound for treatment of bone tumours. Int J Hyperthermia. 2015;31:260–271.
- Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34:1–10.
- Masciocchi C, Conchiglia A, Gregori LM, et al. Critical role of HIFU in musculoskeletal interventions. Radiol Med. 2014;119:470–475.
- Spiker AM, Rotter B-Z, Chang B, et al. Clinical presentation of intra-articular osteoid osteoma of the hip and preliminary outcomes after arthroscopic resection: a case series. J Hip Preserv Surg. 2018;5:88–99.
- Willimon SC, Briggs KK, Philippon MJ. Intra-articular adhesions following hip arthroscopy: a risk factor analysis. Knee Surg Sports Traumatol Arthrosc. 2014;22:822–825.
- Marwan YA, Abatzoglou S, Esmaeel AA, et al. Hip arthroscopy for the management of osteoid osteoma of the acetabulum: a systematic review of the literature and case report. BMC Musculoskelet Disord. 2015;16:1–8.
- Arauz S, Morcuende JA, Weinstein SL. Intra-articular benign osteoblastoma of the acetabulum: a case report. J Pediatric Orthopaed. 1999;8;136–138.
- Albisinni U, Bazzocchi A, Bettelli G, et al. Treatment of osteoid osteoma of the elbow by radiofrequency thermal ablation. J Shoulder Elbow Surg. 2014;23:e1–e7.
- Masciocchi C, Zugaro L, Arrigoni F, et al. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol. 2015;26:1–10.
- Masciocchi C, Arrigoni F, La Marra A, et al. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89:20150356–20150358.
- Schlesinger D, Benedict S, Diederich C, et al. MR-guided focused ultrasound surgery, present and future. Med Phys. 2013;40:080901–080932.
- Napoli A, Bazzocchi A, Scipione R, et al. Noninvasive therapy for osteoid osteoma: a prospective developmental study with MR imaging-guided high-intensity focused ultrasound. Radiology. 2017;285:186–196.
- Napoli A, Anzidei M, Marincola BC, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol. 2013;48:351–358.
