Abstract
Purpose: To compare the predictive value of albumin-bilirubin (ALBI) grade, platelet-ALBI (PALBI) grade and Child-Turcotte-Pugh (CTP) class in patients with large hepatocellular carcinoma (HCC) after transarterial chemoembolization (TACE) combined with microwave ablation (TACE-MWA).
Methods: A total of 349 consecutive HCC patients (89.1% male; mean [± SD] age 53.4 ± 12.27 years) from three medical centers, who underwent TACE-MWA for up to 3 HCCs with maximum diameters of 5.1–8.0 cm between January 2000 and June 2018, were investigated. Overall survival (OS) and progression-free survival (PFS) were analyzed. The prognostic performances of ALBI grade, PALBI grade and CTP class were compared.
Results: TACE procedures were performed using lobaplatin (20–50 mg), epirubicin (30–60 mg), lipiodol (5–25 mL) and gelatin sponge particles (350–560 μm). The end point of the TACE procedure was stasis of blood flow in the feeder artery. The median follow-up duration was 28.0 months, the median OS was 28.0 months (95% confidence interval [CI] 23.55–32.45 months), and the median PFS was 4.8 months (95% CI 4.26–5.34 months). Patients with a ablation margin size of 11–15 mm experienced better PFS than those with a margin size of 6–10 or 0–5 mm (median, 6.5 versus [vs] 4.0 vs 2.3 months; p < .001). PALBI grade demonstrated significantly greater area under the curve values than ALBI grade or CTP class in predicting 1-, 3- and 5-year OS.
Conclusions: PALBI grade provided better predictive value than ALBI grade or CTP class in patients with large HCCs after TACE-MWA.
Introduction
Hepatocellular carcinoma (HCC) is a serious health problem, and ranks as the sixth most common cancer and the third leading cause of cancer-related death in the world [Citation1,Citation2]. More than 700,000 new cases of HCC are diagnosed annually. Transarterial chemoembolization (TACE) has long been recommended as a standard treatment for unresectable HCCs [Citation3,Citation4]. However, previous studies have reported that the incidence of complete tumor response to TACE was only 10–20% according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines [Citation5–8]. Moreover, the median overall survival (OS) of HCC patients who underwent TACE ranged from 16 to 20 months [Citation5–8].
As radical treatments, radiofrequency ablation (RFA) and microwave ablation (MWA) are the two most often performed thermal ablation procedures for liver tumors. Ablation margin size has been validated as an independent prognostic factor of local tumor progression after thermal ablation of liver tumors [Citation9–11]. Wang et al. reported that the risk for local tumor progression decreased by 46% for each 5 mm increase in minimal margin size of RFA for hepatic metastatic disease from colorectal cancer [Citation11]. Compared with RFA, MWA is associated with other advantages, such as more spherical and predictable ablation zones, less susceptibility to the heat sink effect, and is less dependent on tissue properties [Citation12–16]. Nevertheless, when reviewing the performance of MWA in HCC patients, differences among available MWA devices should be considered [Citation17,Citation18].
Previous studies have shown that the combination of TACE and MWA (hereafter, TACE-MWA) can improve OS in patients with large HCCs, with better efficacy than either TACE or MWA alone [Citation19–21]. Therefore, TACE-MWA was introduced as an important treatment strategy for patients with large HCCs. Maluccio et al. reported that bland arterial embolization combined with ablation was effective in treating solitary HCC lesions up to 7 cm in size, and achieved similar OS to surgical resection in selected patients. Moreover, their data indicated that the Okuda stage was associated with OS [Citation22,Citation23].
In HCC patients, OS is a composite clinical endpoint due to the mutual influence of prognostic factors. Hepatic function is an independent predictor of OS in patients with HCCs [Citation24,Citation25]. Unfortunately, the vast majority of HCC patients exhibit hepatic dysfunction, despite the absence of overt hepatic cirrhosis. In current clinical practice, the Child-Turcotte-Pugh (CTP) classification system is a widely used tool for assessing hepatic function in patients with HCC. However, the CTP classification was originally developed for patients with hepatic cirrhosis. The albumin-bilirubin (ALBI) grade is a prognostic nomogram that emerged from a multivariate screen of routine clinicopathological variables in a large, international cohort of HCC patients, further validated in a separate group of cirrhotic patients without cancer [Citation26]. The ALBI grade incorporates two laboratory parameters – serum albumin and bilirubin concentration – and can provide a simple and objective method for assessing hepatic function with good prognostic performance in patients with HCC [Citation26–29]. Based on the ALBI grade, the platelet-ALBI (PALBI) grade was recently developed to account for the effect of portal hypertension, with platelet count acting as a surrogate for the severity of portal hypertension [Citation28,Citation30]. Previous studies have validated ALBI and PALBI grades as significant predictors of OS in HCC patients who underwent surgical resection, RFA, TACE or sorafenib therapy [Citation26,Citation28,Citation30,Citation31].
