Abstract
Purpose: To characterize the T cell receptor (TCR) repertoire, serum cytokine levels, peripheral blood T lymphocyte populations, safety, and clinical efficacy of hyperthermia (HT) combined with autologous adoptive cell therapy (ACT) and either salvage chemotherapy (CT) or anti-PD-1 antibody in patients with previously treated advanced solid tumors.
Materials and methods: Thirty-three (33) patients with ovarian, pancreatic, gastric, colorectal, cervical, or endometrial cancer were recruited into the following therapeutic groups: HT + ACT (n = 10), HT + ACT + anti-PD-1 inhibitor (pembrolizumab) (n = 11) and HT + ACT + CT (n = 12). Peripheral blood was collected to analyze TCR repertoire, measurements of cytokines levels and lymphocyte sub-populations before and after treatment.
Results: The objective response rate (ORR) was 30% (10/33), including three complete responses (CR) (9.1%) and seven partial responses (PR) (21.2%) and a disease control rate (DCR = CR + PR + SD) of 66.7% (22 of 33). The most common adverse reactions, blistering, subcutaneous fat induration, local heat-related pain, vomiting and sinus tachycardia, were observed in association with HT. IL-2, IL-4, TNF-α, and IFN-γ levels in peripheral blood were significantly increased among the clinical responders (p < 0.05) while IL-6 and IL-10 were elevated among those with progressive disease (p < 0.05). Peripheral blood CD8+/CD28+ T cells increased (p = 0.002), while the CD4+/CD25+/CD127+Treg cells decreased after therapy (p = 0.012). TCR diversity was substantially increased among the clinical responders.
Conclusions: Combining HT with ACT plus either CT or anti-PD-1 antibody was safe, generated clinical responses in previously treated advanced cancers, and promoted TCR repertoire diversity and favorable changes in serum IL-2, IL-4, TNF-α, and IFN-γ levels in clinical responders.
Introduction
Immunotherapy with anti-PD-1 and anti-PD-L1 antibodies is revolutionizing the treatment of many cancers; however, the majority of patients do not have tumor responses to PD-1/PD-L1 inhibitors in part due to inadequate intratumoral T cell infiltration. Cellular therapeutics may be an option for addressing this challenge. Recently, adoptive cell therapy (ACT) with cytokine-activated autologous mononuclear cells has shown anti-cancer activity alone and in combination with chemotherapy (CT) [Citation1–3].
Hyperthermia (HT) has direct antitumor activity but also, when applied in the range of 40 °C–43 °C and higher, can act as a thermal sensitizer for radiation and CT [Citation4,Citation5]. The Thermotron radiofrequency (RF)-8 device (Yamamoto Vinita, Osaka, Japan) delivers 8-MHz RF-based deep HT [Citation6]. The device requires a pair of capacitive electrodes placed on opposite sides of the body to treat superficial, subsurface, or deep-seated tumors, especially at depths of 5–7 cm. Dielectric heat with high power is produced after rapid changes in the electric field to reach the goal temperature in a specific region [Citation7]. Since the 1990s, increased local control or overall survival has been reported in multiple randomized trials combining Thermotron RF-8 with radiotherapy (RT) in the settings of advanced cervical cancer [Citation8,Citation9], head and neck cancer [Citation10], and non-small cell lung cancer [Citation7,Citation11].
Although HT has demonstrated synergy with RT and CT, few studies have reported the combination of HT with cancer immunotherapy. HT could play a significant role in immunomodulation through at least two pathways. First, HT enhances the expression of heat shock proteins (HSPs), involved in various immunological processes and as antigen chaperones to antigen-presenting cells (APCs) [Citation12,Citation13]. Moreover, HT can increase tumor antigen exposure or release for capture by dendritic cells (DCs), resulting in activation of tumor-specific cytotoxic T cells [Citation14,Citation15]. Therefore, we wished to explore the feasibility of combining HT with cancer immunotherapy, including ACT and immune checkpoint blockade, and to test whether HT would augment the anti-tumor effects of the immunotherapy. We performed a prospective phase I study to evaluate the safety and preliminary evidence of clinical efficacy of HT combined with ACT and either CT or pembrolizumab. The immune response to this therapy was characterized by changes in the T cell receptor (TCR) repertoire, serum cytokine levels and lymphocyte subpopulations.
