Abstract
Objective: To evaluate the outcomes of subsequent pregnancies in patients with a history of cesarean scar pregnancy (CSP) treated with high intensity focused ultrasound (HIFU) followed by ultrasound-guided dilation and curettage (USg-D&C).
Methods: A retrospective analysis was performed on data collected from 154 patients with CSP who were treated by HIFU followed by USg-D&C in Suining Central Hospital between January 2015 and January 2018. Among them, 28 patients wanted to conceive following treatment. Baseline characteristics, treatment results, intraoperative hemorrhages during USg-D&C, post-curettage serum beta human chorionic gonadotropin (β-hCG) levels and vaginal bleeding were investigated. Subsequent pregnancy outcomes, including intervals between pregnancy and treatment of CSP, complications during pregnancy, and outcomes of newborns were evaluated.
Results: All patients with CSP were successfully treated by HIFU combined with USg-D&C. Of the 28 CSP patients who intended to conceive after the treatment, 23 patients (82.14%) successfully conceived. The average interval between conception and HIFU treatment was 18.38 ± 10.04 months. Eighteen patients (78.26%) had an intrauterine pregnancy, in which 12 had delivery by cesarean section, 1 had an ongoing pregnancy, and 5 had an abortion in the first trimester. Among the other 5 women, 3 had tubal ectopic pregnancy and 2 had recurrent CSP. These five patients underwent laparoscopy within the first trimester.
Conclusion: HIFU followed by USg-D&C is an effective and safe treatment for patients with CSP who wish to conceive. Prospective multi-center studies with larger sample sizes and longer follow-up periods are needed to compare this treatment with others.
Introduction
Cesarean scar pregnancy (CSP) is a rare ectopic pregnancy that refers to the implantation of a gestational sac within the scar of a previous cesarean delivery [Citation1]. The incidence rate has been reported to be between 1:1800 and 1:2216 [Citation2,Citation3]. Over the last few decades, the cesarean section (CS) rate has increased worldwide. In China, the proportion of CSs performed was over 50% in the last decade [Citation4]. Once a diagnosis is made, CSPs should be terminated early and properly because of the risk of life-threatening hemorrhages, hypovolemic shock, uterine rupture, and even maternal death [Citation5]. Due to the rarity of CSP and the paucity of clinical evidence, there is no consensus on the management of CSP. Surgical approaches have a curative effect, completely removing the lesion. However, surgical treatments are invasive and may cause post-operative pelvic adhesion or abnormal uterine shape change [Citation6]. Uterine artery embolization (UAE) is a minimally-invasive option for patients with CSP which has been reported to have a high success rate and has seen more clinical applications in recent years, but the high success rate is also accompanied by a high complication rate (nearly 50%). Severe adverse effects such as infection, infertility and ovarian dysfunction in the long term may occur after UAE. High intensity focused ultrasound (HIFU) is a non-invasive therapeutic technique. This technique has been widely applied in treating benign uterine diseases, such as uterine fibroids, adenomyosis, and placenta accreta [Citation7,Citation8]. HIFU has also been used to treat CSP. Huang et al. first reported results of HIFU combined with ultrasound-guided dilation and curettage (USg-D&C) for patients with CSP [Citation9]. Later, results from several groups have shown the safety and effectiveness of HIFU combined with USg-D&C or hysteroscopy for the treatment of CSP [Citation10,Citation11]. Since HIFU treatment is performed under the guidance of ultrasound, the lesion can be ablated without damage to the surrounding structures [Citation12]. Thus, HIFU has the advantages of not leaving a new scar on the uterus and possibly significantly shortening the preparation period required for subsequent pregnancy after treatment when compared with surgery. Several studies have shown that HIFU can be safely used to treat patients with uterine fibroids who wish to conceive [Citation13–15]. The results showed that patients who were treated with MR-guided focused ultrasound surgery (MRgFUS) or ultrasound-guided HIFU can achieve successful pregnancy and labor without additional obstetric risks [Citation16–18]. However, no subsequent pregnancy outcomes of patients with CSP treated by HIFU combined with USg-D&C were reported. Therefore, we performed a retrospective study to evaluate the subsequent pregnancy outcomes of patients with CSP treated by HIFU combined with USg-D&C in order to provide evidence for clinical practitioners to give optimal reproductive suggestions to CSP patients.
