Abstract
Percutaneous ablation is an increasingly applied technique for the treatment of localized renal tumors, especially for elderly or co-morbid patients, where co-morbidities increase the risk of traditional nephrectomy. Ablative techniques are technically suited for the treatment of tumors generally not exceeding 4 cm, which has been set as general consensus cutoff and is described as the upper threshold of T1a kidney tumors. This threshold cutoff is being challenged, but with still limited evidence. Percutaneous ablation techniques for the treatment of renal cell carcinoma (RCC) include radiofrequency ablation, cryoablation, laser or microwave ablation; the main advantage of all these techniques over surgery is less invasiveness, lower complication rates and better patient tolerability. Currently, international guidelines recommend percutaneous ablation either as intervention for frail patients or as a first line tool, provided that the tumor can be radically ablated. The purpose of this article is to describe the basic concepts of percutaneous ablation in the treatment of RCC. Controversies concerning techniques and products and the need for patient-centered tailored approaches during selection among the different techniques available will be discussed.
Introduction
Ablation used to treat kidney tumors was first described as being a novel technique in the early 80s when absolute ethanol was injected directly into the tumoral vasculature [Citation1]. In parallel to the advent of chemical ablation, technological evolution offered its alternatives to surgery with particular emphasis on thermal based ablative tools such as focused ultrasound, laser, cryo-therapy and radiofrequency (RF). The latter techniques, in particular, have become popular in the past 20 years. The most recent evolution of ablative techniques to treat renal tumors nowadays includes microwave (MW), irreversible electroporation and radiosurgery. The main advantage of all these techniques over surgery is less invasiveness and better patient tolerability, especially if performed percutaneously. Yet if technical contraindications hamper percutaneous access, ablation can be performed intra-operatively and allows for maximized parenchymal sparing [Citation2].
According to the GLOBOCAN 2018 statistics, kidney cancer’s incidence is 8th among cancers in Europe with more than 130,000 new cases and more than 54,000 deaths per year. Early detection and detection due to incidental imaging findings has led to an increased interest in alternatives to surgical resection to treat small sized tumors such as percutaneous ablation. Small renal masses (SRMs) encompass a wide range of benign and malignant tumors and are defined by having a size less than or equal to 4 cm [Citation3]. Although in most of the cases, the masses are confirmed as being renal cell carcinomas (RCCs), up to 25% are benign renal cortical tumors (i.e., oncocytoma, atypical angiomyolipoma) and another 25% are diagnosed as masses with limited metastatic potential [Citation3]. Historically, surgical resection is the standard of care for the treatment of localized renal tumors and partial nephrectomy has been labeled superior to radical nephrectomy for T1 tumors, in terms of long-term renal function and overall survival, while maintaining equivalent oncologic outcomes [Citation3].
Ablative techniques are technically suited for the treatment of tumors generally not exceeding 4 cm, which has been set as general consensus cutoff and is described as the upper threshold of T1a kidney tumors [Citation4]. This threshold cutoff is being challenged, but with still limited evidence [Citation5]. Although not yet established as routine technique, renal tumor biopsy should always be considered to confirm or rule out malignancy [Citation3,Citation6]. One of the major concerns in the use of ablation regards the lack of definitive histopathology of the ablated lesion, which contributes to determining patient prognosis. While intra-intervention biopsy should be standardized, strict imaging follow-up is strongly recommended in this setting in order compensate this potential limitation [Citation7]. Compared to surgery, thermal ablation has been shown to have a lower rate of complication, shorter recovery and additionally spares renal parenchyma. Documented, dated and probably biased data due to non-uniform patient populations, differences in techniques and retrospective nature reported the risk of a higher local recurrence rate [Citation3]. Inadequate percutaneous access or coagulopathies which cannot be corrected, in addition to lesion size, are the main technical and clinical contraindications of thermal ablation. With the use of ablation, though, target lesion proximity to noble structures (i.e., renal pelvis, ureter, adjacent bowel) can be easily overcome by technical tricks [Citation2,Citation8].
The purpose of this article is to describe the basic concepts of percutaneous ablation in the treatment of RCC. Controversies concerning techniques and products will be addressed. The necessity for an individually tailored approach for the selection of the different techniques and targets will be emphasized.
Patient selection
All cases should be discussed within a multidisciplinary tumor board composed by at least an interventional radiologist, an urologist and an oncologist, as per current guidelines [Citation9]. The ideal candidate for renal tumor ablation is a patient that presents a T1a (≤4 cm) RCC, with contraindications or unwillingness to undergo surgery and with any grade of impairment of renal function. Though pathognomonic features of malignancy at imaging are evident, as detailed previously, pre-ablation biopsy is strongly recommended [Citation9]. Since percutaneous ablation in general clinical practice is performed with moderate sedation (not general anesthesia), impairment of lung function or other relative contraindications to surgery, are not patient exclusion criteria. However, in case of absolute contraindications to surgery (i.e., coagulopathies), percutaneous ablation should be considered with caution [Citation10].
