Abstract
Purpose: Patients with oligometastatic non-small-cell lung cancer (NSCLC) benefit from local control treatments such as surgery or irradiation. The efficacy and safety of microwave ablation (MWA) in these patients was unknown.
Material and methods: Between January 2011 and April 2018, eligible patients were retrospectively enrolled. MWA was conducted for both primary lesions and metastatic lesions in patients with synchronous metastases and in metastatic lesions for patients with metachronous metastases. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were overall survival (OS), technical success, technique efficacy, and complications.
Results: Seventy-nine patients with 103 oligometastatic lesions were enrolled. A total of 20 primary lesions and 96 metastatic lesions were treated with MWA during 101 procedures. Technical success was achieved in all patients. Technique efficacy was achieved in 72 patients (91.1%). The median PFS and OS were 14.0 and 47.8 months, respectively. Forty-four patients (55.7%) developed complications with 21 (29.6%) of these patients developing major complications. All complications were resolved via appropriate medical treatments, and no MWA-related deaths occurred.
Conclusion: MWA was safe and effective for patients with oligometastatic NSCLC.
Introduction
Lung cancer remains the leading cause of cancer-related morbidity and mortality in China [Citation1,Citation2], with non-small-cell lung cancer (NSCLC) being the more common type of small-cell lung cancer. Most patients with NSCLC are diagnosed in the advanced stage, and the overall prognosis of advanced NSCLC remains poor [Citation3,Citation4], despite substantial improvements in treatment response and survival owing to targeted and immune therapies [Citation5–19].
The oligometastatic state was first proposed by Hellman and Weichsel Baum in 1995 and referred to an intermediate status between the locally advanced phase and widely metastatic phase [Citation20]. In general, oligometastases is defined as a state of limited (≤5) metastatic lesions that are eligible for radical local therapy, which could achieve long-term survival [Citation21]. Oligometastases can be divided into two main types: synchronous (i.e., synchronous primary tumor and oligometastases) and metachronous (i.e., oligometastases that develop after radical treatment of primary tumors and is also called oligo-recurrence) [Citation22].
For patients with oligometastases, local therapies, including surgery and irradiation, could be administered with cytoreductive intent. A phase II study reported a median progression-free survival (PFS) and overall survival (OS) of 11.6 months and 23 months, respectively, in 26 patients with oligometastatic NSCLC with ≤5 metastatic lesions that were treated with radical irradiation in both primary and metastatic sites [Citation23]. Recently, two randomized phase III trials reported that among patients with oligometastatic NSCLC, those treated with consolidative local therapy for all tumors and who received maintenance treatment after systemic treatment had superior PFS than those treated with maintenance treatment alone (PFS: 9.7–11.9 vs. 3.3–3.5 months) [Citation24,Citation25].
Microwave ablation (MWA) has been used in treating advanced NSCLC. The median PFS of patients with advanced NSCLC treated with chemotherapy and MWA was 8.6 months [Citation26]. One retrospective study and one prospective study also verified that compared with chemotherapy alone, the combination regimen of MWA and chemotherapy yielded longer PFS (7.3–10.3 vs. 4.8–5.2 months) [Citation27–28]. Compared with stereotactic body radiation therapy, MWA had similar local control rates and shorter treatment intervals. Moreover, most of the MWA-associated complications, including pneumothorax, pleural effusion, hemothorax, and pulmonary infection, are manageable. Ablation-associated death has also been rarely reported [Citation29].
To date, no study has assessed the safety and efficacy of MWA in patients with oligometastatic NSCLC. Thus, this retrospective study aimed to evaluate the efficacy and safety of MWA for oligometastatic NSCLC.
Materials and methods
Inclusion and exclusion criteria
Patients were retrospectively enrolled according to the following inclusion criteria: (1) pathologically confirmed NSCLC; (2) stage IV disease or recurrence after radical surgery or radical local control treatments; (3) ≤5 metastatic lesions; (4) no history of systemic treatment; (5) both primary and secondary pulmonary tumors are located in the lung periphery and measure ≤5 cm in size; (6) all tumor lesions eligible for radical local control treatments, such as surgery, irradiation, or MWA; (7) adequate cardiovascular, pulmonary, hepatic, and renal function to receive both local and systemic treatments; (8) no restriction to the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1, or programed death-ligand 1 status; (9) life expectancy of ≥6 months; and (10) could be followed up. The exclusion criteria were (1) mixed small-cell lung cancer and NSCLC; (2) secondary primary tumors developing within the past 5 years; (3) uncontrolled symptomatic brain metastases; (4) severe interstitial lung disease or acute myocardial infarction within 6 months before randomization; and (5) platelet count <100 000/μL. The study was approved by the ethics review board of Shandong Provincial Hospital affiliated to Shandong University, and written informed consent was obtained from all patients.
