ABSTRACT
Fever is a complex physiological response to pathogen infection and injury. One of the beneficial effects of febrile temperatures is stimulation of immune cell trafficking to the lymphoid organs and inflamed tissues, thereby enhancing immune surveillance during infection and inflammation. This trafficking process consists of a highly ordered adhesion cascade that includes tethering and rolling of immune cells along the vessel walls, chemokine-induced activation, firm arrest and diapedesis. In this review, we summarize the current findings of how febrile temperatures regulate the immune cell trafficking process. Febrile temperatures play multiple roles in the functional regulation of critical biomolecules involved in each step of the ordered adhesion cascade that includes L-selectin, chemokines, and α4 and β2 integrins. A better understanding of febrile temperature-induced regulation of immune cell trafficking will shed light on modulating the immunity to fight against infection and inflammation.
1. Introduction
Trafficking of immune cells from blood circulation to lymphoid organs and inflamed tissues has a crucial role in immune surveillance and host defense [Citation1–3]. The entry of blood-borne naive lymphocytes to lymph nodes preferentially occurs at high endothelial venules (HEVs), and this process is essential for lymphocytes to encounter antigens and antigen-presenting cells, such as dendritic cells [Citation4]. HEVs are composed of plump endothelial cells that bulge into the vascular lumen. Recirculation of antigen-specific lymphocytes through lymph nodes allows them to survey their target antigens in any part of the body. This course of action provides effective immune surveillance against foreign invaders (such as viruses, bacteria, and helminths) and alterations in the body’s own cells (such as abnormal self-antigens in cancer) [Citation2]. During homeostasis, HEVs are found only in the lymphoid organs, but they can develop in non-lymphoid tissues during chronic inflammatory diseases and cancer; thus, they are associated with high levels of lymphocyte infiltration into these tissues [Citation5–7]. Furthermore, the recruitment of neutrophils, monocytes, and some other immune cells to inflamed tissues also requires adhesion to and transmigration through the blood vessel walls [Citation8,Citation9].
Febrile temperature is defined as a body temperature above the normal range, which can result from hyperthermia or fever [Citation4,Citation10]. Hyperthermia is a condition in which an individual’s body produces or absorbs more heat than it dissipates due to failed thermoregulation while the body temperature set-point remains normal [Citation11], whereas fever is a complex physiological response to infection or injury, and the set-point is elevated through cytokine-mediated changes in the hypothalamic regulation [Citation12–14]. Local release of endogenous prostaglandin E2 (PGE2) and pyrogenic cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) act systemically to induce fever [Citation4,Citation15]. An increase of 1–4 °C in core body temperature is associated with improved organism survival and the resolution of many infections [Citation4]. During the last two decades, emerging evidence has shown that febrile temperatures can enhance immune cell trafficking [Citation16,Citation17]. In this review, we summarize and discuss the current understanding of how febrile temperatures regulate the immune cell trafficking process.
2. Adhesion cascade during immune cell trafficking
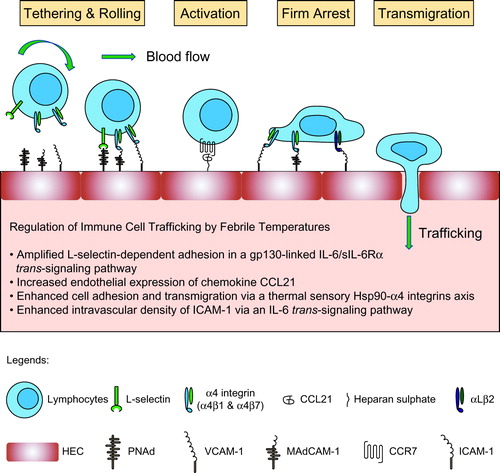
The immune cell trafficking process consists of a highly ordered adhesion cascade that includes tethering and rolling of immune cells along the HEV walls, chemokine-induced activation, firm arrest, and transendothelial migration [Citation3,Citation18,Citation19].
The initial tethering and rolling of lymphocytes are mainly mediated by the interaction between selectins and their ligands. L-selectin (also known as CD62L), expressed by leukocytes, recognizes its counter-receptor (peripheral node addressin, PNAd) on the HEVs to mediate the tethering and rolling [Citation2]. In addition, inactive α4β1, α4β7, and αLβ2 integrins are also able to support lymphocyte rolling by binding to their endothelial ligands, vascular cell adhesion molecule 1 (VCAM-1), mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), and intercellular adhesion molecules (ICAMs) [Citation8,Citation20], respectively.