If pathognomonic features of malignancy at imaging studies are not present but the mass has increased over time, treatment may be justified. In this particular scenario, a pre-interventional percutaneous needle biopsy is cardinal; if biopsy fails to prove malignancy (e.g., uncertainty between oncocytoma and chromophobe tumor), percutaneous ablation could be the first choice of intervention, due to its minor invasiveness respect to surgery. Additional ideal candidates for percutaneous renal ablation are those who have already undergone tumor enucleation, partial- or complete-nephrectomy, for who renal function impairment is very likely and parenchymal sparing is crucial. This group of patients is generally affected by genetic syndromes (i.e., Von Hippel Lindau) that develop multiple renal tumors during the course of their life.
A case-to-case evaluation, based on each individual setting, and pre-interventional imaging interpretation, is key in order to identify potential technical contraindications and plan fine tuning where needed.
Ablation modalities
Various ablative techniques have been applied for the treatment of renal tumors, including RF, MW, laser and cryoablation (CA) [Citation11]. The majority of available literature comes from the experience with RF and CA, but MW is recently gaining more importance due to its higher availability and its intrinsic features. RF ablation (RFA) is delivered through high-frequency alternating current (460–500 kHz) via an RF electrode (available in a great number of shapes and sizes) resulting in ionic agitation which causes frictional heat (temperatures of 60–100 °C) (). When temperatures reach 50 °C cellular destruction commences, followed by protein denaturation and finally coagulative necrosis (of tumoral tissue). Gas formation (100 °C) represents the main limitation for a potential increase of temperature [Citation12]. Microwave energy exerts its effect through dielectric hysteresis and water molecules agitation. Microwave generators have overcome the gas formation limitation by operating at 915 MHz to 2.45 GHz. Advantages of MW over RFA include the ability to achieve higher temperatures (over 100 °C) less affected by ‘heat-sink’ effect caused by the blood stream and any kind of impedance-driven performance producing larger ablation volumes in shorter time; other advantages include less procedural pain and lack of a need for grounding pads placement () [Citation13]. Photoablation/laser ablation has been successfully applied in the treatment of benign and malignant diseases, including the treatment of renal tumors. A collimated, coherent, monochromatic light beam is delivered through a small, precise optical fiber which interacts with biological tissues which determines the focal increase in temperature () [Citation10]. Cryoablation is another well-established technique for renal ablation. The depressurization of a cryogen gas, such as argon, causes a fast decrease in temperature at the tip of an antenna (–160 °C or colder), which by passive thermal diffusion acts on the tumor. Slow and fast freezing produces intracellular and extracellular crystals, respectively (). A process of subsequent freeze-thawing cycles causes cell death through cellular dehydration, vascular thrombosis and membrane rupture [Citation14]. Cryoablation is governed by specific advantages including visibility of the ice-ball and the ability to produce a large-sized ablation zone that can be geometrically designed facilitating a reproducible and predictable ablation shape (by simultaneous use of multiple probes) with minimum patient’s pain and discomfort; all these come in increased cost and time duration.
Figure 1. RFA of a right renal tumor adjacent to the right colon. Before the treatment, hydrodissection has been obtained in order to reduce the risk of bowel damage and perforation. (A) Contrast-enhanced CT demonstrating the enhancing tumor in the right kidney (white arrows). (B–D) A spinal needle has been inserted and dextrose 5% has been infused for hydrodissection. (E) An umbrella-like RF electrode has been inserted in the lesion. (F) Post ablation necrotic zone (white arrows) has covered the whole lesion with no damage to the adjacent colon.
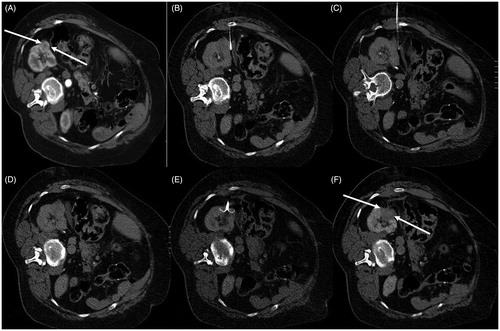
Figure 2. MWA of renal cell carcinoma (biopsy proven) located in the apex of the left kidney. (A) T1-weighted fat saturated sequence post IV contrast medium injection depicting the lesion (arrow) in the apex of the left kidney. (B) Patient was placed in lateral decubitus position – a microwave antenna was inserted and ablation session was performed according to the provided dimensions on terms of energy (W) and time (min). (C, D) Computed tomography scans in arterial (C) and venous (D) phases illustrating complete tumor ablation (arrow).