Materials and methods
Patients
A retrospective analysis was performed on data collected from 154 patients with CSP treated by HIFU combined with USg-D&C in Suining Central Hospital between January 2015 and January 2018. All patients signed an informed consent before each procedure. Among them, 28 patients wanted to conceive following the treatment of CSP. In these 28 patients, 19 were classified as type I, and 9 were classified as type II ().
Table 1. Demographic characteristics of CSP patients with reproductive requirement underwent HIFU followed by USg-D&C (n = 28).
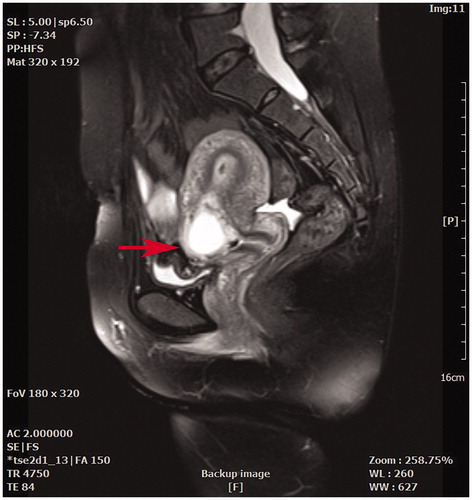
The inclusion criteria were as follows: (1) a prior history of CS delivery; (2) amenorrhea and positive urine pregnancy test; (3) a gestational sac located in the anterior lower uterine segment; (4) diagnosis of CSP confirmed by transvaginal or transabdominal ultrasound and pelvic magnetic resonance imaging (MRI) () [Citation19]; and (5) a desire to conceive in the future and an age less than 45 years old.
Figure 1. A sagittal view magnetic resonance imaging showed a gestational sac embedding at a previous cesarean section scar (red arrow).
Exclusion criteria included: (1) previous treatment for trophoblastic disease and other disorders; (2) previous treatment for CSP before HIFU; and (3) cervical pregnancy or other ectopic pregnancy.
HIFU ablation and USg-D&C combined therapy
The procedure of USgHIFU has been described in a previous publication [Citation20]. To summarize, the patients were requested to have a three-day bowel preparation, including a bland diet of semi-liquid and liquid food, a 12-h fasting period, and an enema the morning before HIFU. To reduce the risk of skin burn, skin preparation was required, including shaving the hair from the umbilicus to the upper margin of the pubic symphysis, degreasing, and degassing with 75% ethanol or degassed water. A catheter was inserted into the bladder to control its volume.
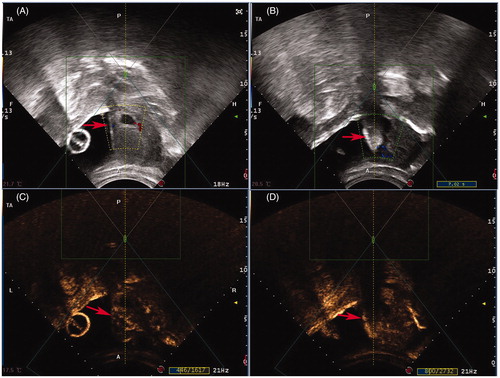
HIFU ablation was performed using a JC or JC200 focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co. Ltd, Chongqing, China). Patients were positioned prone on the HIFU table of the system with the lower abdominal wall in contact with degassed water. The treatment was performed under conscious sedation with fentanyl (1 μg/kg) and midazolam hydrochloride (20 μg/kg). At the beginning of the HIFU ablation procedure, the focus was placed close to the embedding area of the gestational sac in the center slice with sonication power ranging from 300 to 400 W. Two milliliters (23.6 mg/mL) of contrast-enhanced micro-bubble agent (SonoVue, Bracco, Milan, Italy) was intravenously injected into one hand 10 min before and immediately after HIFU treatment for evaluation. The termination criteria for HIFU ablation were as follows: (1) a significant gray scale change in the embedding area, as shown by real-time ultrasound images (); (2) a significantly reduced blood flow signal in the embedding area, as shown by color Doppler; or (3) a significant reduction in the blood supply in the embedding area, as shown by contrast-enhanced ultrasound ().