MWA procedure
MWA was performed using the MTC-3C MWA system (Vison-China Medical Devices R&D Center) with computed tomography (CT) guidance (Lightspeed 64 V, GE General Electric). The frequency was 2450 ± 50 MHz, and the adjustable output level of the continuous wave ranged from 0 to 100 W. The effective length was 100, 150 and 180 mm, and an outside diameter of 16, 18 or 19 G was used. A water circulation cooling system was used to reduce the surface temperature of the antenna. For tumors larger than 3.5 cm, two ablation antennae were applied. Local anesthesia (lidocaine) and preemptive analgesia (morphine) were administered before the procedure. The detailed methodology of MWA has been described in previous studies [Citation27,Citation28]. An immediate post-ablation ground glass opacity of 5 mm larger than the tumor was required. A second or even third ablation was performed for those with progression in the ablation lesions.
Treatments after MWA
Patients with sensitive mutations were treated with EGFR-tyrosine kinase inhibitors (TKIs) (e.g., gefitinib or erlotinib for EGFR-positive mutations) or ALK inhibitors (e.g., crizotinib for EML4-ALK fusion) until disease progression or intolerable toxicities occurred. For those without mutations or unknown mutation status, platinum-based (cisplatin and carboplatin) doublet chemotherapy or single-drug chemotherapy was administered. Chemotherapy was repeated every 3 weeks for up to 6 cycles. Patients that did not progress after 6 cycles were deemed eligible for maintenance therapy.
Response evaluation
Contrast-enhanced CT (CECT) was conducted every month for the first three months after MWA and then every three months thereafter. Treatment response was evaluated using CECT conducted every 6 weeks for patients treated with chemotherapy and every 2 months for those treated with targeted therapies. Treatment response to chemotherapy, targeted therapy, or irradiation was evaluated according to the Response Evaluation of Criteria in Solid Tumors version 4.0 [Citation30]. A tumor treated according to the protocol and that recovered completely as determined at the time of the procedure was deemed a ‘technical success’. ‘Technique efficacy’ referred to a defined prospective time point when ‘complete ablation’ of macroscopic tumor was achieved as noted on follow-up imaging [Citation31]. Complete ablation (i.e., lesion disappearance, complete cavitation formation, fibrotic progression or scar formation, solid nodule involution or no change, and/or atelectasis presenting no contrast-enhanced signs on CT images) was considered technical efficacy. Incomplete ablation was indicated by incomplete cavernous formation with some remaining solid or liquid components; partial fibrosis or fibrotic lesions with solid residues; and/or solid nodules with unchanged or increased size displaying irregular peripheral or internal enhancement signs on CT images [Citation32].
Statistical analyses
The primary study endpoint was PFS, which was calculated from the time of MWA to the date of disease progression or death, whichever came first. Time to local failure was calculated from the date of MWA to progression of previously treated metastatic lesions.
The Chi-square test was used to evaluate correlation. Kaplan–Meier curve with log-rank test was used for the univariate analyses, and factors with p values <.2 in univariate analyses were used in multivariate analyses. Cox regression analyses with stepwise forward regression were used for the multivariate analyses. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Both tests were two sided, and a p value of <.05 was deemed significant.
Results
Patient characteristics
From 1 January 2011 to 31 April 2018, 79 patients with oligometastatic NSCLC were retrospectively enrolled. The patients were predominantly men (n = 59, 74.7%), and the mean age was 64.9 (range, 33–81) years. Of the 79 patients, 69 (87.3%) and 10 (12.7%) had an ECOG PS of 1 and 0, respectively. The most frequent histology was adenocarcinoma (n = 54, 68.5%), and the majority of patients had metachronous oligometastases (n = 59, 74.7%). The most common treatment of the primary tumor in patients with metachronous oligo-disease was surgery (n = 49), followed by MWA (n = 5), and irradiation (n = 2). Excluding the M stage, most patients had T1 and T2 (n = 61, 77.2%) disease, and the majority (n = 70, 88.6%) had N0 or N1, including 49 postoperative patients.
Fifty-eight patients had 1 metastatic lesion, 18 had 2 lesions, and 3 patients had 3 lesions. The lung was the predominant metastatic site (n = 81), followed by the liver (n = 9), brain (n = 4), adrenal gland (n = 3), bone (n = 2), pleura (n = 2), and lymph node (n = 2). A total of 116 tumor lesions (including 20 primary tumors from synchronous oligometastases) were treated with MWA in 101 procedures, with the mean tumor lesion of 1.1 (range, 1–3) for each procedure. A total of 4 brain metastases, 1 bone metastasis, and 1 lymph node metastasis were treated with irradiation. One lymph node metastasis was treated with radioactive iodine-125 implantation.