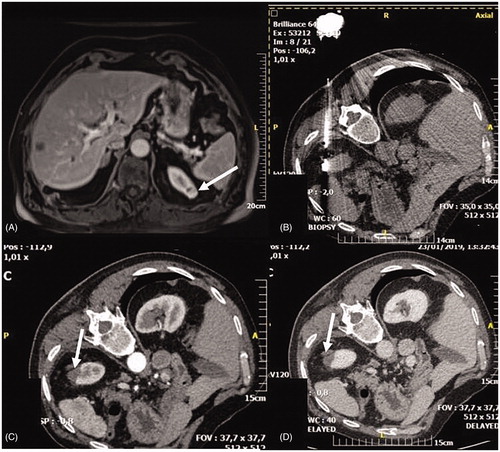
Figure 3. Patient with previous right nephrectomy for RCC who developed a small 9 mm new lesion in the left kidney and was successfully ablated with image guided laser ablation. (A) Contrast-enhanced CT demonstrating a 9 mm posterior enhancing tumor (arrow) in the left kidney. (B) Ultrasound scan demonstrating the left kidney lesion (arrow). (C) Ultrasound scan demonstrating the positioning of the tip of the laser fiber in the middle of the lesion to be treated (arrow). (D) Ultrasound scan demonstrating hyperechoic area due to gas formation during treatment (arrows). (E) Contrast-enhanced ultrasound demonstrating the absence of enhancement at the level of the treated lesion (arrows). (F) Contrast-enhanced MRI at 3 months demonstrating the complete treatment of the renal lesion (arrows = ablative area).
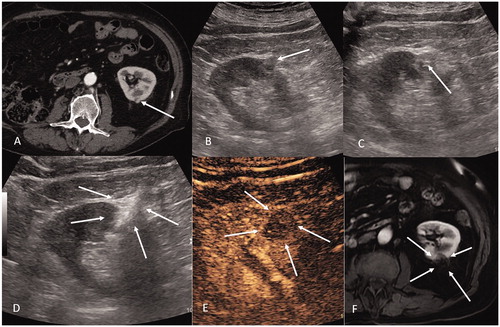
Figure 4. Percutaneous cryoablation of renal clear cell tumor of the right kidney. (A) Arterial phase contrast-enhanced CT (supine position) shows a hypervascular lesion (arrow) in the medial mesorenal region of the right kidney. (B) Same tumor presented in image (a) two cryoablation probes are inserted within the tumor through a posterior retroperitoneal approach. (C) Arterial phase contrast-enhanced CT (supine position) shows a gross hypervascular lesion, which demonstrated to be a clear cell tumor (arrow) in the lower pole of the right kidney. (D) 7 mm-MIP axial reconstruction shows three cryoablation probes positioned within the tumor through a postero-lateral retroperitoneal approach. (E) Same lesion presented in images C and D: sagittal reconstruction shows the three probes oriented in three different planes (arrow) of space in order to shape the ice ball in the three dimensions.
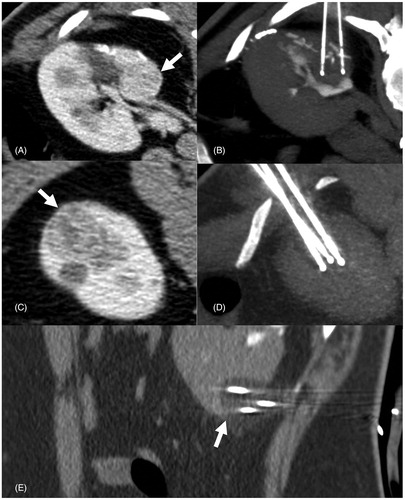
Image-guidance for ablation
RCC ablation is assisted by state-of-the art imaging. US remains the most frequently used and available imaging method to guide renal ablation and assess technical success in certain cases with the aid of US contrast-medium administration (CEUS) [Citation15–18]. Moreover, US has the priceless advantage to provide real-time image guidance, which is extremely useful to follow each step of the tumor ablation procedure. Conversely, US is limited by inherent patient and characteristics and gas formation limits a proper visualization of the ablation area, making post-procedural assessment difficult [Citation15–18]. Limitations are linked to patient and tumor conformation as well as proper visualization of the ablation zones post-procedure or due to gas. Isoechoic tumors or tumors located in deep regions may also be difficult to visualize [Citation15–18].
CT and the more recent CBCT, provide a larger field of view, are less limited by the patient structure or body region, and can provide an easier post-procedural assessment, especially after contrast-medium administration [Citation16]. However, both CT and CBCT require radiation exposure and high operator experience to guide non-axial approaches. MR, due to its characteristics, may see to represent an ideal imaging aid, since it lacks ionizing radiation, provides for good anatomical definition as well as real time visualization of the procedure. MR guidance for ablation, though, requires a complex environment and its use is limited to a few highly specialized centers [Citation9]. Finally, PET/CT has the advantage of merging anatomical and functional information, and has been proposed as a valid alternative in certain interventional procedures in which lesions are poorly visible at conventional imaging [Citation19,Citation20]. In recent years, real time fusion of multiple imaging modalities has been implemented and successfully applied in the guidance of tumor ablation [Citation15–20]. This strategy is highly promising, since it merges advantages of different imaging modalities succumbing their limitations, allowing performance of a successfully ablation even of complex lesions without a relevant increase in the operatory environment complexity [Citation15–20].