Chemokine-induced activation is the critical step to switch from the rolling to firm arrest [Citation21]. The homeostatically expressed chemokine ligands CC-chemokine ligand 21 (CCL21), CXC-chemokine ligand 12 (CXCL12), and CXCL13 are the crucial factors for lymphocyte extravasation through the HEVs in lymph nodes. Naive T lymphocytes express CC-chemokine receptor 7 (CCR7) and CXC-chemokine receptor 4 (CXCR4), which are the receptors for CCL21 and CXCL12, respectively. Furthermore, naive B lymphocytes express CXCR5, which is the receptor for CXCL13, in addition to CCR7 and CXCR4 [Citation3,Citation22,Citation23]. During this process, chemokines activate integrins by rapidly triggering an inside-out signaling network that regulates the binding of intracellular effector proteins (e.g., talin or kindlin) to the cytoplasmic domains of integrins [Citation24,Citation25]. Binding of the effector proteins converts the inactive integrin in the low-affinity (bent) conformation into its active form, characterized by an extended conformation with high affinity for the ligands [Citation26,Citation27].
Activated α4 and β2 integrins (e.g., α4β1, α4β7, αLβ2, and αMβ2) mediate firm cell arrest on the endothelium by binding to their distinct endothelial ligands (VCAM-1, MAdCAM-1, and ICAM-1) with high affinity.
The final transmigration step across HEVs involves adhesion molecules, including α4β1, αLβ2, VCAM-1, ICAM-1, ICAM-2, platelet endothelial cell adhesion molecule-1 (PECAM-1), junctional adhesion molecule 1 (JAM-1), and JAM-2 [Citation28,Citation29].
3. Febrile temperatures amplify L-selectin-dependent lymphocyte adhesion and trafficking
By binding to PNAd, L-selectin mediates the initial tethering and rolling of immune cells along the vessel walls. Treatment of lymphocytes with the fever-range temperatures (38–41 °C) strongly increases L-selectin-dependent lymphocyte adhesion to cryosections of lymph node HEVs in vitro and trafficking to peripheral lymph nodes (PLNs), mesenteric lymph nodes (MLNs), and Peyer’s patches (PPs) in short-term homing assay [Citation17,Citation30]. Moreover, exposure of mice to fever-range whole-body hyperthermia (WBH) or LPS/turpentine-induced fever stimulates lymphocyte homing to secondary lymphoid tissues in an L-selectin-dependent manner [Citation17].
Investigation of the mechanisms underlying the thermal regulation of L-selectin adhesion shows that fever-range hyperthermia neither increases L-selectin surface density on lymphocytes nor alters the expression of PNAd on HEVs [Citation30,Citation31]. Further study demonstrates that IL-6 cooperates with a soluble form of IL-6 receptor-α (sIL-6Rα) and the membrane-anchored gp130 to amplify L-selectin adhesion in response to thermal stimulation [Citation32,Citation33]. The MEK1-ERK1/2 signaling pathway acts as the downstream of gp130-linked IL-6/sIL-6Rα trans-signaling to increase L-selectin/cytoskeleton interactions and L-selectin avidity/affinity in lymphocytes [Citation4,Citation32]. Furthermore, 11 amino acids on the C-terminus of L-selectin are essential to mediate the association with the cytoskeleton [Citation34].
4. Febrile temperatures enhance endothelial expression of chemokine CCL21
The interaction between chemokine CCL21 displayed on the lumenal surface of HEVs with Gα1-protein-coupled chemokine receptor CCR7 on lymphocytes triggers integrin activation and the subsequent firm cell arrest [Citation1,Citation3,Citation22]. Indeed, fever-range WBH treatment substantially increases the intravascular presentation of CCL21 without affecting the weak-to-nondetectable staining for other homeostatic chemokines (CXCL12 and CXCL13) on HEVs [Citation31]. However, there is no change in CCR7 expression on T lymphocytes after thermal stress in vitro and in vivo. This observation excludes a role for increased expression of chemokine receptors in enhancing T lymphocyte homing after thermal stress [Citation35].