To our knowledge, however, no studies to date have compared the predictive value of the ALBI grade, PALBI grade and CTP class in patients with large HCCs after TACE-MWA. In this study, we hypothesized that the prognostic performance of the PALBI grade was better than that of the ALBI grade or CTP class in predicting long-term OS. Hence, the purpose of this study was to compare the predictive value of the ALBI grade, PALBI grade and CTP class in patients with large HCCs after TACE-MWA.
Materials and methods
Patients and study design
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center, the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (Guangdong, China) and the Fujian Provincial Hospital of Fujian Medical University (Fujian, China). Clinicopathological data from 641 patients, who underwent TACE-MWA for large HCCs between January 2000 and June 2018, were retrospectively reviewed. The follow-up was terminated on June 30, 2018. For patients who underwent TACE-MWA, TACE was performed first, followed by MWA 1–2 weeks later. MWA treatments were performed using local anesthesia and moderate intravenous sedation, and TACE treatments were performed using local anesthesia. If necessary, pethidine (60–75 mg) and valium (10 mg) were injected intramuscularly 5 min before either the MWA or TACE procedure to relieve pain and to achieve sedation. After the treatments, oxycodone hydrochloride controlled-release tablets or bonding transdermal fentanyl was used ‘on demand’ for management of abdominal pain. To avoid the occurrence of serious complications or adverse events, such as hepatic dysfunction, liver abscesses, or gastrointestinal hemorrhage, among others, the timing of MWA treatment depended on recovery after TACE. Among the 641 patients, approximately 75% (480/641) were treated with MWA within 2 weeks after undergoing TACE. The patients were discussed at multidisciplinary expert meetings, which included interventional radiologists, oncologists, hepatologists and pathologists, to determine therapeutic strategies for those with large HCCs.
In this study, HCC patients were diagnosed according to the criteria defined by the American Association for the Study of Liver Disease and the European Association for the Study of Liver. The clinical stage was confirmed according to the Barcelona Clinic Liver Cancer guidelines. The presence of hepatic cirrhosis was confirmed based on hepatitis B virus (HBV)/hepatitis C virus (HCV) infection and the findings from computed tomography (CT)/magnetic resonance imaging (MRI) examinations. Inclusion criteria for the present study were as follows: ≥18 years of age; Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; hepatic function of CTP class A or B; ≤3 HCC lesions that were 5.1–8.0 cm in maximum diameter; absence of evidence of intrahepatic vascular invasion and extrahepatic metastasis; absence of a history of surgical resection or other interventional treatments (e.g., 125I seed implantation, RFA, cryoablation, or percutaneous ethanol injection); absence of severe coagulation dysfunction (e.g., prothrombin activity <40%, international normalized ratio >1.26 and/or platelet count <50 × 109/L); absence of general infection, serious dysfunction of the heart or kidney, chronic obstructive pulmonary disease, recent stroke, or other uncontrolled comorbidities; absence of a combination of other malignancies; no previous targeted therapy (e.g., sorafenib) or immunotherapy (e.g., PD-1/PD-L1 antibody); and complete treatment and follow-up data.
Equipment
The Allura Xper FD 20 (Philips Healthcare, Best, Amsterdam, the Netherlands) digital subtraction angiography (DSA) instrument was used for the TACE procedures. A 64-slice spiral CT scanner (SOMATOM 64 Sensation, Siemens, Munich, Germany) was used for MWA puncture guidance and image acquisition. The microwave ablation system was a water-cooled microwave apparatus (Kangyou Institute, Nanjing, China) equipped with a monopole microwave antenna (16–18 G). Consumables included the puncture needle, the artery catheter sheath, the angiography catheter (Terumo, Tokyo, Japan), and the micro-catheter (Terumo, Tokyo, Japan), lipiodol (Lipiodol Ultrafluide; Guerbet, Aulnay-Sous-Bois, France), gelatin sponge particles (Alicon, Hangzhou, China), and chemotherapeutics including lobaplatin and epirubicin.
TACE procedure
TACE was performed under DSA guidance. Hepatic artery angiography was performed using a 5 Fr catheter (RH or Yashiro) to assess location, number, size and blood supply of the target tumors. Subsequently, a 2.7/2.8 Fr micro-catheter was super-selectively inserted into the tumor-feeding arteries. A solution of lobaplatin (20–50 mg [0.5 mg/mL]) was infused into the tumor-feeding arteries via micro-catheter, followed by slow injection of an emulsion of epirubicin (30–60 mg) mixed with lipiodol (5–25 mL). The dosages of chemotherapeutics and embolization emulsion were dependent on body weight and tumor status. Finally, gelatin sponge particles (350–560 μm) mixed with contrast agent were administered into the tumor-feeding vessels. The endpoint of the TACE procedure was defined as static flow in the tumor arteries and full saturation of the feeding arteries. Postoperative treatments, including recovery of hepatic function, prevention of infection and symptomatic treatment(s), were performed. Liver and kidney function, and tumor marker levels were evaluated 3–7 days after TACE and before MWA.