Figure 2. Real-time monitoring ultrasound and contrast enhanced ultrasound images obtained from a patient with CSP before and after HIFU. (A) Pre-HIFU ultrasound image showed a gestational sac (red arrow) embedding in the CSP scar; (B) Real-time ultrasound image showed the significant gray scale change (red arrow) in the embedding area of the CSP scar immediately after HIFU treatment; (C) Pre-HIFU contrast-enhanced ultrasound showed enhancement in myometrium of CSP scar (red arrow) around gestational sac; (D) Post-HIFU contrast-enhanced ultrasound showed no enhancement in myometrium of CSP scar (red arrow).
The patients underwent USg-D&C under general anesthesia 3 days after HIFU ablation. Patients were placed in the lithotomy position and transabdominal ultrasound imaging (DC-60, Mindray Medical Co. Ltd, Shenzhen, China) was used to guide the procedure. A 6.5 mm suction cannula with a vacuum pressure of 40 Pa was placed in the uterine isthmus during the curettage procedure. The ablated trophoblasts and decidual tissues were detached by carefully moving the cannula around the gestational sac embedding area and gently scraping the residue. One milliliter of oxytocin (5 U/mL) was injected into the cervix at the 12 o’clock position.
Follow-up
Serum beta human chorionic gonadotropin (β-hCG) was evaluated 1 day before the patients were discharged from hospital, followed by weekly monitoring in clinics until it returned to normal levels. All patients were followed up by either monthly telephone calls made by our obstetrician and gynecologists or clinical visits for 18 or 24 months. The duration of vaginal bleeding post-curettage, recovery of menstruation, interval between pregnancy and treatment of CSP, outcome of pregnancy, delivery information and neonatal outcome, and complications during pregnancy, intra-partum, and post-partum were recorded.
Statistical analysis
SPSS software (SPSS 22.0, IBMCompany, Chicago, IL) was used for statistical analysis. A one-sample Kolmogorov–Smirnov test was used to obtain statistics of the probability distribution for quantitative data. All normally distributed data are reported as mean ± SD. Non-normally distributed data are reported as median and inter-quartile range (P25–P75).
Results
Demographic characteristics of CSP patients who wanted to conceive
The average age of the 28 CSP patients was 31.18 ± 4.88 years. The average body mass index (BMI) was 21.53 ± 2.48 kg/m2. Among these patients, 8 (28.57%) were asymptomatic, while 2 (7.14%) complained of abdominal pain, 14 (50.00%) complained of vaginal bleeding and 4 (14.29%) complained of both. The median duration of amenorrhea was 44 days. The median pre-treatment serum β-hCG was 19638.20 mIU/mL. Among these patients, 21 (75.11%) had one previous cesarean delivery, 6 (21.43%) had two, and 1 (3.57%) had three. The average interval from the last cesarean section to CSP was 4.79 ± 3.93 years. The average diameter of the gestational sac was 28.69 ± 9.51 mm. The median thickness of the gestational sac embedding myometrium was 4.50 ± 1.43 mm ().
Outcomes of HIFU ablation
Every patient received only one session of HIFU treatment. As shown in , the median HIFU treatment power used was 396.50 W, the average treatment time was 76.93 ± 36.81 min, the average sonication time was 616.04 ± 369.82 s, and the average treatment intensity was 466.12 ± 110.98 s/h.
Table 2. Treatment and follow-up outcomes of CSP patients with reproductive requirement underwent HIFU followed by USg-D&C (n = 28).
Common adverse effects during HIFU treatment include sciatic/buttock pain and treatment area pain. The incidence rate of sciatic/buttock pain was 35.71% (10/28), and the rate of pain in the treated area was 64.29% (18/28). One patient (3.57%) experienced emesis during HIFU treatment. After HIFU treatment, no patient reported any severe HIFU-related adverse events, such as nerve injury, bowel injury or skin burn.