Patients and methods
Patients
The study (ClinicalTrials.gov identifier, NCT03757858; https://register.clinicaltrials.gov/) was registered and approved by the Regional Ethical Review Board for Capital Medical University Cancer Center, Beijing, China. Patients were treated according to the Declaration of Helsinki's ethical principles for medical research involving human subjects. All patients provided informed written consent prior to study entry. Patients were required to have a documented diagnosis of an advanced solid tumor and to have experienced disease progression after at least two lines of treatment. Other inclusion criteria included an age of 18–75 years, a life expectancy of greater than 12 weeks, Eastern Cooperative Oncology Group performance status (ECOG-PS) [Citation16] of 0–1, adequate organ function, and evaluable lesions using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines [Citation17].
Treatment groups
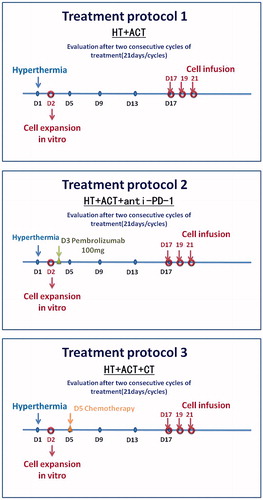
Patients were enrolled prospectively into one of three treatment groups: HT + ACT, HT + ACT + anti-PD-1 (pembrolizumab) and HT + ACT + CT. This study was non-randomized and the treatment arm was chosen based on a consideration of patients’ previous treatments, their ability to tolerate a particular therapy, and risks of specific toxicities after evaluation by two independent physicians as we have published in a previous study [Citation3]. In the HT + ACT + CT group, six patients received paclitaxel combined with carboplatin, three patients received oxaliplatin combined with capecitabine, two patients received nanoparticle albumin-bound paclitaxel and one patient received oral S-1.
Hyperthermia
The patients were treated in the supine position with Thermotron RF-8. The Thermotron machine required paired electrodes for heating. For a proper heat-up process, tight skin contact was crucial. The device was designed with double movable joints (gantry and electrodes) with a maximal tilting angle of 15°. Computed tomography films were used for tumor localization. An optimal treating position of the patient was based on maximal coverage of electrodes and by his or her comfort. A smaller electrode was placed near the tumor in order to focus the power. According to common practice with the Thermotron RF-8, the operator started from 150 W and increased the output by 25–50 W per minute until the patient complained of discomfort, a process called “output-limiting symptoms” [Citation18]. A sensor catheter with three temperature detectors was attached to the skin on the lateral abdomen and the average of the skin surface temperatures from the four points was monitored during treatment. The skin cooling was set at 25 °C and could be gradually decreased to 10 °C or lower to decrease patient discomfort. When pain occurred, the output was decreased by 100 W. The output was increased by 50 W again or de-escalated back and forth depending on whether the symptoms reappeared. This was the optimal output dose maintained for the next 40 min. More details of the operations and procedures that were followed may be found in the manufacturer’s instructions as previously reported [Citation19].
Serum cytokine determination by cytometric bead array (CBA)
Measurement of serum interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) was performed using cytometric bead array (CBA) kits (BD Biosciences) according to the manufacturer’s instructions. Samples were analyzed with flow cytometer-incorporated FCAP Array version 3.0 software (Soft Flow, USA).
Preparation of cytokine-induced killer (CIK) cells
The cytokine-induced killer (CIK) product was generated ex vivo as described in detail previously [Citation1]. Nucleated peripheral blood cells were mobilized by injection of GM-CSF 5 μg/kg/day (Qilu Pharmaceutical, China) until the level of mononuclear cells in peripheral blood reached 1.5 × 109/L. Peripheral blood (50 mL) was collected and the lymphocyte fraction was separated by density gradient centrifugation over Ficoll 400. The lymphocytes were cultured for an average of 14 days in media containing gamma interferon, anti-CD3 antibody, and IL-2 to produce the CIK cells. All cell products met quality control requirements.