EGFR and ALK status were evaluated in 47 and 37 patients, respectively. Among them, 12 patients had EGFR mutations and no patients had positive ALK fusion genes. Regarding treatments after MWA, 49 patients received chemotherapy, 10 received targeted therapy, and the other 20 were only observed. The baseline characteristics of the patients and treatment regimens are detailed in .
Table 1. Baseline characteristics and treatments of enrolled patients (N = 79).
Treatment response to MWA
Technical success was achieved in all patients, and technical efficacy was achieved in 72 patients (91.1%). Of the 7 patients who did not achieve technical efficacy, 3 received MWA, 1 received irradiation, and 3 received no subsequent local treatments.
PFS
The median PFS was 14.0 months (95% CI, 10.3–17.8). A total of 52 patients had tumor progression; of these, 5 had recurrent lesion in the ablation zones, 27 had new metastatic tumor lesions, 14 had both recurrent lesions in the ablation zones and new metastatic tumor lesions, and 6 patients died. Patients with the following characteristics had superior PFS: female sex (43.2 months (95% CI, 28.1–58.2) vs. 10.3 months (95% CI, 4.9–15.8) in males, p = .004); ECOG PS of 1 (14.2 months (95% CI, 9.0–19.4) vs. 6.5 months (95% CI, 4.7–8.2) in ECOG PS of 0, p = .046); adenocarcinoma (14.7 months (95% CI, 7.7–21.7) vs. 10.3 months (95% CI, 1.4–19.3) in non-adenocarcinoma, p = .026); metachronous disease (16.1 months (95% CI, 11.1–21.1) vs. 7.1 months (95% CI, 6.3–7.8) in synchronous, p = .007); underwent surgery for primary tumors (18.2 months (95% CI, 4.7–31.7) vs. 10.3 months (95% CI, 1.7–19.0) in irradiation or MWA, p = .013); complete ablation via MWA (14.6 months (95% CI, 10.0–19.3) vs. 2.4 months (95% CI, 2.3–2.5) in incomplete ablation via MWA, p = .000); underwent observation post-MWA (32.2 months (95% CI, 17.7–46.8) vs. 7.4 months (95% CI, 3.3–11.5) in chemotherapy and 18.2 months (95% CI, 8.5–27.9) in targeted therapy, p = .026); and achieved complete ablation via MWA (14.6 months (95% CI, 10.0–19.3) vs. 2.4 months (95% CI, 2.3–2.5) in incomplete ablation via MWA, p = .000) (, ).
Figure 1. Kaplan–Meier analyses of PFS. (A): sex; (B): ECOG PS; (C): pathology; (D): synchronous/metachronous oligometastases; (E): primary tumor treatment; (F): response to MWA; (G): treatment post-MWA.
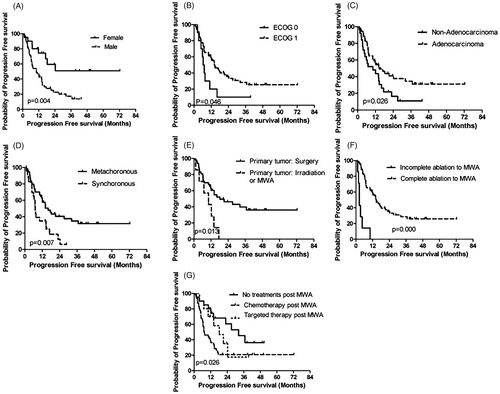
Table 2. Univariate analysis of PFS and OS.
The above factors with a significant difference in the univariate analysis were included in the multivariate analyses. Female sex (HR: 3.887, p = .019), metachronous disease (HR: 9.480, p = .010), surgery for primary tumor sites (HR: 3.613, p = .016), and complete ablation via MWA (HR: 0.024, p = .000) were independent prognostic factors for PFS ().
Table 3. Cox-regression multivariate analysis of PFS and OS.
Among the 19 patients with local progression, 16 received local treatments, i.e., MWA (n = 15 patients) and irradiation (n = 1 patients). As for the 41 patients who developed new lesions, 14, 5, and 1 patients were treated with MWA, irradiation, and radioactive iodine-125 implantation as local treatments, respectively. The most common site for the development of new lesions after ablation were the lungs (n = 21), followed by the lymph node (n = 6), bone (n = 5), brain (n = 5), liver (n = 4), adrenal gland (n = 3), chest wall (n = 1), pleural space (n = 1), and meninges (n = 8) (including 7 patients with two new lesions).
