Abstract
Objectives: To compare the clinical outcomes of ultrasound-guided laser ablation (LA) and surgery for treatment of solitary papillary thyroid microcarcinoma (PTMC).
Methods: A total of 81 consecutive patients with solitary PTMC were included in this retrospective study. Among them, 36 received LA and 45 underwent surgery. Surgery was performed by hemithyroidectomy with unilateral central neck dissection. The follow-up consisted of a physical examination, neck ultrasonography, chest X-ray or CT scan and thyroid function tests. The procedure time, hospital stay, complication and recurrence rates were compared between the two groups after treatment.
Results: The follow-up period for the LA and surgical group were 49.2 ± 4.5 months (range, 30–54 months) and 48.5 ± 6.2 months (range, 24–54 months), respectively. The mean hospital stay and procedure time in the LA group were shorter than those in the surgical group. After LA, the largest diameter and average volume decreased from 4.7 ± 1.4 mm to 0.2 ± 0.8 mm, and from 43.2 ± 38.8 mm3 to 0.7 ± 4.1 mm3 (p < .05 for both), respectively. The complication rates and recurrence rates did not differ between the LA group (2.8% [1 of 36] and 5.6% [2 of 36]) and the surgical group (6.7% [3 of 45] and 6.7% [3 of 45]) (p > .05 for both). No distant metastasis occurred in the either group during the follow-up period.
Conclusions: Compared with hemithyroidectomy with unilateral central neck dissection, ultrasound-guided LA was also a safe and effective therapy for treating solitary PTMC, and it may be considered as a treatment alternative for patients who are ineligible or refusal to undergo surgery.
Introduction
Papillary thyroid microcarcinoma (PTMC) is defined as a papillary thyroid carcinoma (PTC) 10 mm or less in maximum dimension [Citation1]. Due to the extensive use of ultrasound (US) examination and US-guided fine-needle aspiration biopsy (US-FNAB), the incidence of incidentally discovered PTMCs has rapidly increased in recent decades [Citation2,Citation3]. PTMC usually does not become clinically evident and is associated with an excellent prognosis, either because the rate of progression is very slow or because the tumor does not progress. The majority of these small nodules can be reported as overdiagnosis. The American Thyroid Association guidelines suggested that an active surveillance can be considered in PTMC patients without local invasion or clinically evident metastases, and in patients who have high surgical risk, short remaining life span or concurrent medical issues requiring priority treatment [Citation4]. However, local spread or nodal metastases occurred in some cases with long-time follow-up [Citation5]. Moreover, the tumor could evoke considerable patient anxiety when it is left untreated. Although traditional surgery may facilitate accurate staging of the patient and the low recurrence rate, complications from surgery such as injury to the recurrent laryngeal nerve and parathyroid might exceed the degree of morbidity of the disease itself. In this setting, imaging-guided therapies could play a key role, representing potentially less invasive treatment alternatives to operation, thus being able to provide treatment to the PTMC patients, but lowering its invasiveness [Citation6].
In recent years, image-guided ablations including ethanol injection, radiofrequency ablation (RFA) and microwave ablation (MWA) have attracted much interest in the minimally invasive treatment of various tumors such as neoplasms of the liver, kidney and lung [Citation7–10], and these techniques have been regarded as an alternative to surgery. Several studies showed that RFA and MWA have been used to treat thyroid nodules and metastatic lymph nodes from PTC, yielding favorable results [Citation11–13]. US-guided laser ablation (LA) has also been demonstrated to be an effective method to reduce the volume of benign thyroid nodules [Citation14,Citation15], and to treat malignant diseases such as early hepatocellular carcinoma and metastatic lymph nodes from PTC [Citation16,Citation17]. The safety and effectiveness of LA in PTMC were reported in few studies [Citation18–20]. Moreover, the mean follow-up time was relatively short. To the best of our knowledge, no previous studies have compared LA and surgery for PTMC. The aim of the present study was to compare the clinical outcomes in patients with solitary PTMC who underwent LA with those who underwent hemithyroidectomy and unilateral central neck dissection.