TCR repertoire analysis by next generation sequencing
DNA was extracted from peripheral blood using a Qiagen DNA FFPE kit, DNA blood kit, or DNA blood mini kit (Qiagen) according the manufacturer’s instructions. Genomic DNA (200 ng) from each sample was subjected to a semi-nested PCR with two separate reactions involving the same Vβ consensus primer. Negative controls (samples without DNA) were included after each sample. Thirty cycles were carried out with a primer annealing temperature of 60 °C (40 s) for the initial five cycles and 57 °C (40 s) for the remaining 25 cycles. PCR products were made into sequencing libraries (Illumina Inc., San Diego, CA) by ligation of indexed adapters. Purified libraries were mixed in equimolar ratios and directly sequenced in pools of 10–12 cases on MiSeq (Illumina Inc) using 2 × 150 base pair paired-end reads. Samples were analyzed with high-throughput deep sequencing of the TCR V-beta CDR3 region with the Illumina Genome Analyzer from Adaptive Biotechnologies (Seattle, WA) using the ImmunoSEQ immune profiling system [Citation20,Citation21]. This analysis results in a 5× sequence coverage for T cells from 3.6 μg of genomic DNA (e.g., an input of 200,000 T cell genomes results in an output of 1,000,000 sequences, depending on the proportion of T cells). The product was sequenced and organized providing in-frame and out-of-frame sequences. An algorithm was applied to the in-frame sequences for collapsing reads and resulting in unique in-frame rearrangement of the CDR3 genes. For visualization, the geometric means for each set of samples was calculated per clonal bin and normalized to range from 0 to 1. The Shannon diversity index was calculated in order to evaluate the diversity of TCR V-beta CDR3 sequences for all patients. The Shannon diversity index was defined as: where pi is the proportion of sequence relative to the total N sequences. It accounted for both richness and relative abundance (evenness) of the TCR V-beta CDR3 sequences present in each sample. The larger the Shannon diversity index, the more diverse the distribution of the TCR V-beta CDR3 sequences [Citation22].
Flow cytometric analysis
We used the following fluorochrome-conjugated antibodies: CD3 PerCP-Cy5.5, CD4 FITC, CD8 FITC, CD25 PE, CD28 PE, CD56 PE (Beckman). We detected subpopulation of PBMCs by flow cytometric analysis as described previously [Citation1]. Briefly, cells were re-suspended in staining buffer and then stained with primary antibody at 4 °C for 30 min in the dark. Stained cells were centrifuged for 10 min at 1500 rpm at room temperature and subsequently washed twice in staining buffer prior to FACS analysis. Three-color flow cytometric analysis was run to determine cell phenotype using a Cytometry FC500 and CXP analysis software (Beckman-Coulter USA).
Adverse event and response assessments
Clinical and laboratory examinations were carried out within 7 days before enrollment and each cycle of treatment. Adverse events were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE v4.0). Response to treatment was assessed by the Response Evaluation Criteria in Solid Tumors 1.1 (www.cancer.gov/) using tumor measurements obtained from computed tomographic (CT) scans. We refer to the patients with disease control (complete response [CR] + partial response [PR] + stable disease [SD]) as the remission group.
Statistical methods
Continuous variables were expressed as mean ± SD (standard deviation) and compared using both paired Student’s t test and two-tailed unpaired Student’s t test; categorical variables were compared using χ2 or Fisher analysis. Shannon index and Clonality were used to evaluate the TCR repertoire. All statistical evaluations were carried out using SPSS (Statistical Package for the Social Science, version 15.0; SPSS Inc., Chicago, IL) and GraphPad Prism 5 (Version 5.01; GraphPad Software, Inc., USA). A value of p < 0.05 was considered to be statistically significant in all the analyses.