Ablation procedure
Different crucial steps lead to successful ablations; planning, targeting, monitoring and result assessment. A personalized treatment planning including careful choice of the best ablation modality, patient positioning, image-guidance and type of anesthesia is critical to achieve a successful procedure and avoid complications. In this perspective, practical algorithms for procedure planning have been proposed, such as the ABLATE approach (A, axial tumor diameter; B, bowel proximity; L, location within the kidney; A, adjacency to the ureter; T, touching renal sinus fat; and E, endophytic or exophytic position) [Citation21]. Particularly important is the diameter of the tumor, directly related to ablation success and bleeding risk [Citation22,Citation23]. After a careful case-to-case evaluation, planning the procedure includes also identifying the ideal anesthesiologic regimen for the procedure. Several technical aspects should be considered for a successful renal ablation, from patient positioning, to the need of stent insertion for pyeloperfusion or need for hydro or pneumo-dissection to protect surrounding critical structures [Citation24]. Proper ablation needs to also consider protective techniques aimed at reducing damage to adjacent structures. Saline hydrodissection and CO2 insufflation are two commonly used to isolate the kidney during the ablation, but sometimes just a proper patient positioning aided by vacuum mattress can be sufficient to displace surrounding structures. In addition, a slight traction of the electrode, known as ‘torqueing’, can be applied during ablation. When the excretory system is at risk for damage, cold pyeloperfusion can be performed through a ureteral single J connected to a cold fluid bag [Citation19]. For a proper tumor targeting, the ideal image guidance method should be carefully chosen.
Once the chosen device has been properly inserted in the target tumor, ablation must be monitored thoroughly to assess ablation technical success and monitor potential complications. Real time US can show early signs of complete ablation, coverage of the target tumor by hyperechoic area due to gas formation, and early bleeding. Once the ablation is completed, contrast-enhanced imaging such as CEUS and/or contrast-enhanced CT can provide more precise information regarding the completeness of ablation, and eventually guide further treatment in the same operative session [Citation25]. Advantages of MRI peri-operative control include the tumor visibility without the need for contrast medium injection, the ability of multiplanar, near-real time advancement of the probe toward the target and the lack of ionizing radiation [Citation26].
Clinical outcomes
Regardless the technique used, clinical outcomes are invariably associated with dimensions of the renal tumor. Ablations of T1a (≤4 cm) lesions show technical success approaching 100% and excellent primary local control. In a recent paper, Talenfeld et al. evaluated retrospectively T1a lesions treated either with RF-, cryo- or MW ablation reporting similar outcomes of total or partial nephrectomy (5-year cancer-specific survival 95–98%), but observing less periprocedural complications, less unplanned hospital readmissions, reduced 30- and 90-day mortality and a lower risk of long-term renal insufficiency [Citation27]. Another recent retrospective analysis performed on a large patient cohort with long-term follow up confirmed 3-, 5- and 9-year specific and nonspecific cancer survival rates not to be statistically significant among thermal ablation, partial and total nephrectomy [Citation28]. These recent insights, along with detailed economic analysis supporting the convenience of minimally invasive procedures have been confirmed in numerous studies, including recent meta-analysis [Citation22,Citation23,Citation29–31]. Within the T1a classification, tumors larger than 3 cm have been associated with higher incidence of recurrence, with a disease-free survival of 68% compared to 97% of tumors ≤3 cm [Citation32]. Even though survival rates are comparable between RF, cryo- and MW ablation, MW ablation has achieved shorter procedural times and required less sedation than cryo- and RFA. However, no compelling evidence suggest that one technique is superior to any of the other [Citation9,Citation22,Citation27].
For T1b lesions (≤7 cm), 5-year overall survival rates were similar in a recent study comparing RFA and CA (78% vs. 82%), even though CA was associated with higher primary technical efficacy (65% vs. 95%) [Citation5]. Radiofrequency ablation achieved notable results when compared to partial nephrectomy, with a 5-year overall survival of 85.5% vs. 96.6%, a 5-year cancer-specific survival of 92.6 vs. 96.6% and a 5-year disease-free-survival of 81.0% vs. 89.7% [Citation33]. In a small patient sample, MW ablation achieved excellent primary efficacy in lesions >4 cm with no local tumor progression after 1-year follow-up [Citation34]. In addition to careful patient selection and the availability of state-of-the art image-guidance, the interventional radiologist’s experience must be considered and most-likely still represents the fundamental element in order to achieve excellent results [Citation35].
Complications
Reported complications include renal infection, hemorrhage, pneumothorax, thermal damage to surrounding structures (ureter, bowel, genitofemoral nerve, psoas muscle) and track seeding [Citation36,Citation37]. Most commonly, complications post-percutaneous ablation of renal masses are associated to bleeding or non-target thermal injury; hematoma formation rate is ∼6% while that of massive bleeding requiring transfusion is <1% [Citation9]. Post-ablation syndrome is an accepted, self-limiting condition occurring in about 9% of patients; a combination of fever and flu-like symptoms (myalgia, malaise, mild pain at the site of ablation) should not be considered as a complication but as an expected side effect [Citation38,Citation39]. Complications are assessed through standard classifications scales such as the revised Clavien–Dindo classification [Citation40] and proposed scores by the main radiological societies [Citation41,Citation42]. 13–17% are classified as minor complications and 1.8–6% major complications, which require non-medical therapy [Citation5,Citation43,Citation44]. Cumulative 30-day non-urologic complication rate has been reported to be 6% for patients treated with percutaneous ablation vs. 29–30% for patients treated with partial or total nephrectomy. Rates of acute renal failure were found to be lower for percutaneous ablation (3% vs. 7–11%) [Citation27,Citation45]. Meta-analytic pooled incidences concerning major and minor complications of MW ablation reported rates of 1.8% and 17.5%, respectively [Citation30].