Materials and methods
Patients
This retrospective study was approved by our institutional review board, and informed consent from the patients was waived. Patients enrolled in our study should fulfill the criteria as follows: (1) solitary PTC confirmed by US-FNAB or postoperative pathology, (2) lesion with largest diameter measured <10 mm, (3) the lesion away from the thyroid capsule not less than 3 mm, (4) the lesion with no massive calcifications, which was defined as the largest diameter of a strong echo more than 2 mm, (5) the thyroid function within normal limits, (6) surgical patients (surgical group) had undergone hemithyroidectomy and ipsilateral central compartment node dissection, and no lymph node metastasis was confirmed by postoperative pathology. For the patients (LA group) who underwent ablation due to high surgical risk or other reasons, LA was only performed for one lesion in each patient.
The exclusion criteria of patients included: (1) other types of thyroid carcinoma were confirmed by US-FNAB, (2) coexisting other thyroid nodules with malignant US features, (3) PTMC with cystic component, which may be related to slow volume reduction after LA treatment, (4) clinically apparent multi-centricity, (5) imaging examinations showing cervical lymph node or distant metastasis, (6) tumor in the isthmus, (7) pregnancy, (8) coagulation disorder.
Pretreatment assessment
Before each treatment, all patients underwent ultrasound examination and US-FNAB, which were performed by one of two radiologists with >10 years of experience in thyroid US imaging. The size, site, sonographic appearances of the nodules and cervical lymph nodes were carefully evaluated with real-time US systems (Mylab Twice, Esaote, Italy; Mylab 90, Esaote, Italy, and Resona 7, Mindray, China), equipped with linear transducers (4–13 MHz).
Three orthogonal dimensions of the lesion were measured, and the volume was calculated as follows: V = L × W × D × 0.524. Where, V is the volume, L, the length and W, D, the width and depth. The ultrasonic characteristics of the nodule included location, border, echogenicity, shape and the presence of microcalcification. Location was classified into two groups: right or left. The border was divided as well-defined or ill-defined. If more than 50% of its border was not clearly demarcated, the nodule was considered ill-defined. Compared with the adjacent normal thyroid tissue, the echogenicity was classified as hypoechoic and non-hypoechoic. The anteroposterior/transverse (A/T) diameter ratio was evaluated by comparing the height (anteroposterior diameter) and width (transverse diameter) of the tumor measured perpendicular and parallel to the ultrasound beam. According to the ratio, the shape was divided as taller than wide (A/T ≥ 1) or wider than tall (A/T < 1). Microcalcification was defined as the largest diameter of a strong echo smaller than 2 mm and was classified as present or absent. The presence of Hashimoto’s thyroiditis, which was diagnosed based on the increased levels of antithyroglobulin antibody and antithyroid peroxidase antibody, was also evaluated, and it was classified as present or absent.
LA procedures
Before LA, all patients were informed of the processes and possible complications, and written informed consent was obtained. LA procedures were performed in an outpatient clinic and carried out by the same two doctors according to a previous study [Citation15]. The patients were supine on an operation table with hyperextended neck. After local anesthesia was obtained with 2–5 mL of 2% lidocaine, hydrodissection technique was achieved to protect the vital organs (common carotid artery, recurrent laryngeal nerve, trachea, or esophagus) by means of injecting a mixture of 2% lidocaine and physiological saline solution (1:8 dilution).
All the nodules were treated with ‘transisthmic approach’ [Citation21]. Under real-time US guidance, the introducer needle (21-G) was penetrated into the target tumor. After the withdrawal of the core needle, a plane-cut optic fiber with a diameter of 300 μm and a length of 1.5 m was placed in the same position through the sheath of the introducer needle. The guide needle was withdrawn at a distance of 5 mm so that the tip of the fiber remained just in contact with the target tumor (). The fiber was connected to a continuous-wave neodymium yttrium-aluminium-garnet (Nd:YAG) laser source at a wave length of 1064 nm. The LA apparatus was an optical beam-splitting device (EchoLaser X4, Esaote, Florence, Italy). A power output of 3 W or 4 W was used, depending on the position of the nodule and pretreatment nodule volume. After LA treatment, contrast enhanced ultrasound (CEUS) was performed by injecting 2.4 mL Sonovue (Bracco, Milan, Italy) to evaluate the local response to ablation therapy.
Figure 1. (a) A 45-year-old man had a suspicious hypoechoic lesion (arrow) measured 5.3 × 3.7 × 3.0 mm in the right thyroid lobe. (b) A 300-m plane-cut optic fiber was inserted through the introduced needle (arrow head). During the ablation, the typical hyperechoic region (arrow) occurred surrounding the tip of the fiber due to formation of gas.