Ultrasound-guided suction curettage results and follow-up
The average blood loss in ultrasound-guided suction curettage procedure was 24.72 ± 19.13 ml. One patient had massive vaginal bleeding (500 ml) during curettage; the procedure was then terminated to resolve the complication by compression with a balloon, and a second curettage was conducted successfully 5 days later with 20 ml of blood loss. The average duration of post-curettage vaginal bleeding was 7.65 ± 6.96 days, the median serum β-hCG level 1 week after curettage was 2264.30 mIU/mL, and the average time for serum β-hCG return to normal level was 31.96 ± 19.38 days.
Subsequent pregnancy outcomes of the CSP patients treated with HIFU and USg-D&C
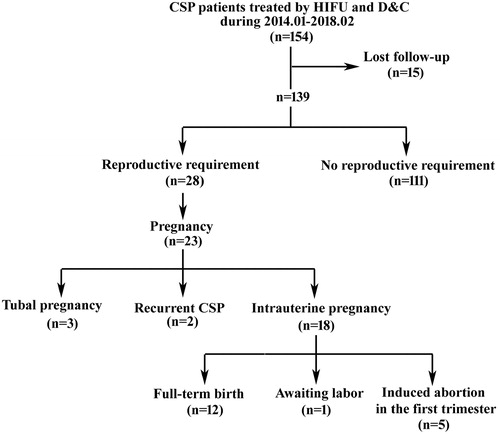
All patients were followed-up for at least 18 months. As shown in , among the 154 patients treated, 28 patients attempted to conceive following treatment, whereas the rest had no desire for future pregnancy. At the end of the follow-up period, 23 out of those 28 patients (82.14%) conceived. The average interval between conception and HIFU treatment was 18.38 ± 10.04 months. Among these pregnancies, 18 (64.29%) were intrauterine, 3 (10.71%) were tubal, and 2 (7.14%) were recurrent CSPs (). Of the18 patients with intrauterine pregnancy, 13 women chose to continue with the pregnancy, whereas the other 5 women chose induced abortion. Of the 13 patients who decided to continue with their pregnancies, one had mild anemia and another had intrahepatic cholestasis during pregnancy. Twelve of them underwent cesarean delivery at terms (≥37 weeks), and 1 has a non-going pregnancy at the time of this paper’s writing. The 12 newborn babies were healthy; all of them had an Apgar score of 10 both 1 min and 5 min after birth. The average weight of the newborns was 3225.00 ± 235.00 g (). No complications occurred. The three patients with tubal ectopic pregnancies and the two patients with recurrent CSPs had accepted laparoscopic excision of the ectopic lesion within the first trimester.
Table 3. Pregnancy outcomes of CSP patients after HIFU and USg-D&C (n = 23).
Table 4. Delivery outcomes of CSP patients with intrauterine pregnancy after HIFU and USg-D&C (n = 12).