Controversies
Comparing different ablation techniques
In current clinical practice, the most often used percutaneous ablation techniques are RFA and CA while microwave ablation (MWA) is increasingly being used in the treatment of RCC [Citation9]. Percutaneous irreversible electroporation is a new emerging focal ablation technique but there is limited experience in its use for RCC treatment and high intensity focused ultrasound is used in experimental settings [Citation10,Citation46]. Each technique has advantages and disadvantages that have to be considered by the interventional radiologist in each particular setting to achieve best patient outcome. In literature, no randomized prospective clinical trials have compared different ablation techniques for treatment of RCC [Citation47]. Radiofrequency ablation and CA are the techniques that are advised by current guidelines and are considered equivalent as they both have excellent technical success rates and low tumor recurrence rates (even in cases of repeated ablation) [Citation8,Citation10,Citation48]. Evidence in literature suggests that there are no significant outcome differences regarding overall complications, metastasis-free rates and cancer-specific survival between RFA and CA [Citation8,Citation23]. In comparison to CA, RFA costs less, procedure time is shorter and bleeding complication rate is lower, yet CA is less harmful to the renal collecting system [Citation46,Citation49,Citation50]. Microwave-based techniques present practical advantages over radiofrequencies, but their more recent development is coupled with limited literature compared to RFA and CA, i.e., lack of mid- and long-term follow-up and RCT [Citation12,Citation51]. Available evidence on MWA in renal tumors suggests that MWA has a similar or slightly better technical efficacy rate and local tumor recurrence rate than RFA or CA with comparable complications rates and lower incidence of major complications [Citation30]. In comparison to RFA, MWA procedure times are shorter and the procedure is less influenced by the heat-sink effect also providing larger ablation zones [Citation43,Citation46,Citation51]. Advantages of CA include the ability to monitor ablation with imaging, the potential treatment of T1b RCCs and the treatment of centrally located or very close to vulnerable structures, such as the spinal column and the bowel, RCCs [Citation46,Citation52,Citation53].
Percutaneous ablation vs. surgery or active surveillance
The gold standard of treatment of small RCCs remains surgical resection (radical or partial nephrectomy) but percutaneous ablation is a solid-based alternative technique for the treatment of small RCCs showing comparable oncological outcome results to partial nephrectomy and minimal risk of severe complications [Citation45,Citation54]. In the literature, there is only one randomized controlled trial (RCT) with a small number of patients comparing partial nephrectomy and MWA and there is no level 1 evidence regarding the comparison of the different ablation techniques with surgery for stage T1a RCC [Citation55,Citation56].
Thermal ablation, on contrary to surgery can be performed without the need of general anesthesia and is a nephron-sparing technique facilitating preservation of renal function similar to partial nephrectomy [Citation57]. Published data have shown that major complication rates were significantly lower or similar in thermal ablation treatment compared to partial nephrectomy and overall lower complication rates following thermal ablation [Citation22,Citation58,Citation59]. MWA was shown to have significantly fewer complications and less blood loss than partial nephrectomy and is considered to be as safe as RFA in RCC treatment [Citation55,Citation56]. Deterioration of renal function has been shown to be significantly greater in case of surgery than in case of ablation techniques in many published studies suggesting the feasibility of ablation treatment in patients with impaired baseline renal function [Citation22,Citation60].
Thermal ablation exhibits metastasis-free and cancer-specific survival rates comparable to surgery [Citation23,Citation57]. Published data have shown that local recurrence-free survival was worse for thermal ablation than surgery but secondary efficacy of thermal ablation (repeat ablation) showed no difference compared with surgery [Citation23]. Oncologic outcomes diminish as RCC’s size exceeds 3 cm in diameter [Citation31,Citation61]. Comparison of MWA with partial nephrectomy revealed similar relapse-free survival and cancer-specific survival rates [Citation55]. Ablation techniques have been shown to be more cost-effective than partial nephrectomy due to their minimal-invasive approach and less cost and procedure time [Citation58,Citation62].
In Clinicaltrials.gov using search parameters ‘percutaneous ablation’, only three completed studies are available in which renal masses were ablated using the percutaneous approach. The CONSERVE trial, a feasibility, multicenter RCT comparing surgery to needle ablation techniques in patients with SRMs (4 cm) was concluded in 2015 but results were never published. Results still lack for other two registered studies: the RF-REIN trial, which evaluated the efficacy of RF in the treatment of renal tumors in which 310 patients were enrolled, and the CRYOREIN trial, which evaluated the efficacy of percutaneous CA for renal tumors <4 cm in patients not candidates for partial nephrectomy, respectively, indicated as concluded in 2015 and 2016. Concerning intra-operative ablation, a large multi-institutional European registry (EURECA) which involved 808 patients with T1a RCC treated with laparoscopic-assisted CA, revealed satisfactory long-term oncological outcomes in a non-direct comparison with surgical resection [Citation2].