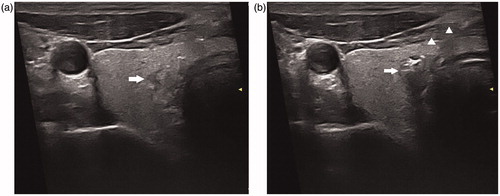
Surgical procedure
Before surgery, written informed consent was obtained from all patients. Thyroidectomy was performed by the same surgical team. The patient was under general anesthesia with the neck hyperextended. A 2–3 cm transverse arc incision above the sterna notch was performed, and the midline was opened. After the recurrent laryngeal nerve and parathyroid gland were identified and preserved, the unilateral thyroid gland was removed. Dissection of ipsilateral central compartment lymph nodes including the prelaryngeal, pretracheal and paratracheal basins was achieved after hemithyroidectomy. Following all specimens removal, the area was carefully examined for bleeding. After hemostasis was complete, the incision was closed with sutures, and the specimens were sent for pathologic examination.
Post-ablation care and follow-up
Following LA or surgery the possible complications such as hematoma, voice change, dysphagia, infection, tracheal injury and hypoparathyroidism were carefully evaluated. In the LA group, the patients were closely monitored for about 60 min before discharge. At subsequent follow-up, US examination was performed 1 h, 7 days, 1, 3 and 6 months after treatment and every half year thereafter with focus on the size of the ablated zone and development of recurrent lesions in the thyroid and metastatic lymph nodes in the neck. US-FNAB was also conducted at 1, 6 and 12 months after LA if the ablation zone was still visible.
In the surgical group, patients were generally hospitalized for 1 or 2 days after surgery. On US imaging, new suspicious lesions and metastatic lymph nodes were evaluated at 1, 3, 6 months after surgery and every half year thereafter. In both groups, thyroid function tests were performed in all patients during each follow-up period. Chest X-ray or computed tomography (CT) was performed once a year. US-FNAB was also performed when new suspicious thyroid nodules or abnormal cervical lymph nodes developed during the follow-up. The thyroxine suppression therapy was administered to patients in order to control the value of serum thyroid-stimulating hormone below 2.0 mIU/L. All US examinations and US-FNABs were performed by one of the two radiologists with more than 10 years of experience in thyroid US imaging.
Data analysis
At follow-up, technical feasibility, technical success, rate of complications and technique efficacy were analyzed. Technical feasibility of LA was defined as the ability to correctly target the nodules and perform the ablation as planned. Technical success of LA was defined as the complete absence of enhancement in the treated tumor at immediate CEUS after ablation. Complications were classified as minor or major according to the Proposal for Standardization of Terminology and Reporting Criteria [Citation21]. Technique efficacy: there was no regrowth of treated tumors at each follow up, and the US-FNAB results of the ablated zones at 1, 6 and 12 months showed no viable neoplastic cells.
The data were analyzed statistically by using SPSS (version 17.0). Quantitative data were expressed as mean ± standard deviation. Continuous outcomes of the two groups were compared by using Mann–Whitney U-test or paired t-tests, and categorical data from the two groups were compared by the χ2 test or Fisher’s exact test, as appropriate. The recurrence-free survival curves of the two groups were generated using the Kaplan–Meier method and were compared by using the log-rank test. p < .05 was considered statistically significant.
Results
Baseline clinical and ultrasonographic characteristics of participants
From January 2014 to December 2014, 36 patients with solitary PTMC who were treated by US-guided LA in our department were included for analysis in this study as the LA group. During the same period, 45 patients who underwent surgery in chronological order were enrolled in our study as the surgical group. The baseline clinical information and sonographic features of the nodules are summarized in . There were no significant differences between the two groups.
Table 1. Patients and PTMCs characteristics in the two groups.
Treatment response after LA
The mean energy applied during LA was 1120.9 ± 301.8 joule (range, 600–1800 joule), and the mean duration of application was 284.4 ± 73.7 s (range, 150–450 s).
We correctly targeted the small nodules in 36/36 (100%) cases, and all the ablations were performed as preoperatively planned. Immediate post-ablation CEUS showed no perfusion in the ablated zones, which completely encompassed the target tumors after treatment () (Technical success = 100%).