Results
Patient characteristics
Thirty-three (33) patients with previously treated, advanced solid tumors, including ovarian, pancreatic, gastric, colorectal, cervical, and endometrial cancer, were enrolled into one of three treatment combinations: HT + ACT (n = 10), HT + ACT + PD-1 (pembrolizumab) (n = 11) and HT + ACT + CT (n = 12). The details of their clinical characteristics are presented in . The detailed treatments administered to the patients are shown in . All patients received two cycles of HT (five times in a 21 day period) plus two cycles of ACT. The mean temperatures achieved with HT in each group were: HT + ACT: 39.2 ± 1.7 °C; HT + ACT + PD-1: 39.7 ± 1.8 °C; and HT + ACT + CT: 40.5 ± 2.1 °C.
Table 1. Demographics and baseline characteristics of patients (N = 33).
Clinical effectiveness assessment
The objective response rate (RR) was 10 of 33 (30.3%), including 3 (9.1%) with a CR and 7 (21.2%) with a PR (). The disease control rate (DCR) across the 33 enrolled patients was 22 of 33 (66.7%) (). The disease control rate (DCR) was 7 of 10 (70%), 6 of 11 (55%), and 9 of 12 (75%) in HT + ACT, HT + ACT + PD-1 (pembrolizumab) and HT + ACT + CT groups respectively. Patients with ovarian cancer and colorectal cancer had the most favorable clinical responses with ORR and DCR of 50% and 83.3% in patients with ovarian cancer, and 42.8% and 74.3% in patients with colorectal cancer ().
Table 2. Clinical responders and non-responders distribution among enrolled patients.
Serum cytokine levels after treatment
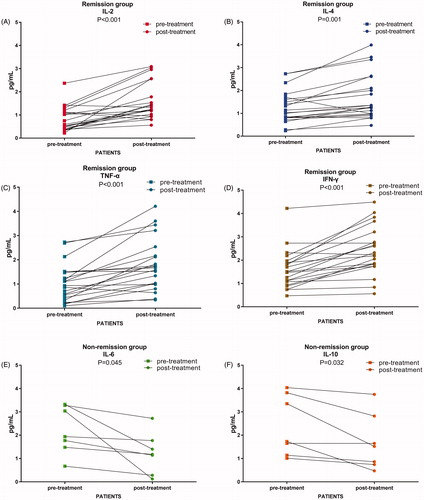
We measured serum IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ levels in specimens obtained before and after all study treatments. IL-2, IL-4, TNF-α, and IFN-γ significantly increased after treatment compared with baseline in patients with disease control (CR + PR + SD) (p < 0.05, ), whereas IL-6 and IL-10 were increased in patients with progressive disease (p < 0.05, ).
Assessment of TCR diversity
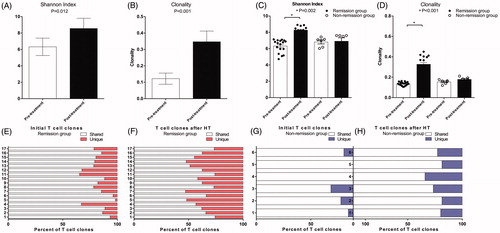
In order to calculate the TCR diversity of circulating T cells, the Shannon diversity index [Citation23] and TCR clonality [Citation24] were used to characterize the diversity of CDR3 TCR Vβ sequences of T cell samples of 23 of 33 patients from whom there was an adequate amount of specimen to perform next generation sequencing (NGS). There were significant differences in the Shannon index (, p = 0.012) and clonality (, p < 0.001) between the baseline T cells (pre-treatment) and T cells after treatment (post-treatment). We then performed TCR stratification analysis with respect to the clinical outcomes. There were significant differences in the Shannon index (, p = 0.002) and clonality (, p < 0.001) between the T cells at baseline and T cells after treatment in those with disease control, but there were no differences between baseline and post-treatment T cell clonality in those with progressive disease. Unique subclones were significantly increased after treatment and the shared subclones were significantly decreased in those with disease control (). In the group with progressive disease, no significant changes were observed in the subclones ().