MWA procedure
Before the MWA procedure, a fine metal marker was placed on the body surface over the target tumor with the patient in a supine position. If the target tumor was located in challenging segments of the liver, such as segment I, patients were positioned prone or on their left side to obtain an effective path for puncture, and to avoid damage to important vessels or other normal organs. After routine preparation before the procedure, patients were put into deep sedation. A plain CT scan was first taken to confirm the puncture path and location of the target lesion. The puncture site was anesthetized using 2% lidocaine, and an MWA electrode probe was then inserted along the path to reach the opposite edge of tumor lesion through its center. After confirming the location of the MWA electrode probe, MWA treatment was performed. Microwave power was set to 55–70 W and the procedure lasted for 10–20 min. Subsequently, the MWA electrode probe was removed, and a final CT scan was taken to reexamine the ablation zone and to determine whether serious complications, such as abdominal bleeding or massive pneumothorax, occurred. During the MWA procedure, vital signs, such as heart rate, blood pressure and oxygen saturation, were monitored. After MWA treatment, liver protective, anti-inflammatory and symptomatic treatments were prescribed.
Assessment of clinical efficacy and safety
In the present study, the primary endpoint was OS, and the secondary endpoint was progression-free survival (PFS). OS was defined as the date of the first TACE session to the date of death or last date of follow-up. PFS was measured from the date of treatment initiation to tumor progression, death, or last follow-up, whichever came first. Tumor progression was defined as the occurrence of new lesions or local tumor recurrence diagnosed based on imaging, and cytological analysis or biopsy (if necessary). Local tumor response and recurrence, or new lesions, were assessed using contrast-enhanced CT/MRI examinations. According to the mRECIST guidelines, an objective response (OR) was defined as a complete response (CR) and partial response (PR), and disease control (DC) included CR, PR and stable disease (SD). Ablation margin size was evaluated by registration of post- and pre-MWA CT images. During the registration process, unenhanced, arterial phase, and portal phase images of the pre-MWA CT were first used for creating a color map. Then, portal phase CT images of post-MWA and pre-MWA were registered to assess the ablation size. Treatment-related complications were recorded. Major complications were defined as events that caused substantial morbidity or led to hospital admission or prolonged hospital stay. Furthermore, complications related to the TACE-MWA procedures were evaluated according to the criteria defined by the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification system of complications [Citation32].
Follow-up
Contrast-enhanced CT/MRI scans were performed 1–2 weeks before the initial TACE treatment to record and evaluate tumor status in the included patients. After TACE-MWA treatment, follow-up was performed at clinical visits at monthly intervals. Physical examination, laboratory tests (e.g., total bilirubin, serum albumin, prothrombin time and serum tumor marker levels) and contrast-enhanced CT/MRI were performed. Tumor response to TACE-MWA was evaluated using contrast-enhanced CT/MRI every 4–6 weeks after treatment. Local tumor control was assessed by the multidisciplinary team of radiologists and oncologists. If there was no residual tumor and tumor progression, the follow-up tests were prolonged to every 3 months. If residual tumor and/or tumor progression were observed, repeat MWA or TACE, 125I seed implantation, cryoablation, percutaneous ethanol injection, or sorafenib therapy were performed based on a consensus decision made by the multidisciplinary team in accordance with the evaluation of tumor status based on CT/MR imaging.
Definition of ALBI grade and PALBI grade
The ALBI score was calculated before interventional treatment using appropriate clinical parameters and recommended methods. The ALBI score was calculated using the following equation: ALBI score = (log10 bilirubin [μ mol/L] × 0.66) + (albumin [g/L] × –0.085). The ALBI grade was defined as follows: grade 1 (ALBI score ≤–2.60); grade 2 (–2.60 < ALBI score ≤–1.39); and grade 3 (ALBI score >–1.39) [Citation27]. The PALBI score was calculated using the following equation: PALBI score = 2.02 × log10 bilirubin − 0.37 × (log10 bilirubin)2 − 0.04 × albumin − 3.48 × log10 platelets + 1.01 × (log10 platelets)2. The PALBI grade was defined as follows: grade 1 (PALBI score ≤−2.53); grade 2 (–2.53 < PALBI score ≤–2.09); and grade 3 (PALBI score >–2.09) [Citation30,Citation33].