Figure 2. (a) Before LA, transverse ultrasound image revealed a hypoechoic nodule (arrow) with an ill-defined border 56.2 mm3 in volume, which was confirmed as a papillary thyroid microcarcinoma. (b) After LA, the necrotic area (arrow) 684.6 mm3 in volume was significantly larger than the target tumor on CEUS. (c) Ultrasound examination showed that the ablated lesion completely disappeared at the last follow up.
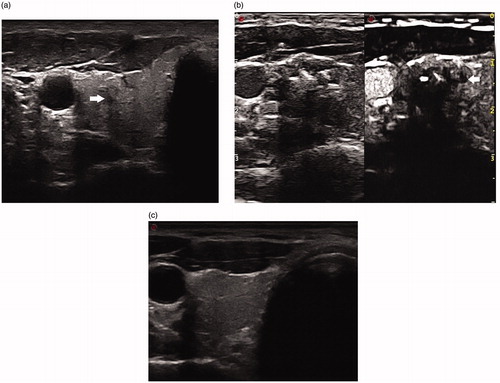
One hour after LA, the average maximum diameter and volume of the ablated zone on gray-scale imaging significantly increased (p = .000 for both; ). At the 7th day follow-up, the largest diameter and volume of the ablated zone were still larger than those before treatment. Subsequently, the size significantly decreased at each follow-up.
Table 2. Size and volume change of the ablated zone in the LA group.
No major complications were observed in all the cases. Minor complication occurred in one case. The patient had increased serum thyroid hormones and decreased serum thyroid stimulating hormone levels at 1 month after LA, but this patient recovered without treatment within 2 months.
The final follow-up obtained was at 49.2 ± 4.5 months (range, 30–54 months). At the last follow-up, the mean value of largest diameter was reduced from 4.7 ± 1.4 mm (mean ± standard deviation) to 0.2 ± 0.8 mm (p = .000), and the mean volume decreased from 43.2 ± 38.8 mm3 to 0.7 ± 4.1 mm3 (p = .000; ). US examination showed that 34 (94.4%) of the 36 ablated lesions completely disappeared (), and 2 (5.6%) remained as scar-like zones. Technique efficacy was achieved in 36/36 (100%) cases.
Overall, recurrence occurred in 5.6% (2/36) patients. In one patient, a metastatic lymph node in the lateral compartment was detected and confirmed by US-FNAB at 30 months after LA. No suspicious nodules were newly detected in the thyroid. Total thyroidectomy with central and lateral cervical lymph node dissection was subsequently performed. In the other patient, two small suspicious tumors in the contralateral lobe were detected at 42 months after LA. Both lesions were confirmed as PTMC by FNAB, and therefore surgery was planned for the patient.
Treatment response after surgery
The mean follow-up period for the surgical group was 48.5 ± 6.2 months (range, 24–54 months). Among the 45 patients who underwent surgery, one patient developed transient hypocalcemia. Hoarseness was observed in two patients, and they recovered spontaneously at 3 and 6 months, respectively. Three patients (6.7%) exhibited a recurrence. At 30 months after surgery, one patient exhibited a small nodule in the contralateral lobe, which was suspicious for PTMC. It was subsequently confirmed by US-guided FNAB. Then, hemithyroidectomy with unilateral central neck dissection was performed. In the other two patients, small lymph nodes in the ipsilateral central compartment were detected at 24 and 30 months after surgery. US-FNAB was performed, and its findings confirmed metastatic lymph nodes from PTC.
Comparison of treatment response and complications in the LA and surgical groups
The total complication rates were 2.8% (1/36) and 6.7% (3/45) for the LA and surgical groups, respectively, and there was no significant difference (p = .625, ). Treatment response after surgery versus LA is summarized in . The mean hospital stays and procedure time of LA group were 3.6 ± 0.7 h and 25.9 ± 7.0 min, which were shorter than those of surgical group (62.0 ± 11.4 h and 74.2 ± 12.5 min; p = .000 for both). In the surgical group, the number of patients who received thyroxine suppression treatment after surgery (45 of 45 [100%]) was greater than that after LA treatment (19 of 36 [52.8%]; p = .000). No distant metastasis was detected in both groups. The time lag for recurrence was similar in the two groups (LA group: mean 36.0 ± 8.5 months; surgical group: mean 28.0 ± 3.5 months; p = .395). No statistical difference in the overall recurrence-free survival rate was obtained between the groups (, p = .822). For the LA group, the 1- and 3-year recurrence-free survival rates were 100% and 97.2%, respectively, and the corresponding values for the surgical group were 100% and 93.3%, respectively.