OS
The median OS was 47.8 months (95% CI, 40.4–55.2). Patients who underwent surgery for primary tumors had superior OS than those who underwent other treatments (55.3 months (95% CI, 46.4–64.3) vs. 37.2 months (95% CI, 26.3–48.1) in those who underwent irradiation or MWA, p = .007). Moreover, patients with the following characteristics tended to have superior OS: (1) female sex (56.9 months (95% CI, 44.7–69.2) vs. 45.1 months (95% CI, 36.4–53.7) in males, p = .051); (2) synchronous disease (51.3 months (95% CI, 42.3–60.3) vs. 39.6 months [95% CI, 26.8–52.3] in metachronous disease, p = .052); and (3) achieved complete ablation via MWA (49.2 months [95% CI, 41.4–57.1] vs. 27.3 months (95% CI, 10.0–44.5) in incomplete ablation via MWA, p = .079) (, ).
Figure 2. Kaplan–Meier analyses of OS. (A): sex; (B): synchronous/metachronous oligometastases; (C): primary tumor treatment; (D): response to MWA.
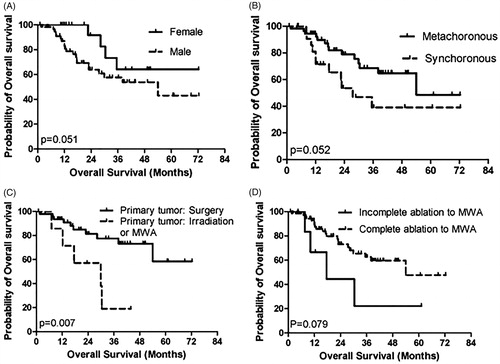
These factors were included in the multivariate analysis, and the results showed that only surgery for primary tumors was an independent prognostic factor for OS (HR: 3.679; 95% CI, 1.202–11.261; p = .022) ().
Safety
A total of 44 patients (55.7%) developed complications. The number of major and minor complications and adverse events were 21, 24, and 12, respectively. The complications included pneumothorax (n = 22), pleural effusion (n = 16), bleeding (n = 13), post-ablation syndrome (n = 7), pulmonary infection (n = 7), cardiovascular disease (n = 3), and bronchial fistula (n = 2). Eleven patients with pneumothorax or pleural effusion were treated via chest tube insertion, and 7 patients with pulmonary infection were treated with anti-bacterial or anti-fungal treatments. All patients recovered after the treatment. No ablation-associated death occurred during the peri-ablation period. Only age was correlated with the occurrence of complications (). Those aged 65 years old or more tended to more commonly develop complications (68.4%) compared with those less than 65 years old (43.9%, p = 0.028).
Table 4. Correlation between baseline characteristics and MWA associated complications (N = 79).
Discussion
To the best of our knowledge, this is the first retrospective study to assess the safety and efficacy of MWA for oligometastatic NSCLC. Technical success was achieved in all patients, and technique efficacy was achieved in majority of the patients. Although complications developed during the peri-ablation period, no treatment-associated death occurred, and all complications were managed accordingly.
Oligometastatic disease of NSCLC is an intermediate phase between locally advanced stage and advanced multiple metastases. Among patients with oligometastatic NSCLC, those who underwent systemic plus cytoreductive treatments, particularly, irradiation or surgery, achieved better survival.
Collen et al. reported that patients with oligometastatic NSCLC having ≤5 metastatic lesions who were treated with irradiation to all tumor lesions had a median PFS and OS of 11.2 and 23 months, respectively [Citation23]. Gomez et al. and Iyengar et al. showed that patients with oligometastatic NSCLC had longer PFS (9.7–11.9 months) and OS (13.5–20.0 months) when treated with maintenance treatments and radical local treatments [Citation24,Citation25]. Iyengar et al. also showed that the use of stereotactic body radiation therapy with erlotinib as a second- or subsequent-line therapy for unresected patients with oligometastatic stage IV NSCLC resulted in high PFS and OS (14.7 and 20.4 months, respectively) [Citation33]. Ruysscher et al., in a phase II study comprising 40 patients with NSCLC and synchronous oligometastases, showed that radical surgery and irradiation yielded a median PFS and OS of 12.1 and 13.5 months, respectively [Citation34]. Griffioen et al. reported that their cohort of 61 patients with 1–3 synchronous metastases treated with irradiation with a radical intent to all sites of the disease had PFS and OS of 6.6 and 13.5 months, respectively [Citation35]. A systematic review of 49 studies that included 2176 patients with oligometastatic NSCLC reported a PFS of 12.0 (range, 4.5–23.7) months and OS of 14.8 (range, 5.9–52) months [Citation36]. The median PFS was similar with that reported in a previous study, while the OS was superior than that reported in most studies.