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Quantitative data are expressed as frequency, mean ± standard deviation (SD), or median with corresponding 95% confidence interval (CI). The Mann-Whitney U test was used to compare continuous variables, and either Pearson’s χ2 test or Fisher’s exact test was used to compare categorical data. Cumulative survival curves of OS and PFS were generated using the Kaplan-Meier method. For OS analyses, patients who remained alive at the date of last follow-up were considered to be ‘censored.’ In terms of PFS, time was censored at the date of death or last follow-up without progression. The cumulative survival curves of the factors assessed using univariable analysis were compared using the log-rank test. Statistically significant (p < .1) factors identified in the univariate analysis were entered into a Cox proportion hazards regression model to identify independent predictors of survival. In the multivariable analyses, CTP class, ALBI grade and PALBI grade were included in three separate models. The area under the receiver operating characteristic curve (AUC), equivalent to concordance index, was calculated to test discriminatory powers for predicting 1-, 3- and 5- year mortality rates. For all tests, p < .05 was considered to be statistically significant.
Results
Patient characteristics
A total of 349 patients were ultimately enrolled (89.1% male; mean [± SD] age, 53.4 ± 12.27 years range, 19–79 years). Ninety (25.8%) and 259 (74.2%) patients had an ECOG performance status score of 0 and 1, respectively, 273 (78.2%) had a history of HBV/HCV infection, and 156 (44.7%) developed hepatic cirrhosis. There were 86 (24.6%), 182 (52.1%) and 81 (23.3%) patients with ALBI grade 1, grade 2 and grade 3, respectively. There were 135 (38.7%), 106 (30.4%) and 108 (30.9%) patients with PALBI grade 1, grade 2 and grade 3, respectively. There were 261 (74.8%) and 88 (25.2%) patients with the CTP class A and B. In terms of tumor factors, HCCs in all included patients were 5.1–8.0 cm in maximum diameter, and 45% (159/349) exhibited multiple tumor lesions. When stratified according to ablation margin size, there were 90 (25.8%), 119 (34.1%) and 140 (40.1%) patients with a minimal margin size of 0–5, 6–10 and 11–15 mm, respectively. Baseline patient characteristics are summarized in .
Table 1. Baseline patient characteristics stratified by ALBI grade.
Tumor response to TACE-MWA
The local tumor response to TACE-MWA was assessed according to the mRECIST guidelines. Analysis revealed that CR, PR, SD and progressive disease (PD) were noted in 58 (16.6%), 211 (60.5%), 41 (11.7%) and 39 (11.2%) patients, respectively. The DC and OR rates were 88.8% and 77.1%, respectively. Among 79 patients who experienced PD, 58 (73.4%) experienced local tumor recurrences and 21 (26.6%) experienced new tumor lesions.
OS and PFS
Over an 18-year follow-up period, the median follow-up duration was 28.0 months (range, 4.0–221 months), 253 patients died, and 96 survived at their last visit. The median OS was 28.0 months (95% CI 23.55–32.45 months), and the cumulative 1-, 3-, 5- and 10-year OS rates were 85.2%, 43.4%, 24.5% and 8.6%, respectively (). The median PFS was 4.8 months (95% CI 4.26–5.34 months), and the cumulative 1-, 2-, 3- and 5-year PFS rates were 21.8%, 9.7%, 3.7% and 0.3%, respectively ().
Figure 1. Kaplan-Meier curves of overall survival in all 349 patients with large HCCs after TACE-MWA.
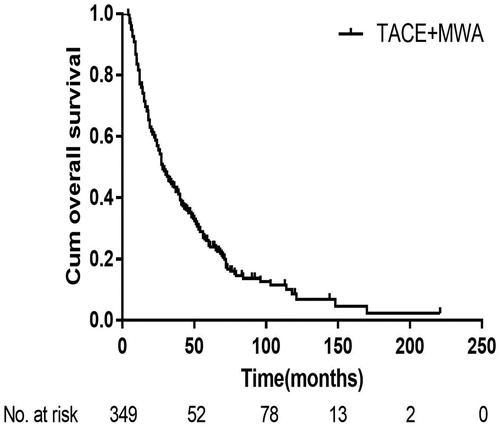
Figure 2. Kaplan-Meier curves of progression-free survival (A) and the progression-free survival stratified by the ablation margin size (B) in all 349 patients with large HCCs after TACE-MWA.
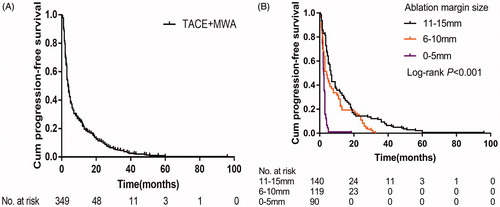
Stratified according to ablation margin size, patients with a margin size of 11–15 mm experienced better median PFS than those with a margin size of 6–10 or 0–5 mm (6.5 versus [vs] 4.0 vs 2.3 months; p < .001) . For patients with a margin size of 11–15, 6–10 and 0–5 mm, the respective cumulative 1-, 3- and 5-year PFS rates were 33.6%, 9.3% and 3.6%; 22.7%, 0% and 0%; and 1.1%, 0% and 0% (p < .001) ().