Figure 3. The graph demonstrated recurrence-free survival curves for patients treated with surgery and LA.
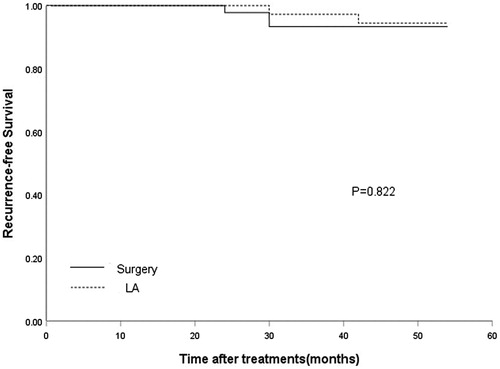
Table 3. Complications after surgery versus LA.
Table 4. Treatment response after surgery versus LA.
Discussion
A rapidly increasing incidence of thyroid cancer, which is almost exclusively attributable to PTMC, has been reported in many countries during the past several decades. Due to the indolent nature, the loco-regional recurrence rates, distant recurrence rates and disease-specific mortality rates of PTMC after surgery are very low [Citation4]. Whether it is beneficial to eliminate these small thyroid nodules at all is unclear. However, Kwon et al. [Citation22] reported that 14% patients increased in tumor volume more than 50% during follow-up, and a small percentage of PTMC presented with regional lymph node and distant metastases. There is currently no clinical features that can definitively differentiate the small number of PTMC patients that will develop clinically significant progression from the larger population of people with indolent PTMCs [Citation23–26]. Moreover, it is usually difficult for patients to decide to observe the tumors regularly because of considerable psychological burdens when PTMC is identified by FNAB. If image-guided ablation could locally eliminate these small tumors, it may be an alternative therapy for the patients who are ineligible or refusal to undergo surgery due to high surgical risk or other reasons.
Of the image-guided thermal ablations, LA was firstly proposed as a therapeutic tool in PTMC [Citation18], and it was confirmed as a feasible treatment for PTMC by means of accurate pathological and immunohistochemical analysis [Citation19]. Recently, several studies showed that RFA and MWA have also been applied to treat PTMC with promising results [Citation11,Citation12]. Compared to RFA and MWA methods, LA seems to be more advantageous for treating the tumors in the neck. First, the introducer needle (21-G) was less invasive than the 16-G antenna used for MWA and 17-G needle for RFA. The small needle is much easier to reach the target. Therefore, it could theoretically reduce problems in such a complex region. In addition, thermal energy of laser is deployed precisely and predictably. The zone of necrosis obtained after LA is relatively small, which may minimize the risks of thermal injury to the vital organs around the thyroid.
In our study, LA was performed in an outpatient clinic, and the procedures completed rapidly without general anesthesia. Compared with the surgical group, the mean procedure time and hospital stays were significantly shorter in the LA group. Our results demonstrated that LA might be an effective therapy for destroying solitary PTC without capsule invasion that was less than 10 mm. The technical success and efficacy of LA were revealed by the radiologic and pathologic findings after treatment. On conventional US, the ablated zone completely encompassed the target tumor, and it was confirmed by CEUS. The FNAB results at 1, 6 and 12 months demonstrated that there was no evidence of tumor residue and regrowth of the treated lesion. In the current study, the completed disappearance rate was 94.4% in the LA group. Previous studies reported 22.2% (four of 18 ablation zones) [Citation11] and 10.2% (ten of 98 ablation zones) [Citation12] completed disappearance rates of PTMC treated by MWA and by RFA, respectively. The difference might be due to the limited number of the tumors in the previous studies. Moreover, the average follow-up time in the current study was 49.2 ± 4.5 months, which is much longer than those in the studies by Yue et al. (11.0 months) [Citation11] and by Zhang et al. (18.0 months) [Citation12].