Figure 3. TCR diversity were compared in different group by paired t test. (A, B) Alterations of Shannon index and clonality in all patients; (C, D) Shannon index and clonality significantly changed in tumor remission group, while Shannon index and clonality did not significantly change in Non remission group; (E, F) Unique TCR subclones increased and the shared TCR subclones decreased in patients with tumor remission; (G, H) The unique and the shared TCR subclones had no significant changes in patients of non-remission group.
Peripheral blood T lymphocyte phenotype
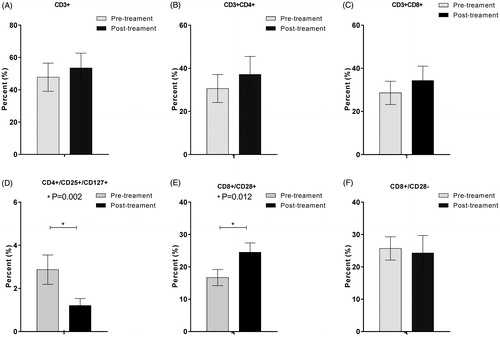
Phenotypic analysis of peripheral blood before the treatment and at the end of the first cycle of HT demonstrated that the CD8+/CD28+ T cell subsets were increased after treatment (p = 0.002), while the CD4+/CD25+/CD127+ cell subsets were significantly decreased after treatment (p = 0.012) ().
Figure 4. Peripheral blood T cell phenotype measurements via cytometry before and after the treatment. CD8+/CD28+ (E) T cell subset was significantly increased after the combined treatment (p < 0.05); CD4+/CD25+/CD127+ (D) cell subset was significantly decreased after the combined treatment (p < 0.05); CD3+ (A), CD3+/CD4+ (B), and CD3+/CD8+ (C) and CD8+/CD28− (F) cell subsets were not significantly changed after the combined treatment (p > 0.05).
Treatment-associated toxicity
All acute and delayed adverse events from the three groups are listed in . The majority of the toxicities was associated with the CT. The most common adverse events (AEs) associated with HT during the study were blistering (3/33, 9.1%), subcutaneous fat induration (4/33, 12.1%), local heating pain (3/33, 9.1%), vomiting (1/33, 3.0%), and accidental sinus tachycardia (1/33, 3.0%). All grades of treatment-related AEs occurred in 15 of 33 patients (45.5%), and most of them were grade 1 or 2 (13 of 15 patients, 86.7%). Grade 3 or 4 treatment-related AEs were observed in only two patients (6.1%). There were no unanticipated significant differences in adverse events among the groups with and without anti-PD-1 antibody. In particular, there were no immune-mediated adverse events.
Table 3. Summary of hematological and treatment-associated adverse events.
Discussion
Hyperthermia combined with other standard anti-cancer treatments has been demonstrated to improve survival [Citation25–28]. Physical HT delivers energy that penetrates into tumor, modifies the tissue constitution, alters vascularization, and releases tumor antigens. Antigen exposure and release caused by HT can induce T cell-mediated immune responses [Citation15]. Therefore, we designed this study to evaluate the safety and preliminary evidence of clinical efficacy of HT combined with ACT. Further, we have assessed the addition of CT or anti-PD-1 antibody (pembrolizumab) to the HT/ACT since previous studies have demonstrated benefit for HT with CT [Citation9].
We confirmed the safety of the HT/ACT combinations. HT toxicity does not overlap with toxicities associated with CT, ACT, or PD-1 immune inhibitor. Adverse reactions included blistering, subcutaneous fat induration, local heat-related pain, vomiting, and sinus tachycardia. There were no occurrences of severe adverse events. Of the different malignancies, recurrent or metastatic ovarian cancers showed the best clinical responses to these combinational therapies during this study.
Changes in the TCR repertoire can be used as indicators of the activation of T cell clones and have been reported to be associated with response to immune checkpoint blockade and survival of patients with cancer [Citation29–31]. In this study, TCR diversity was augmented after combined HT with ACT in the patients with disease controlled. Moreover, TCR subclone analysis demonstrated that an increase in unique subclones was associated with improved clinical outcomes after the combined treatments.