5. Febrile temperatures regulate integrin-mediated lymphocyte adhesion and transmigration
α4 and β2 integrins involve in all the steps during lymphocyte homing and thus have essential roles in regulating lymphocyte trafficking to the lymphoid organs and inflamed tissues. The expression of α4 integrin ligands (VCAM-1 and MAdCAM-1) and β2 integrin ligands (ICAMs) on the blood vessels is homeostatic and also inducible upon inflammation [Citation9,Citation36]. Emerging evidence shows that febrile temperatures regulate α4- and β2-integrin-mediated lymphocyte adhesion and transmigration via distinct mechanisms.
Fever-range hyperthermia treatment (40 °C, 12 h) significantly enhances α4β7 integrin-dependent adhesion of murine TK1 lymphoma cells and human peripheral blood lymphocytes (PBLs) to HEV cryosections in vitro [Citation37]. Similarly, fever-range WBH treatment of mice also causes α4β7 integrin-dependent lymphocyte redistribution in lymphoid tissues [Citation17], without affecting the expression of α4β7 integrin on lymphocytes [Citation17,Citation37] or MAdCAM-1/VCAM-1 on HEVs [Citation31,Citation35].
A recent study reveals a mechanism underlying the functional regulation of α4 integrin using fever-range hyperthermia treatment and an infection-induced mouse model of fever [Citation35]. Febrile temperatures (≥38.5 °C) can efficiently enhance the expression of heat shock protein 90 (Hsp90) in T lymphocytes [Citation35,Citation38]. Hsp90 binds to the cytoplasmic tail of α4 and induces association of talin and kindlin-3 with the cytoplasmic tails of integrin β subunit, triggering α4 integrin activation via inside-out signaling. Moreover, the N- and C-terminus of one Hsp90 molecule can simultaneously bind to two α4 tails, resulting in dimerization and clustering of α4 integrins on the plasma membrane and subsequent activation of the FAK-RhoA signaling pathway in T lymphocytes, thereby promoting T lymphocyte adhesion and transmigration. This regulation of α4 integrin function does not require the ATPase activity of Hsp90, suggesting that this function is distinct from Hsp90’s chaperone function, which requires the energy released from ATP hydrolysis.
Abolishing Hsp90-α4 interaction in vivo inhibits WBH-induced T lymphocyte trafficking to draining lymph nodes [Citation35]. Moreover, in Salmonella typhimurium infection-induced mouse model of fever [Citation39], disruption of Hsp90-α4 interaction in the mice markedly decreases the number of infiltrated T lymphocytes and increases bacterial dissemination in the small intestine 5 days after the oral administration of S. typhimurium [Citation35]. Thus, Hsp90-α4 integrin axis is a thermal sensory pathway that promotes T lymphocyte trafficking to inflamed tissues and facilitates the clearance of bacterial infection. It is noteworthy that fever-induced Hsp90 expression in T lymphocytes can last for at least 48 h, which enables a persistent regulation of α4 integrin function even after the temperature drops back to the normal range [Citation35]. In addition to T lymphocytes, Hsp90-α4 integrin axis can also enhance the trafficking of other α4 integrin-expressing immune cells, such as monocytes, and B lymphocytes [Citation35].
ICAM-1 and ICAM-2 are two major ligands for β2 integrins during steady-state trafficking of lymphocytes [Citation40,Citation41]. Although febrile temperatures do not increase the surface density and ligand-binding affinity of integrin αLβ2 [Citation30,Citation37], fever-range thermal stress has been shown to enhance endothelial expression of ICAM-1 [Citation31,Citation42–44]. In WBH-treated mice, the ICAM-1 expression on HEVs is strongly upregulated via an IL-6 trans-signaling pathway to facilitate αLβ2-integrin-dependent lymphocyte adhesion to and transmigration across the HEV walls [Citation31]. Conversely, the intravascular density of ICAM-2 is not altered by thermal stress. Thus, the “HEV axis” works as a thermally sensitive alert system by amplifying ICAM-1 density to promote β2-integrin-dependent lymphocyte trafficking to the lymphoid organs [Citation31,Citation45].
6. Perspective
In this review, we summarize the mechanisms underlying the regulation of immune cell trafficking by febrile temperatures caused by hyperthermia or fever. It is noteworthy that hyperthermia is different from fever. For example, peripheral blood vessels constrict during fever, whereby the body heat is conserved, whereas they dilate to dissipate the heat in hyperthermia [Citation46–48]. This difference may prompt a major revision of the mechanisms underlying the regulation of immune cell trafficking.