Although recurrent tumors have not been reported during the follow-up periods after RFA [Citation12] and after MWA [Citation11], our results demonstrated a recurrence rate of 5.6% (two of 36 treated patients). In one patient, a metastatic lymph node was confirmed after LA, and two new PTMCs in the contralateral lobe were detected in the other patient. However, no distant metastasis was observed. Recurrence was also detected in the surgical group, and the recurrence rate was 6.7%. There might be a trend toward a difference in the time lag for recurrence between the two groups, however, the difference was not statistically significant. There was also no statistical difference in the overall recurrence-free survival rate between the groups. The main US parameters in predicting malignancy in thyroid nodules included composition, echogenicity, shape, margin and microcalcification [Citation4], and some of these indicators were independent predictors of lymph node metastases. In the present study, there were no significant differences of sonographic appearances between the two groups. Several clinical factors have been identified to be responsible for cancer recurrence in PTMC, including gender, age, tumor size and initial presence of lymph node metastases [Citation27,Citation28]. PTMC with lymph node metastases on imaging examinations was excluded in our study, and there was no difference between the two groups in age, gender and tumor size. Therefore, for the management of PTMC, LA may be a good alternative to surgery based on the efficacy.
The complications of bleeding, sepsis and hypothyroidism associated with thyroid surgery have been overcome after a long journey from the start of thyroidectomy [Citation29]. At present, the main complications are hypoparathyroidism and vocal fold paralysis. The reported rate of hypocalcemia after surgery ranged from 3 to 7.2% [Citation29,Citation30], and that of vocal fold paralysis ranged from 0.2 to 5% [Citation31,Citation32], which were comparable with our results. In our surgical group, temporary hypocalcemia occurred in one patient. Because the parathyroid glands were less vascularized and more damaged in the patients who received hemithyroidectomy with unilateral central neck dissection, the incidence of temporary hypocalcemia was higher compared with those who received hemithyroidectomy alone. However, permanent hypocalcemia was not associated with unilateral central neck dissection. There were two cases with temporary hoarseness caused by vocal cord paralysis in our surgical group. Vocal fold paralysis secondary to damage to the recurrent laryngeal nerve is regarded as a major complication, resulting in glottic insufficiency, further limiting the patient’s quality of life. In our LA group, hypocalcemia did not occur, and, to the best of our knowledge, it has not been reported in the PTMC cases treated by RFA and MWA [Citation11,Citation12]. However, transient hoarseness has been reported after MWA and RFA [Citation12,Citation33]. It did not develop in our LA group. This might be due to the small tumor size and predictable ablated zone produced by LA. In addition, the hydrodissection technique was also used in our study, which was regarded as a thermal barrier to the recurrent laryngeal nerve. In our LA group, hyperthyroidism developed in one patient, showing elevated thyroid hormones and decreased serum thyroid stimulating hormone levels. This has also been reported by Valcavi et al. [Citation14] and Gambelunghe et al. [Citation34], which is possibly induced by thyroid tissue damage and associated with a transient and self-limited nature. In general, there was also no statistical difference in the complication rate between the two groups. Therefore, for the management of PTMC, LA may be a safe alternative to surgery.
Despite the efficacy and safety of LA for PTMC, there were some limitations to this therapy. Multifocality was reported to be unilateral or bilateral in 20–40% of patients with PTMC [Citation35], however, it could not be absolutely excluded without surgical excision. LA could not eradicate these occult tumors that are invisible on US, and multifocality is also significantly associated with lymph node metastasis and disease recurrence in PTMC. Additionally, there are limitations in using conventional US for detecting metastatic lymph nodes, especially those in the central compartment. As previously reported [Citation36], the sensitivity of preoperative US for diagnosing central lymph node metastasis is only 10.9%. In the LA group, there was no guarantee that all patients had no central lymph node metastasis. Therefore, the decision to perform LA instead of surgery to treat PTMC should be made cautiously.
Several limitations of our study should be acknowledged: (1) It might not be able to demonstrate clinically meaningful differences because of the small sample size in both groups and the relatively short follow-up period; (2) This study was retrospective and nonrandomized, and it could not avoid selection bias. A further randomized controlled trial of larger sample size should be performed; (3) Because the follow-up protocol for the work-up of distant metastasis only consisted of chest radiography and physical examination, hidden metastasis could not be absolutely excluded in the patients; (4) In the LA group, the pathological results were obtained from US-FNAB, which could cause false-negative and false-positive results. The surgical patients had histology-confirmed N0 disease and are more accurately staged compared to the LA patients who did not have histology-confirmed PTMC, which is nonequivalent for the two groups selected for comparison.