In present study, we had observed that the serum IL-6 and IL-10 increased in the patients without response to the treatments. However, in patients responding to the combined treatments, the expression of IL-2, IL-4, TNF-α, and IFN-γ were enhanced. IL-6 and IL-10 had been reported to be critical for the progression of various cancers. IL-6 could regulate proliferation, invasion and angiogenesis [Citation32] and was associated poor prognosis for patients with advanced cancer [Citation33]. Moreover previous reports had demonstrated that HT could induce acute IL-6-mediated trafficking of T cells to the tumor microenvironment and lymph nodes and boost T cell-mediated antitumor immune responses [Citation34–36]. The reasons why some patients had increased IL-6 and IL-10 might relate to challenges in achieving high temperatures during the HT procedure. Incomplete, moderate levels of HT can induce the proliferation, migration and invasion of tumor cells [Citation37,Citation38]. qRT-PCR screening has shown that uncompleted or moderate HT is able to increase the expression of IL-6 and IL-10, while silencing these cytokines could reverse moderate HT-induced proliferation and invasion [Citation39].
For the quantitative assay of peripheral blood lymphocyte phenotype, we have found that CD8+CD28+ T cells were significantly increased after the combined treatments. CD28 is a co-stimulatory molecule which plays multiple roles in the activation, proliferation, and survival of T cells [Citation40,Citation41]. Conversely, CD8+CD28− T cells are associated with inflammatory disorders and are over-represented in tumor microenvironments and the circulation of cancer patients. Both active and suppressive antitumor immune responses have been ascribed to CD8+CD28− T cell populations [Citation42,Citation43]. We also observed that CD4+CD25+CD127+ regulatory T cell subsets were significantly decreased after treatment. Those findings of an increased percentage of CD8+CD28+ T cells and decreased CD4+CD25+CD127+ T cells support our hypothesis that HT combined with ACT was capable of stimulating T cell-mediated anti-tumor activity and inhibiting production of the suppressive T cell subpopulation.
In summary, we have provided preliminary data showing that combining HT with ACT has a convincing safety profile and demonstrates encouraging clinical responses in patients with advanced solid tumors. TCR repertoire, peripheral blood T cell populations, and serum cytokine levels may be regarded as potential biomarkers for predicting the outcomes of HT combined with ACT.
Disclosure statement
The authors who have taken part in this study declare that they have nothing to disclose regarding funding or conflict of interest with respect to this manuscript.
Additional information
Funding
References
- Ren J, Gwin WR, Zhou X, et al. Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy. Oncoimmunology. 2017;6:e1264563.
- Qiao G, Wang X, Zhou L, et al. Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: a prospective study. Clin Cancer Res. 2019;25:1494–1504.
- Jiang N, Qiao G, Wang X, et al. Dendritic cell/cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: a prospective study. Clin Cancer Res. 2017;23:5066–5073.
- Roti Roti JL. Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events. Int J Hypertherm. 2008;24:3–15.
- Crezee J, van Leeuwen CM, Oei AL, et al. Biological modelling of the radiation dose escalation effect of regional hyperthermia in cervical cancer. Radiat Oncol. 2016;11:14.
- Abe M, Hiraoka M, Takahashi M, et al. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer. 1986;58:1589–1595.
- Ohguri T, Imada H, Yahara K, et al. Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: the radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys. 2009;73:128–135.
- Harima Y, Nagata K, Harima K, et al. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. 2001. Int J Hypertherm. 2009;25:338–343.
- Harima Y, Ohguri T, Imada H, et al. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hypertherm. 2016;32:801–808.
- Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Can Res Ther. 2010;6:492–496.
- Mitsumori M, Zeng ZF, Oliynychenko P, et al. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Clin Oncol. 2007;12:192–198.
- Court KA, Hatakeyama H, Wu SY, et al. HSP70 inhibition synergistically enhances the effects of magnetic fluid hyperthermia in ovarian cancer. Mol Cancer Ther. 2017;16:966–976.