Prognostic factors of OS and PFS
Univariate analyses revealed that ALBI grade (p < .001), PALBI grade (p < .001), CTP class (p < .001), ECOG performance status (p < .001), tumor number (p < .001), albumin level (p < .001), total bilirubin (p < .001) and alpha-fetoprotein level (p < .001) were significantly associated with OS (). Multivariate analyses revealed that ALBI grade (ALBI grade 2, hazard ratio [HR] 0.18 [95% CI 0.11–0.29; p < .001], ALBI grade 3, HR 0.41 [95% CI 0.29–0.57; p < .001]; PALBI grade (PALBI grade 2, HR 0.35 [95% CI 0.23–0.53; p < .001], PALBI grade 3, HR 0.46 [95% CI 0.32–0.67; p < .001]; CTP class, HR 2.16 [95% CI 1.49–3.15; p < .001]; ECOG performance status; and alpha-fetoprotein level were independent predictors of OS ().
Table 2. Univariable and multivariable predictors of overall survival.
In terms of PFS, univariate analyses revealed that ALBI grade (p < .001), PALBI grade (p < .001), CTP class (p < .001), patient age (p = .085), ECOG performance status (p < .001), tumor number (p < .001), albumin level (p < .001), total bilirubin (p = .017), alpha-fetoprotein level (p = .088), TACE session (p = .001), MWA session (p = .001) and ablation margin size (p < .001) were significantly associated with PFS (). Multivariate analyses revealed that ALBI grade (ALBI grade 2, HR 0.30 [95% CI 0.19–0.47; p < .001], ALBI grade 3, HR 0.57 [95% CI 0.43–0.78; p < .001]; PALBI grade 2, HR 0.24 [95% CI 0.42–0.71; p < .001], PALBI grade 3, HR 0.61 [95% CI 0.47–0.82; p < .001]; ECOG performance status, tumor number and ablation margin size were independent predictors of PFS ().
Table 3. Univariable and multivariable predictors of progression-free survival.
Predictive value of the ALBI grade, PALBI grade and CTP class in OS
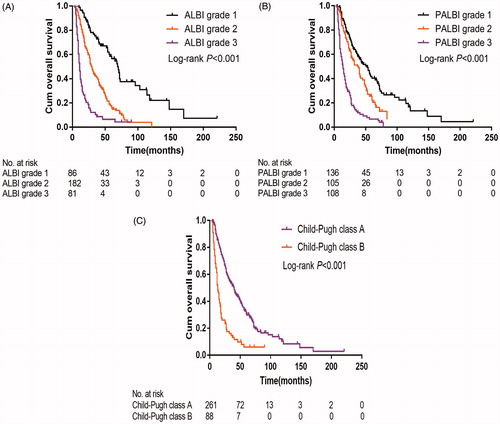
Patients with ALBI grade 1 experienced better median OS than those with ALBI grade 2 or grade 3 (69.0 vs 29.0 vs 11.0 months; p < .001) ( and ). Similarly, the median OS of patients with PALBI grade 1 was significantly better than in those with PALBI grade 2 or grade 3 (52.0 vs 37.0 vs 14.0 months; p < .001) ( and ). Patients with CTP class A experienced better median OS than those with the CTP class B (40.0 vs 13.0 months; p < .001) ( and ).
Relationship between ALBI grade, PALBI grade and CTP class in OS and PFS
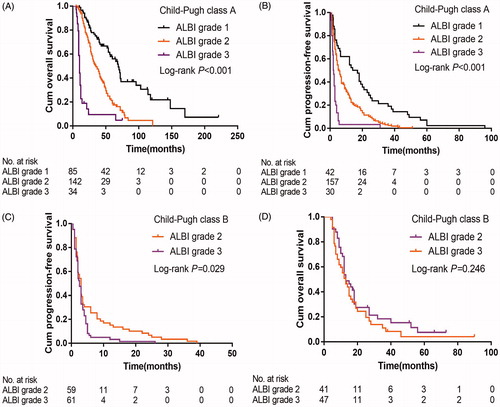
In the subgroup of those with CTP class A, patients with the ALBI grade 1 experienced better median OS (69.0 vs 35.0 vs 10.0 months; p < .001) () and PFS (15.5 vs 5.5 vs 2.1 months; p < .001) () than those with ALBI grade 2 or grade 3. In the subgroup analysis of CTP class B, patients with ALBI grade 2 demonstrated better PFS than those with ALBI grade 3 (3.0 vs 2.5 months; p = .029) (). However, there were no significant differences in OS (p = .246) ().
Figure 4. Kaplan-Meier curves of overall survival (A&C) and progression-free survival (B&D) in patients with the CTP class A (A&B) and CTP class B (C&D) stratified by the ALBI grade. ALBI: albumin-bilirubin; CTP: Child-Turcotte-Pugh.
Regarding PALBI grade, in the subgroup analysis of those with CTP class A, patients with PALBI grade 1 experienced better median OS (54.0 vs 40.0 vs 18.0 months; p < .001) () and PFS (6.5 vs 6.0 vs 2.5 months; p = .001) () than those with PALBI grade 2 or grade 3. In the subgroup analysis of patients with CTP class B, there were no significant differences in OS (p = .132) () and PFS (p = .531) (). A summary of the relationships between ALBI grade, PALBI grade and CTP class in assessing survival is presented in .