The regulation of immune cell trafficking by thermal stress is explained by the nature of the adhesion molecules on both immune cells and HEVs. Febrile temperatures regulate each step of the adhesion cascade differently (). Firstly, L-selectin-dependent lymphocyte tethering and rolling are amplified in a gp130-linked IL-6/sIL-6Rα trans-signaling pathway. Secondly, fever promotes α4-positive immune cell adhesion and transmigration via a thermal sensory pathway comprising Hsp90-α4 integrins. By the binding of Hsp90, α4 integrins are activated via inside-out signaling. Meanwhile, α4 integrin dimerization and clustering is induced on the cell membrane to activate the FAK-RhoA signaling pathway. Thirdly, febrile temperatures “preferentially” enhance the intravascular density of two molecules involved in homeostatic trafficking (CCL21 and ICAM-1). These two molecules act cooperatively to optimize the binding activity of αLβ2 integrin. In addition, thermal induction of ICAM-1 involves an IL-6 trans-signaling pathway. Taken together, febrile temperatures systemically regulate immune cell delivery to HEVs, thereby promoting immune surveillance during infection and inflammation.
Hsps are cytoprotective proteins that are constitutively expressed and also rapidly induced under proteotoxic stress conditions such as heat [Citation4,Citation49]. Although Hsps were originally discovered in the context of heat shock (42–45 °C), they are also induced by febrile temperatures (38–41 °C) in mammalian cells [Citation49,Citation50]. Multiple lines of evidence have demonstrated that Hsps, especially Hsp70 and Hsp90, are involved in the febrile temperature-induced regulation of both innate and adaptive immunity [Citation4,Citation51–53]. However, the direct participation of Hsps in immune cell trafficking is poorly understood. A recent study shows that fever promotes T lymphocyte trafficking via a thermal sensory Hsp90-α4 integrin pathway [Citation35]. By inducing selective binding of Hsp90 to α4 integrins, but not β2 integrins, fever increases α4-integrin-mediated T lymphocyte adhesion and transmigration. Additionally, another study finds that Hsp70 could associate with the cytoplasmic domain of integrin β7 subunit [Citation54]. Furthermore, Hsp40, Hsp60, and Hsp70 bind to both α4 and β2 integrins [Citation35], suggesting that these Hsps may involve in the constructive regulation of integrin-mediated cell adhesion and migration by thermal stress, which needs further investigation.
Immune cell trafficking participates in all kinds of autoimmune diseases (such as multiple sclerosis, inflammatory bowel disease, lupus, rheumatoid arthritis, etc.) and even cancer. Based on the mechanisms discussed in this review, it might be possible to promote immune cell trafficking to enhance the immune response against infections and cancer, or suppress it during chronic inflammation and autoimmune disorders. Thereby, modulation of immune cell trafficking may help developing disease management strategies [Citation55–57].
Acknowledgement
The authors gratefully acknowledge the support of SA-SIBS scholarship program.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by grants from the National Natural Science Foundation of China [31525016, 31830112, 31601129, 31701219], Program of Shanghai Academic Research Leader [19XD1404200], Personalized Medicines-Molecular Signature-based Drug Discovery and Development, the Strategic Priority Research Program of the Chinese Academy of Sciences [XDA12010101], China Postdoctoral Science Foundation [2016M601670], the CAS/SAFEA International Partnership Program for Creative Research Teams.
References
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66.
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773.
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349.
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457.
- Drayton DL, Liao S, Mounzer RH, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353.
- Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687.
- Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689.
- Barreiro O, Sanchez-Madrid F. Molecular basis of leukocyte-endothelium interactions during the inflammatory response. Rev Esp Cardiol. 2009;62:552–562.
- Ogoina D. Fever, fever patterns and diseases called ‘fever’ – a review. J Infect Public Health. 2011;4:108–124.
- Dalal S, Zhukovsky DS. Pathophysiology and management of fever. J Support Oncol. 2006;4:9–16.
- Mackowiak PA. Pathophysiology and management of fever – we know less than we should. J Support Oncol. 2006;4:21–22.
- Robins HI, Brandt K, Longo WL. Pathophysiology and management of fever revisited. J Support Oncol. 2006;4:265–266 (author reply 266).
- Walter EJ, Hanna-Jumma S, Carraretto M, et al. The pathophysiological basis and consequences of fever. Crit Care. 2016;20:200.
- Appenheimer MM, Chen Q, Girard RA, et al. Impact of fever-range thermal stress on lymphocyte-endothelial adhesion and lymphocyte trafficking. Immunol Invest. 2005;34:295–323.