Another interesting topic to consider is the comparison between active surveillance and ablation: a randomized, feasibility trial comparing ablation vs. active surveillance (SURAB) concluded that the hypothesized recruitment rate was lower than expected and therefore the criteria for disease progression were not met and demonstrated that the times were not mature for conducting and completing such a trial [Citation63]. Active surveillance trials and potentially programs could lead to a better and broadened view of the actual behavior of small renal tumors, yet not clearly understood.
Recent guidelines
Percutaneous ablation for RCC is considered as a treatment alternative to partial nephrectomy and is advised by various published guidelines. In 2015, the European Association of Urology published guidelines stating that thermal ablation is indicated in case of small RCCs in elderly patients with co-morbidities considered unfit for surgical treatment, patients with a genetic predisposition to develop multiple tumors and patients with bilateral tumors or with a solitary kidney and a high risk of complete loss of renal function following partial nephrectomy [Citation64]. It was also stated that due to the quality of the available data (level of evidence 3) no definite conclusions could be reached regarding morbidity and oncological outcomes for RFA and CA [Citation64].
The National Comprehensive Cancer Network (NCCN) Clinical Practice guidelines in oncology published in 2017, stated that ablative techniques can be considered only in selected patients with clinical stage T1a RCCs, are associated with a higher local recurrence rate than conventional surgery and also highlighted the lack of a randomized phase III comparison of ablative techniques with surgery [Citation48]. The guidelines also stated that biopsy may be considered to obtain or confirm a diagnosis of malignancy and guide surveillance and ablation [Citation48].
The 2017 American Society for Clinical Oncology (ASCO) guidelines on the management of small RCCs stated that percutaneous thermal ablation should be considered for patients with tumors that are located such that complete ablation can be achieved and TA should be reserved for carefully selected and counseled patients [Citation3]. This recommendation was supported by intermediate quality evidence and was considered to have a moderate strength [Citation3]. Biopsy is recommended prior or at the time of ablation in order to guide surveillance [Citation3].
In 2017, the American Urological Association (AUA) published updated guidelines stating that thermal ablation should be considered as an alternate approach for management of cT1a renal masses <3 cm in diameter and a percutaneous approach is preferred [Citation8]. The guidelines suggested that RFA and CA are equivalent and have similar oncologic efficacy [Citation8]. The above recommendations were supported by grade C evidence [Citation8]. It was also stated that a renal mass biopsy should be performed prior to ablation for diagnostic purposes and to guide surveillance and a strong recommendation (evidence level grade B) was made regarding counseling about thermal ablation that should include information about an increased likelihood of tumor persistence or local recurrence after primary ablation which may be addressed with repeat ablation if further intervention is elected [Citation8].
As the level and quality of evidence regarding percutaneous ablation techniques remains low, and this is stated in the aforementioned guidelines, there is need for level 1 data comparing the available treatment strategies for small RCCs to clearly establish the role of thermal ablation in the management of these tumors. The main concern which has hampered the widespread adoption of thermal ablation to treat renal tumors is the lack of data proving sustained oncological efficacy. The absence of RCTs, the heterogeneity of patient populations investigated, indications, histopathology and methods described in the literature do not consistently provide for the above. However, in the past couple of years, literature has been laden with substantiated intermediate and long-term follow-up data; reviews and series, mainly from retrospective observational studies in which thermal ablation, mainly RFA and CA, is compared to surgery still considered gold-standard [Citation12,Citation24,Citation52,Citation58,Citation65–68]. In addition, percutaneous thermal based techniques are compared to surgery (nephrectomy, partial nephrectomy), to laparoscopy and among themselves [Citation27,Citation69–76]. The most recent European Society of Medical Oncology (ESMO) guidelines suggest that percutaneous ablation techniques (incl. RFA, MWA and CWA) are options for patients with small cortical tumors <3 cm in diameter especially in cases of co-morbidities [Citation77].
Conclusions
Percutaneous ablation of RCC is a safe and efficacious technique for the treatment of T1a renal masses. As size grows larger than 4 cm in T1b tumors addition of trans-arterial embolization can be suggested in order to enhance percutaneous ablation. Percutaneous ablation procedures for the treatment of RCC are low cost, of short duration of hospitalization with low complication rates when compared to surgical approaches. Further evaluation of the technique against surgical therapies in a comparative randomized mode is warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ellman BA, Parkhill BJ, Curry TS, et al. Ablation of renal tumors with absolute ethanol: a new technique. Radiology. 1981;141:619–626.
- Nielsen TK, Lagerveld BW, Keeley F, et al. Oncological outcomes and complication rates after laparoscopic-assisted cryoablation: a European Registry for Renal Cryoablation (EuRECA) multi-institutional study. BJU Int. 2017;119:390–395.
- Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American society of clinical oncology clinical practice guideline. J Oncol Pract. 2017;35:668–680.
- Brierley JD, Gospodarowicz MK, Wittekind C, editors. The TNM classification of malignant tumours. 8th ed. Hoboken, New Jersey: Wiley-Blackwell; 2107.