Figure 5. Kaplan-Meier curves of overall survival (A&C) and progression-free survival (B&D) in patients with the CTP class A (A&B) and CTP class B (C&D) stratified by the PALBI grade. PALBI: platelet-albumin-bilirubin; CTP: Child-Turcotte-Pugh.
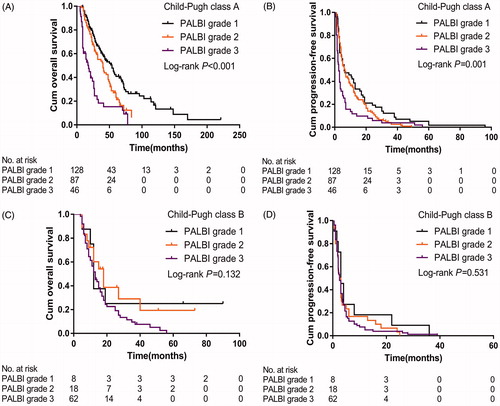
Table 4. Overall survival and progression-free survival stratified by ALBI grade, PALBI grade and CTP class.
Comparison of ALBI grade, PALBI grade and CTP class in predicting OS
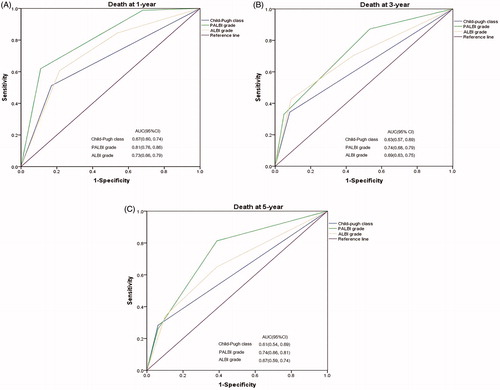
The discriminatory capabilities of PALBI grade, ALBI grade and CTP class were quantified using AUC values. PALBI grade had higher AUC values than ALBI grade or CTP class in predicting 1-, 3- and 5-year OS (). The 1-, 3- and 5-year AUC values for PALBI grade were 0.81 (95% CI 0.76–0.86), 0.74 (95% CI 0.68–0.79) and 0.74 (95% CI 0.66–0.81), respectively. The 1-, 3- and 5-year AUC values for ALBI grade were 0.73 (95% CI 0.66–0.79), 0.69 (95% CI 0.63–0.75) and 0.67 (95% CI 0.59–0.74), respectively. The 1-, 3- and 5-year AUC values for CTP class were 0.67 (95% CI 0.60–0.74), 0.63 (95% CI 0.57–0.69) and 0.61 (95% CI 0.54–0.69), respectively.
Complications
There were no treatment-related deaths. Hepatic dysfunction was the most common complication, including elevation of biochemical test results and manifestations of ascites or icterus during the follow-up period. Among all patients, major complications related to TACE-MWA procedures were observed in 9 (2.6%), including liver abscess (n = 2), subcapsular hematoma (n = 1), intercostal artery hemorrhage (n = 1), intrahepatic artery hemorrhage (n = 2), refractory pleural effusion (n = 1) and biloma (n = 2). Minor complications were observed in 77 (22.1%) patients, including mild abdominal pain, fever, nausea, vomiting, fatigue and elevated liver enzyme levels. All minor complications were transient and resolved within 3–7 days. According to the CIRSE classification system of complications, the incidence of grade 1, grade 2, grade 3 and grade 4 complications were 18.6% (65/349), 3.7% (13/349), 2.0% (7/349) and 0.6% (2/349), respectively. There were no grade 5 or grade 6 complications.
Discussion
In the present study, TACE-MWA procedures were performed in 349 patients with HCCs, which yielded acceptable 3-, 5- and 10-year OS rates of 43.4%, 24.5% and 8.6%, respectively. Prognostic analyses revealed that PALBI grade, ALBI grade, CTP class, ECOG performance status, and alpha-fetoprotein level were independent predictors of OS. Furthermore, our data revealed that PALBI grade was a better prognostic tool than ALBI grade or CTP class in predicting OS of patients with large HCCs after TACE-MWA.