In conclusion, compared with surgery, US-guided LA was also a safe therapy for treating solitary PTMC with good therapeutic effect. Hence, it might be a treatment alternative to hemithyroidectomy with unilateral central neck dissection for patients with solitary PTMC who are ineligible or refusal to undergo surgery due to high surgical risk or other reasons.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987; discussion 987–988.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
- Pellegriti G, Scollo C, Lumera G, et al. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004;89:3713–3720.
- Mauri G, Sconfienza LM. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int J Hyperthermia. 2017;33:489–490.
- Li X, Liang P, Yu J, et al. Role of contrast-enhanced ultrasound in evaluating the efficiency of ultrasound guided percutaneous microwave ablation in patients with renal cell carcinoma. Radiol Oncol. 2013;47:398–404.
- Chi J, Ding M, Shi Y, et al. Comparison study of computed tomography-guided radiofrequency and microwave ablation for pulmonary tumors: a retrospective, case–controlled observational study. Thorac Cancer. 2018;9:1241–1248.
- Iniguez-Ariza NM, Lee RA, Singh-Ospina NM, et al. Ethanol ablation for the treatment of cystic and predominantly cystic thyroid nodules. Mayo Clin Proc. 2018;93:1009–1017.
- Du J, Li HL, Zhai B, et al. Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol. 2015;41:2400–2411.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30:150–157.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26:1581–1587.
- Zhou W, Chen Y, Zhang L, et al. Percutaneous microwave ablation of metastatic lymph nodes from papillary thyroid carcinoma: preliminary results. World J Surg. 2019;43:1029–1037.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20:1253–1261.
- Spiezia S, Vitale G, Di Somma C, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003;13:941–947.
- Pacella CM, Francica G, Di Lascio FM, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. JCO. 2009;27:2615–2621.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39:1023–1030.
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917–920.
- Valcavi R, Piana S, Bortolan GS, et al. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23:1578–1582.
- Zhou W, Jiang S, Zhan W, et al. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: preliminary results. Eur Radiol. 2017;27:2934–2940.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618.
- Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center's experience in Korea. J Clin Endocrinol Metab. 2017;102:1917–1925.
- Giordano D, Gradoni P, Oretti G, et al. Treatment and prognostic factors of papillary thyroid microcarcinoma. Clin Otolaryngol. 2010;35:118–124.
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35.
- Niemeier LA, Kuffner Akatsu H, Song C, et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer. 2012;118:2069–2077.
- Roh JL, Kim JM, Park CI. Central cervical nodal metastasis from papillary thyroid microcarcinoma: pattern and factors predictive of nodal metastasis. Ann Surg Oncol. 2008;15:2482–2486.
- Pedrazzini L, Baroli A, Marzoli L, et al. Cancer recurrence in papillary thyroid microcarcinoma: a multivariate analysis on 231 patients with a 12-year follow-up. Minerva Endocrinol. 2013;38:269–279.
- Wang WH, Xu SY, Zhan WW. Clinicopathologic factors and thyroid nodule sonographic features for predicting central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study of 1204 Patients. J Ultrasound Med. 2016;35:2475–2481.
- Gupta S, Vasu Reddy C, Chettri ST, et al. Clinicopathological features and complications of thyroid operations: a single centre experience. Indian J Otolaryngol Head Neck Surg. 2013;65:140–145.
- Son HJ, Kim JK, Jung YD, et al. Comparison of outcomes between hemithyroidectomy alone and hemithyroidectomy with elective unilateral central neck dissection in patients with papillary thyroid microcarcinoma. Head Neck. 2018;40:2449–2454.
- Friguglietti CU, Lin CS, Kulcsar MA. Total thyroidectomy for benign thyroid disease. Laryngoscope. 2003;113:1820–1826.
- Ernandes-Neto M, Tagliarini JV, Lopez BE, et al. Factors influencing thyroidectomy complications. Braz J Otorhinolaryngol. 2012;78:63–69.
- Xu B, Zhou NM, Cao WT, et al. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. WJCC. 2018;6:936–943.
- Gambelunghe G, Fede R, Bini V, et al. Ultrasound-guided interstitial laser ablation for thyroid nodules is effective only at high total amounts of energy: results from a three-year pilot study. Surg Innov. 2013;20:345–350.
- Zheng W, Wang K, Wu J, et al. Multifocality is associated with central neck lymph node metastases in papillary thyroid microcarcinoma. CMAR. 2018;10:1527–1533.
- Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006;30:91–99.