- Ostberg JR, Repasky EA. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int J Hyperthermia. 2000;16:29–43.
- Evans SS, Wang WC, Bain MD, et al. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727–2733.
- Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591.
- Picker LJ. Mechanisms of lymphocyte homing. Curr Opin Immunol. 1992;4:277–286.
- Schurpf T, Springer TA. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30:4712–4727.
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702.
- Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370.
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371.
- Ye F, Snider AK, Ginsberg MH. Talin and kindlin: the one-two punch in integrin activation. Front Med. 2014;8:6–16.
- Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517.
- Hogg N, Patzak I, Willenbrock F. The insider's guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–426.
- Sun Z, Costell M, Fassler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21:25–31.
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388.
- Boscacci RT, Pfeiffer F, Gollmer K, et al. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116:915–925.
- Wang WC, Goldman LM, Schleider DM, et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160:961–969.
- Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7:1299–1308.
- Chen Q, Wang WC, Bruce R, et al. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20:59–70.
- Appenheimer MM, Girard RA, Chen Q, et al. Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur J Immunol. 2007;37:2856–2867.
- Evans SS, Schleider DM, Bowman LA, et al. Dynamic association of L-selectin with the lymphocyte cytoskeletal matrix. J Immunol. 1999;162:3615–3624.
- Lin C, Zhang Y, Zhang K, et al. Fever promotes T lymphocyte trafficking via a thermal sensory pathway involving heat shock protein 90 and alpha4 integrins. Immunity. 2019;50:137–151 e136.
- Norris P, Poston RN, Thomas DS, et al. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991;96:763–770.
- Evans SS, Bain MD, Wang WC. Fever-range hyperthermia stimulates alpha4beta7 integrin-dependent lymphocyte-endothelial adhesion. Int J Hyperthermia. 2000;16:45–59.
- Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114.
- Mathur R, Oh H, Zhang D, et al. A mouse model of Salmonella typhi infection. Cell. 2012;151:590–602.
- Lehmann JC, Jablonski-Westrich D, Haubold U, et al. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol. 2003;171:2588–2593.
- Gerwin N, Gonzalo JA, Lloyd C, et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyperresponsiveness. Immunity. 1999;10:9–19.
- Chen Q, Fisher DT, Kucinska SA, et al. Dynamic control of lymphocyte trafficking by fever-range thermal stress. Cancer Immunol Immunother. 2006;55:299–311.
- Fisher DT, Vardam TD, Muhitch JB, et al. Fine-tuning immune surveillance by fever-range thermal stress. Immunol Res. 2010;46:177–188.
- Chen Q, Appenheimer MM, Muhitch JB, et al. Thermal facilitation of lymphocyte trafficking involves temporal induction of intravascular ICAM-1. Microcirculation. 2009;16:143–158.
- Vardam TD, Zhou L, Appenheimer MM, et al. Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine. 2007;39:84–96.
- Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39:139–148.
- Xu Y, Choi J, Hylander B, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia. 2007;23:513–527.
- Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612.
- Hasday JD, Thompson C, Singh IS. Fever, immunity, and molecular adaptations. Compr Physiol. 2014;4:109–148.
- Ostberg JR, Kaplan KC, Repasky EA. Induction of stress proteins in a panel of mouse tissues by fever-range whole body hyperthermia. Int J Hyperthermia. 2002;18:552–562.
- Di YP, Repasky EA, Subjeck JR. Distribution of HSP70, protein kinase C, and spectrin is altered in lymphocytes during a fever-like hyperthermia exposure. J Cell Physiol. 1997;172:44–54.
- Chatterjee M, Jain S, Stuhmer T, et al. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728.
- Sato N, Yamamoto T, Sekine Y, et al. Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochem Biophys Res Commun. 2003;300:847–852.
- Chan YC, Greenwood DR, Yang Y, et al. Leukocyte integrin α4β7 associates with heat shock protein 70. Mol Cell Biochem. 2015;409:263–269.
- Evans SS, Fisher DT, Skitzki JJ, et al. Targeted regulation of a lymphocyte-endothelial-interleukin-6 axis by thermal stress. Int J Hyperthermia. 2008;24:67–78.
- Fisher DT, Chen Q, Skitzki JJ, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–3859.
- Kraybill WG, Olenki T, Evans SS, et al. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperthermia. 2002;18:253–266.