TACE has long been recommended as the first-line treatment for unresectable HCCs. In attempts to improve the clinical efficacy of TACE, TACE-MWA has been validated as a better treatment strategy than TACE alone in several randomized controlled trials (RCTs) [Citation34]. However, a limited number of studies reported the long-term outcomes of TACE-MWA for large HCCs. Zheng et al. reported medium-term outcomes of TACE-MWA in 92 patients with large HCCs, with a 3-year OS rate of 32.6% [Citation35]. In that study, 44 (47.8%) patients exhibited HCC lesions with a maximum diameter >10 cm, and tumor size was confirmed as a significant predictor of OS. This may explain why the 3-year OS rate in our patients was higher than in their patients. Hu et al. reported a survival analysis involving 84 patients with large HCCs with maximum diameters of 5.0–10.0 cm who underwent TACE-MWA [Citation36]. They found that alpha-fetoprotein level, hepatic function and tumor number were significant prognostic factors for OS. These findings are consistent with those of our study. Elnekave et al. compared the clinical efficacy of the combination of transarterial embolization (TAE) and ablation (TAE-ablation) with that of surgical resection for the treatment of solitary HCCs <7 cm [Citation37]. The authors reported that patients who underwent TAE-ablation had a median OS of 54 months. However, approximately 70% of the patients in that study exhibited HCC lesions <5.5 cm, and all had CTP class A hepatic function. As such, it would be inappropriate to directly compare our results with those of their study. However, according to the previous studies, it is clear that TACE-MWA is superior to TACE alone for the treatment of solitary or a limited number of large HCCs.
In our study, we found that PALBI grade was superior to ALBI grade and CTP class in predicting OS of patients with large HCCs after TACE-MWA. These findings are consistent with those of previous studies [Citation30,Citation38]. The CTP classification was originally developed to assess hepatic function in patients with hepatic cirrhosis. However, in our study, 193 (55.3%) patients exhibited no evidence of hepatic cirrhosis, which may have decreased the predicted efficacy of CTP class. In addition, the clinical application of CTP class was limited by some constraints. Due to the challenges in assessing minimal ascites or encephalopathy, the subjective assessment of hepatic encephalopathy and ascites, and inter-relationships between serum albumin level and ascites by physicians, may decrease the accuracy of CTP class in evaluating hepatic function. It indicated that not all constituents of the CTP class have equal accessibility and reproducibility. The cutoff levels for albumin, bilirubin and pro-thrombin time are intrinsic drawbacks of CTP classification, which may have ultimately limited accurate prognostication. Furthermore, hepatic cirrhosis-related portal hypertension is a highly lethal factor affecting the long-term prognosis of HCC patients. However, the CTP classification does not include any biomarkers to assess portal hypertension.
To overcome the limitations of CTP classification, the ALBI grade is recommended as an important biomarker for assessment of hepatic function in patients with HCCs. Compared with CTP class, the ALBI grade is associated with three important features making it simple, objective, and accurate. Regarding ALBI grade, both serum albumin and bilirubin levels can offer an evidence-based, objective tool for assessment of hepatic function in patients with HCCs. The efficacy of the ALBI grade in assessing hepatic function of HCC patients has been validated in several studies. Kao et al. constructed a nomogram with ALBI grade to assess the OS of patients with early-stage HCCs after RFA, in which personalized long-term OS data were described [Citation31]. Based on ALBI grade, PALBI grade was developed with platelet count acting as an evaluation tool to assess the severity of portal hypertension. Liu et al. also reported that ALBI grade was a good tool to assess hepatic dysfunction in patients with HCC [Citation33]. In that study, the investigators examined HCC patients who underwent liver resection, RFA or TACE, and both ALBI and PALBI grades were validated as adequate models to assess hepatic dysfunction in HCC patients. Our data indicated that PALBI grade had better discriminatory power than ALBI grade and CTP class for prediction of 1-, 3- and 5-year OS. The findings of our study, therefore, were consistent with those of previous studies [Citation33,Citation38].
In the present study, the ablation margin size was evaluated by the registration of post- and pre-MWA CT images. Kim et al. [Citation39] and Shin et al. [Citation40] reported that the registration of post- and pre-ablation CT images was an accurate and useful technique for assessing the safety margin after ablation therapy. Therefore, the method we used in the present study to evaluate the ablation margin was supported by previous studies [Citation39,Citation40]. We found that patients with a minimum ablation margin size of 11–15 mm experienced significantly better median PFS than those with a minimum ablation margin size of 0–5 or 6–10 mm (6.5 vs 4.0 vs 2.3 months). Our data demonstrated that the differences between the two groups were statistically significant (p < .001). Previous studies have shown that ablation margin size is independently associated with the outcomes of thermal ablation for HCCs or hepatic metastases with a maximum tumor size <5.0 cm [Citation9–11]. Wang et al. reported that the risk for local tumor progression decreased by 46% for each 5 mm increase in minimal margin size of RFA for hepatic metastasis of colorectal cancer [Citation11]. Shady et al. reported that an ablation margin <5 cm was a significant predictor of shorter local tumor PFS, and suggested that an ablation margin >5 cm was critical for local tumor control in patients with colorectal liver metastases who underwent RFA or MWA [Citation16]. Few studies have investigated the predictive value of ablation margin in the assessment of outcomes of TACE-MWA for large HCCs.