- Chen T, Guo J, Han C, et al. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449–1459.
- Joshi N, Duhan V, Lingwal N, et al. Adjuvant properties of thermal component of hyperthermia enhanced transdermal immunization: effect on dendritic cells. PloS One. 2012;7:e32067.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. Am J Roentgenol. 2010;195:W221–W228.
- Shoji H, Motegi M, Osawa K, et al. Output-limiting symptoms induced by radiofrequency hyperthermia. Are they predictable?. Int J Hypertherm. 2016;32:199–203.
- Shoji H, Motegi M, Osawa K, et al. A novel strategy of radiofrequency hyperthermia (neothermia) in combination with preoperative chemoradiotherapy for the treatment of advanced rectal cancer: a pilot study. Cancer Med. 2015;4:834–843.
- Sufficool KE, Lockwood CM, Abel HJ, et al. T-cell clonality assessment by next-generation sequencing improves detection sensitivity in mycosis fungoides. J Am Acad Dermatol. 2015;73:228–236.e2.
- Huang H, Sikora MJ, Islam S, et al. Select sequencing of clonally expanded CD8(+) T cells reveals limits to clonal expansion. Proc Natl Acad Sci U S A. 2019;116:8995–9001.
- Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432.
- Rempala GA, Seweryn M. Methods for diversity and overlap analysis in T-cell receptor populations. J Math Biol. 2013;67:1339–1368.
- Qi Q, Liu Y, Cheng Y, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A. 2014;111:13139–13144.
- Fujimoto S, Kobayashi K, Takahashi M, et al. Clinical pilot studies on pre-operative hyperthermic tumour ablation for advanced breast carcinoma using an 8 MHz radiofrequency heating device. Int J Hypertherm. 2003;19:13–22.
- Mochiki E, Shioya M, Sakurai H, et al. Feasibility study of postoperative intraperitoneal hyperthermochemotherapy by radiofrequency capacitive heating system for advanced gastric cancer with peritoneal seeding. Int J Hypertherm. 2007;23:493–500.
- Plan Sangnier A, Preveral S, Curcio A, Kas A, et al. Targeted thermal therapy with genetically engineered magnetite magnetosomes@RGD: Photothermia is far more efficient than magnetic hyperthermia. J Control Release. 2018;279:271–281.
- Youssef I, Amin NP. Radiation therapy, hyperthermia for chest wall recurrence. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC; 2018.
- Faghih Z, Deihimi S, Talei A, et al. Analysis of T cell receptor repertoire based on Vbeta chain in patients with breast cancer. CBM. 2018;22:733–745.
- Gkazi AS, Margetts BK, Attenborough T, et al. Clinical T cell receptor repertoire deep sequencing and analysis: an application to monitor immune reconstitution following cord blood transplantation. Front Immunol. 2018;9:2547.
- Wieland A, Kamphorst AO, Adsay NV, et al. T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol Immunother. 2018;67:1767–1776.
- Gupta SC, Kim JH, Prasad S, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434.
- Shao YY, Lin H, Li YS, et al. High plasma interleukin-6 levels associated with poor prognosis of patients with advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2017;47:949–953.
- Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7:1299–1308.
- Fisher DT, Chen Q, Skitzki JJ, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–3859.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349.
- Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1alpha/VEGFA. PloS One. 2012;7:e37266.
- Dong S, Kong J, Kong F, et al. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med. 2013;11:273.
- Duan XH, Li H, Han XW, et al. Upregulation of IL-6 is involved in moderate hyperthermia induced proliferation and invasion of hepatocellular carcinoma cells. Eur J Pharmacol. 2018;833:230–236.
- Dumitriu IE. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology. 2015;146:185–193.
- Maly K, Schirmer M. Corrigendum to “The Story of CD4 (+) CD28(-) T Cells Revisited: Solved or Still Ongoing?”. J Immunol Res. 2015;2015:251657.
- Filaci G, Fenoglio D, Fravega M, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334.
- Casado JG, Soto R, DelaRosa O, et al. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171.