- Hasegawa T, Yamanaka T, Gobara H, et al. Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: a multi-center study. Jpn J Radiol. 2018;36:551–558.
- Iguchi T, Hiraki T, Tomita K, et al. Simultaneous biopsy and radiofrequency ablation of T1a renal cell carcinoma. Diagn Interv Imaging. 2016;97:1159–1164.
- Iannuccilli JD, Grand DJ, Dupuy DE, et al. Percutaneous ablation for small renal masses-Imaging follow-up. Semin Intervent Radiol. 2014;31:50–63.
- Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529.
- Krokidis ME, Orsi F, Katsanos K, et al. CIRSE guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2017;40:177–191.
- Sartori S, Mauri G, Tombesi P, et al. Ultrasound-guided percutaneous laser ablation is safe and effective in the treatment of small renal tumors in patients at increased bleeding risk. Int J Hyperthermia. 2018;35:19–25.
- Higgins LJ, Hong K. Renal ablation techniques: state of the art. Am J Roentgenol. 2015;205:735–741.
- Stone MJ, Venkatesan AM, Locklin J, et al. Radiofrequency ablation of renal tumors. Tech Vasc Interv Radiol. 2007;10:132–139.
- Cornelis FH, Marcelin C, Bernhard J-C. Microwave ablation of renal tumors: a narrative review of technical considerations and clinical results. Diagn Interv Imaging. 2017;98:287–297.
- Maria T, Georgiades C. Percutaneous cryoablation for renal cell carcinoma. J Kidney Cancer VHL. 2015;2:105–113.
- Mauri G. Expanding role of virtual navigation and fusion imaging in percutaneous biopsies and ablation. Abdom Imaging. 2015;40:3238–3239.
- Monfardini L, Orsi F, Caserta R, et al. Ultrasound and cone beam CT fusion for liver ablation: technical note. Int J Hyperthermia. 2018;35:500–504.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–151.
- Mauri G, Nicosia L, Varano GM, et al. Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation. Insights Imaging. 2017;8:357–363.
- Ryan ER, Sofocleous CT, Schöder H, et al. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology. 2013;268:288–295.
- Mauri G, Gennaro N, De Beni S, et al. Real-time US-18FDG-PET/CT image fusion for guidance of thermal ablation of 18FDG-PET-positive liver metastases: the added value of contrast enhancement. Cardiovasc Intervent Radiol. 2019;42:60–68.
- Schmit GD, Kurup AN, Weisbrod AJ, et al. ABLATE: a renal ablation planning algorithm. Am J Roentgenol. 2014;202:894–903.
- Katsanos K, Mailli L, Krokidis M, et al. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc Intervent Radiol. 2014;37:427–437.
- Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196:989–999.
- Uppot RN, Silverman SG, Zagoria RJ, et al. Imaging-guided percutaneous ablation of renal cell carcinoma: a primer of how we do it. Am J Roentgenol. 2009;192:1558–1570.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209–216.
- Cazzato RL, Garnon J, Shaygi B, et al. How to perform a routine cryoablation under MRI guidance. Top Magn Reson Imaging. 2018;27:33–38.
- Talenfeld AD, Gennarelli RL, Elkin EB, et al. Percutaneous ablation versus partial and radical nephrectomy for T1a renal cancer: a population-based analysis. Ann Intern Med. 2018;169:69.
- Xing M, Kokabi N, Zhang D, et al. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked Population Study. Radiology. 2018;288:81–90.
- Kowalczyk KJ, Choueiri TK, Hevelone ND, et al. Comparative effectiveness, costs and trends in treatment of small renal masses from 2005 to 2007. BJU Int. 2013;112:E273–E280.
- Choi SH, Kim JW, Kim JH, et al. Efficacy and safety of microwave ablation for malignant renal tumors: an updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19:938–949.
- Uhlig J, Kokabi N, Xing M, et al. Ablation versus resection for stage 1A renal cell carcinoma: national variation in clinical management and selected outcomes. Radiology. 2018;288:889–897.
- Johnson BA, Sorokin I, Cadeddu JA. Ten-year outcomes of renal tumor radio frequency ablation. J Urol. 2019;201:251–258.
- Chang X, Zhang F, Liu T, et al. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: long-term clinical and oncologic outcomes. J Urol. 2015;193:430–435.
- Wells SA, Wheeler KM, Mithqal A, et al. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol. 2016;41:1203–1211.
- Mauri G, Sconfienza LM. Is operators’ experience more important than the ablation technique in image-guided thermal ablations? Int J Hyperth. 2017;33:955–956.
- Gervais DA, McGovern FJ, Arellano RS, et al. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424.
- Lee SJ, Choyke LT, Locklin JK, et al. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol. 2006;17:1967–1969.
- Kim KR, Thomas S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin Intervent Radiol. 2014;31:138–148.
- Zhong J, Bambrook J, Bhambra B, et al. Incidence of post-ablation syndrome following image-guided percutaneous cryoablation of renal cell carcinoma: a prospective study. Cardiovasc Intervent Radiol. 2018;41:270–276.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213.