Several studies also demonstrated that the following characteristics were associated with survival: age; primary tumor size; primary tumor histology; AJCC stage of primary lung cancer; number of oligometastases; synchronous or metachronous disease; disease-free interval; extracranial metastases; EGFR or EML4-ALK mutations; type of thoracic resection; induction chemotherapy; planning target volume; and positron emission tomography response [Citation3,Citation24,Citation33,Citation35]. In the current study, we also verified that female sex, metachronous oligometastases, surgery for primary tumor sites, and complete ablation via MWA were independent prognostic factors of PFS, while only surgery for primary tumors was the independent prognostic factor of OS.
In patients with tumor progression of oligometastases, the most common site of new lesions were new regions, followed by lesions in both new regions and known regions and then lesions in known regions. Similar results were obtained in this study, with the brain, liver, and lymph node being the most common sites for new lesions. New lesions developing in other sites, such as the ovary, leptomeninges, thorax, pancreas, and spine, were also reported [Citation33]. Meanwhile, the common areas for recurrent lesions in primary disease sites were the lung, bone, liver, and adrenal gland.
MWA is safe for treating primary or secondary lung cancer. Zheng et al [Citation29] retrospectively enrolled 184 consecutive patients. In their study, 204 MWA sessions were performed for 253 lung tumor lesions. Major complications developed after 42 sessions in 20.6% of the patients, including 32 cases (15.7%) of pneumothorax requiring chest tube placement, 6 cases (2.9%) of pleural effusions requiring chest tube placement; 6 cases (2.9%) of pneumonia, and 1 case (0.5%) of pulmonary abscess. Procedure-related death occurred after 1 session (0.5%). In the current study, majority of patients also developed complications, but these were all resolved with medical treatment, and no procedure-related death occurred.
This study had some limitations. First, seven oligometastatic lesions were treated with radical methods besides MWA. Second, seven primary tumors of metachronous oligometastatic NSCLC were treated with MWA and irradiation. Third, 32 patients did not undergo EGFR tests, and 42 patients did not undergo ALK tests. Fourth, 20 patients refused to undergo post-ablation chemotherapy or targeted treatments.
In conclusion, MWA was effective and safe for patients with oligometastatic NSCLC. Our findings should be verified in future prospective studies.
Acknowledgments
The institutional review board of Shandong Provincial Hospital has approved this study. Informed consent was obtained from all individuals included in the study. The work has not been published elsewhere. All the authors listed have seen and approved the manuscript that is enclosed, contributed significantly to the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens 6 for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98.
- Doshi KH, Shriyan B, Nookala MK, et al. Prognostic significance of pretreatment sodium levels in patients of nonsmall cell lung cancer treated with pemetrexed-platinum doublet chemotherapy. J Cancer Res Ther. 2018;14(5):1049–1053.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957.
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742.
- Katakami N, Takada M, Yoshioka H, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128.
- Zhou B, Tang C, Li J. k-RAS mutation and resistance to epidermal growth factor receptor-tyrosine kinase inhibitor treatment in patients with nonsmall cell lung cancer. J Cancer Res Ther. 2017;13(4):699–701.
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019.
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–1012.
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:1627– 1135.
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924–3933.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10.
- Bergsma DP, Salama JK, Singh DP, et al. Radiotherapy for oligometastatic lung cancer. Front Oncol. 2017;7:210.
- Richard PJ, Rengan R. Oligometastatic non-small-cell lung cancer: current treatment strategies. Lung Cancer (Auckl). 2016;7:129–140.
- Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol. 2014;25:1954–1959.
- Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682.
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464.
- Wei Z, Ye X, Geng D, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a prospective, randomized, control, phase III clinical trial. J Clin Oncol. 2017;35:9048.
- Zheng A, Wang X, Yang X, et al. Major complications 20 after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98:243–248.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours 21 9: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: 22 standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Can Res Ther. 2018;14:730–744.
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32:3824–3830.
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol. 2012;7:1547–1555.
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous 23 oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer. 2013;82:95–102.
- Agolli L, Valeriani M, Nicosia L, et al. Stereotactic ablative body radiotherapy (SABR) in pulmonary oligometastatic/oligorecurrent non-small cell lung cancer patients: a new therapeutic approach. Anticancer Res. 2015;35:6239–6245.