To the best of our knowledge, this was the first investigation to examine and compare PALBI grade, ALBI grade and CTP class in a large, multi-center study involving patients with large HCCs after TACE-MWA. We confirmed that PALBI grade demonstrated better efficacy in predicting OS. Strengths of the present study included its multi-center design, large sample size, and long-term follow-up.
There were, however, some limitations to our study, the first of which was its retrospective design. Although the data regarding survival analyses were accurate and carefully recorded by the reviewers, prospective RCTs are still needed to confirm our proposal and hypothesis. Second, the included patients had large HCCs with a maximum diameter of 5.1–8.0 cm; therefore, our findings need to be validated by investigating patients with HCCs >8.0 cm in diameter. Third, the ablation margins were evaluated using traditional two-dimensional images of post- and pre-MWA CT scans. Recently, new volumetric three-dimensional methods for assessment of ablation completeness or ablation margin have been described [Citation41,Citation42]. Future studies may be needed to validate our results by using these recommended methods.
In conclusion, our multi-center data confirmed that PALBI grade was a better prognostic tool than ALBI grade or CTP class in predicting OS of patients with large HCCs after TACE-MWA.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed was obtained from all individual participants in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641.
- Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68:977–993.
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711.
- Wáng YX, De Baere T, Idée JM, et al. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res. 2015;27:96–121.
- Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503–509.
- Farinati F, Giacomin A, Vanin V, et al. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol. 2012;57:221–222.
- Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol. 2007;188:480–488.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancerliver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278:601–611.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of coloncancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973.
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251:705–711.
- Gillams AR, Lees WR. Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol. 2008;18:672–677.
- Crocetti L, Bozzi E, Faviana P, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33:818–827.
- Shady W, Petre EN, Gonen M, et al. Percutaneous microwave versus radiofrequency ablation of colon cancer liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–275.
- Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203.
- Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterialchemoembolization monotherapy versus combined transarterialchemoembolization-percutaneous microwave coagulationtherapy. J Gastroenterol Hepatol. 2013;28:456–463.
- Chen QF, Jia ZY, Yang ZQ, et al. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-microwave ablation therapy for hepatocellular carcinoma tumors ≤5 cm: a propensity analysis at a single center. Cardiovasc Intervent Radiol. 2017;40:1748–1755.
- Veltri A, Gazzera C, Calandri M, et al. Percutaneous treatment of Hepatocellular carcinoma exceeding 3 cm: combined therapy or microwave ablation? Preliminary results. Radiol Med. 2015;120:1177–1183.
- Maluccio M, Covey AM, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005;16:955–961.
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928.
- Mohammadi H, Abuodeh Y, Jin W, et al. Using the Albumin-Bilirubin (ALBI) grade as a prognostic marker for radioembolization of hepatocellular carcinoma. J Gastrointest Oncol. 2018;9:840–846.
- Hiraoka A, Kumada T, Kudo M, et al. Albumin-Bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child-Pugh classifications. Liver Cancer. 2017;6:204–215.
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimationacross each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66:338–346.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558.
- Hansmann J, Evers MJ, Bui JT, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28:1224–1231.
- Jaruvongvanich V, Sempokuya T, Wong L. Is there an optimal staging system or liver reserve model that can predict outcome in hepatocellular carcinoma? J Gastrointest Oncol. 2018;9:750–761.
- Elshaarawy O, Alkhatib A, Elhelbawy M, et al. Validation of modified albumin-bilirubin-TNM score as a prognostic model to evaluate patients with hepatocellular carcinoma. World J Hepatol. 2019;11:542–552.
- Kao WY, Su CW, Chiou YY, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology. 2017;285:670–680.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141–1146.
- Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol. 2017;32:879–886.
- Katsanos K, Kitrou P, Spiliopoulos S, et al. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0184597.
- Zheng L, Li HL, Guo CY, et al. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19:237–246.
- Hu H, Chen GF, Yuan W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patientswith cirrhosis. Int J Hyperthermia. 2018;34:1351–1358.
- Elnekave E, Erinjeri JP, Brown KT, et al. Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC <7 cm. Ann Surg Oncol. 2013;20:2881–2886.
- Ho CHM, Chiang CL, Lee FAS, et al. Comparison of platelet-albumin-bilirubin (PALBI), albumin-bilirubin (ALBI),and child-pugh (CP) score for predicting of survival in advanced HCC patients receiving radiotherapy (RT). Oncotarget. 2018;9:28818–28829.
- Kim KW, Lee JM, Klotz E, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. Am J of Roentgenol. 2011;196:W565–W572.
- Shin S, Lee JM, Kim KW, et al. Postablation assessment using follow-up registration of CT images before and after radiofrequency ablation (RFA): Prospective evaluation of midterm therapeutic results of RFA for hepatocellular carcinoma. Am J of Roentgenol. 2014;203:70–77.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal abltion of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29:2698–2705.
- Solbiati M, Muglia R, Goldberg SN, et al. A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia. 2019;36:337–343.