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28:1432–1437.e3.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146.
- Zhou W, Arellano RS. Thermal ablation of t1c renal cell carcinoma: a comparative assessment of technical performance, procedural outcome, and safety of microwave ablation, radiofrequency ablation, and cryoablation. J Vasc Interv Radiol. 2018;29:943–951.
- Hui GC, Tuncali K, Tatli S, et al. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19:1311–1320.
- Lourenco P, Bilbey N, Gong B, et al. Percutaneous ablation versus nephrectomy for small renal masses: clinical outcomes in a single-center cohort. Cardiovasc Intervent Radiol. 2018;41:1892–1900.
- Zondervan PJ, Buijs M, De Bruin DM, et al. Available ablation energies to treat cT1 renal cell cancer: emerging technologies. World J Urol. 2019;37:445–455.
- Almassi N, Gill BC, Rini B, et al. Management of the small renal mass. Transl Androl Urol. 2017;6:923–930.
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:804–834.
- Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Intervent Radiol: JVIR. 2012;23:48–54.
- Zargar H, Atwell TD, Cadeddu JA, et al. Cryoablation for small renal masses: selection criteria, complications, and functional and oncologic results. Eur Urol. 2016;69:116–128.
- Filippiadis DK, Gkizas C, Chrysofos M, et al. Percutaneous microwave ablation of renal cell carcinoma using a high power microwave system: focus upon safety and efficacy. Int J Hyperth. 2018;34:1077–1081.
- Patel N, King AJ, Breen DJ. Percutaneous image-guided cryoablation of small renal masses. Abdom Radiol. 2016;41:754–766.
- Buy X, Lang H, Garnon J, et al. Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. AJR Am J Roentgenol. 2013;201:1353–1361.
- Azevedo AAP, Rahal AJ, Falsarella PM, et al. Image-guided percutaneous renal cryoablation: five years experience, results and follow-up. Eur J Radiol. 2018;100:14–22.
- Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106:316–321.
- Krokidis ME, Kitrou P, Spiliopoulos S, et al. Image-guided minimally invasive treatment for small renal cell carcinoma. Insights Imaging. 2018;9:385–390.
- Ward RD, Tanaka H, Campbell SC, et al. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics. 2018;38:2021–2033.
- Yin X, Cui L, Li F, et al. Radiofrequency ablation versus partial nephrectomy in treating small renal tumors: a systematic review and meta-analysis. Medicine. 2015;94:e2255.
- Wang S, Qin C, Peng Z, et al. Radiofrequency ablation versus partial nephrectomy for the treatment of clinical stage 1 renal masses: a systematic review and meta-analysis. Chin Med J. 2014;127:2497–2503.
- Pantelidou M, Challacombe B, McGrath A, et al. Percutaneous radiofrequency ablation versus robotic-assisted partial nephrectomy for the treatment of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2016;39:1595–1603.
- Zagoria RJ, Pettus JA, Rogers M, et al. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77:1393–1397.
- Castle SM, Gorbatiy V, Avallone MA, et al. Cost comparison of nephron-sparing treatments for cT1a renal masses. Urol Oncol. 2013;31:1327–1332.
- Soomro N, Lecouturier J, Stocken DD, et al. Surveillance versus ablation for incidentally diagnosed small renal tumours: the SURAB feasibility RCT. Health Technol Assess. 2017;21:1–68.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924.
- Breen DJ, Railton NJ. Minimally invasive treatment of small renal tumors: trends in renal cancer diagnosis and management. Cardiovasc Intervent Radiol. 2010;33:896–908.
- El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110:510–516.
- Sandbergen L, Guven S, Laguna MP. Can ablation win against partial nephrectomy and become first line therapy in cT1a renal tumours? Curr Opin Urol. 2019;29:70–77.
- Ginzburg S, Tomaszewski JJ, Kutikov A. Focal ablation therapy for renal cancer in the era of active surveillance and minimally invasive partial nephrectomy. Nat Rev Urol. 2017;14:669–682.
- Zagoria RJ, Traver MA, Werle DM, et al. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol. 2007;189:429–436.
- Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol. 2005;185:64–71.
- Kutikov A, Smaldone MC, Uzzo RG, et al. Platinum priority – kidney cancer comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252–259.
- Thompson SM, Schmitz JJ, Thompson RH, et al. Introduction of microwave ablation into a renal ablation practice: valuable lessons learned. Am J Roentgenol. 2018;211:1381–1389.
- Wah TM, Irving HC, Gregory W, et al. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113:416–428.
- Psutka S, McGovern F, Mueller P, et al. 1115 long-term durable oncologic outcomes after radiofrequency ablation for t1a renal cell carcinoma. J Urol. 2012;187:e452.
- Long J-A, Bernhard J-C, Bigot P, et al. Partial nephrectomy versus ablative therapy for the treatment of renal tumors in an imperative setting. World J Urol. 2017;35:649–656.
- Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. Am J Roentgenol. 2013;200:461–466.
- Escudier B, Porta C, Schmidinger M, ESMO Guidelines Committee, et al.Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720.