Discussion
Previous studies have shown that the ages at which CSP occurs are between the twenties and the forties. Therefore, the ideal treatment for CSP would not only expel pregnancy tissue and decrease the risk of catastrophic hemorrhage and uterine rupture, but also preserve the uterus to maintain reproductive function [Citation21]. For CSP patients who wish to conceive in the future, the main concerns are optimal timing for a subsequent pregnancy and the outcome of their pregnancy after the treatment of CSP. Seow et al. reported a patient with CSP who conceived 3 months after treatment with D&C and cervical balloon tamponade: uterine rupture occurred at 38 weeks of gestation, leading to maternal death and stillbirth [Citation22]. This tragic outcome warned us that subsequent pregnancies of patients with a previous CSP should be more cautious. Ben et al. retrospectively analyzed the reproductive outcomes of 38 women (median age of 36 years old) with a previous CSP. Among the 38 patients, 28 underwent surgical evacuation, while the other 10 had conservative treatment for CSP using a local injection of methotrexate. The median interval between CSP treatment and conception was 5.3 months. During the follow-up period, 20 (73.68% of) patients had intrauterine pregnancies and 1 had recurrent CSP. However, 7 (35%) of the 20 patients with an intrauterine pregnancy reported miscarriage in the first trimester [Citation23]. Recently, Chen et al. performed a retrospective study, and their results showed that early diagnosis and treatment of CSP could reduce the risk of infertility and recurrent CSP, and HIFU appears to be superior to UAE in reducing the risk of recurrent CSP [Citation24]. In this study, all patients with a previous CSP had a successful treatment with HIFU followed by USg-D&C. Our results showed that HIFU followed by USg-D&C is safe and effective in the treatment of patients with CSP. Among the 18 (78.26%) patients who had intrauterine pregnancies, 12 of them (66.67%) gave birth to healthy newborns at term. In these 12 patients, 6 had typeICSP, and 6 had type II CSP. All patients who carried pregnancies to term underwent cesarean deliveries as there was a concern about the risk, in the presence of a previous CS scar, of uterine rupture during labor by vaginal delivery. The newborn babies were healthy with normal birth weight. No placenta previa, placenta accreta, uterine rupture or premature delivery was observed. For the five patients with intrauterine pregnancies who had induced abortion in the first trimester, they made the decision because two patients took medicine which may affect embryo development before noticing their pregnancies, and the others had family issues. For the three women who had tubal ectopic pregnancies, two had type I CSP, and one had type II CSP. From their histories, uncontrolled chronic pelvic inflammation before they conceived were found, which may have resulted in an obstruction of a fallopian tube, which interferes with the movement and implantation of fertilized eggs. For the two patients who had recurrent CSP, laparoscopic surgery was performed to repair the defect of the uterus because we found the diverticulum was deep.
Figure 3. Flow chart of subsequent pregnancy outcomes of CSP patients treated with USgHIFU and USg-D&C.
We also noted that the interval between HIFU treatment followed by USg-D&C and conception in this study was much longer than that in previous studies [Citation22,Citation23]. In previous studies, uterine rupture and a high miscarriage rate were observed. These complications might associate with the short interval between CSP treatment and the following conception because of the poor healing after surgical resection of CSP. More studies with larger size of subjects are needed.
This study is limited because this is a retrospective study and several unexpected factors may affect pregnancy outcomes, such as age and accompanying diseases. Another limitation of this study is the relatively small sample size because the incidence of CSP was low and the follow-up time was short. Therefore, it is necessary to perform prospective studies to compare this treatment strategy with others and enroll more patients from many centers with longer follow-up periods to validate our findings.
Conclusions
In summary, our results demonstrated that HIFU combined with USg-D&C is an effective and safe treatment for CSP. Based on the results from our small number of patients, HIFU followed by USg-D&C for the treatment of patients with CSP seems to have few adverse effects on subsequent pregnancy outcomes. To validate this finding, prospective multi-center trials with larger sample sizes and longer follow-up periods are needed to compare this treatment strategy with others.
Disclosure statement
L.Z. is a senior consultant to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253–263.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus-an unusual cause of post-abortalhaemorrhage. A case report. S Afr Med J. 1978;53:142–143.
- Zhai JF, Xu M, Zhang B, et al. Treatments of caesarean scar pregnancy and the corresponding results in ten years. Eur Rev Med Pharmacol Sci. 2015;19:2523–2527.
- Wang X, Hellerstein S, Hou L, et al. Caesarean deliveries in China. BMC Preg Childbirth. 2017;17:54.
- Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2013;25:456–461.
- Awonuga AO, Fletcher NM, Saed GM, et al. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011;18:1166–1185.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124:207–211.
- Zhang C, Jacobson H, Ngobese ZE, et al. Efficacy and safety of ultrasound-guided high intensity focused ultrasound ablation of symptomatic uterine fibroids in Black women: a preliminary study. BJOG. 2017;124:12–17.
- Huang L, Du Y, Zhao C. High-intensity focused ultrasound combined with dilatation and curettage for cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2014;43:98–101.
- Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine. 2015;94:e854.
- Zhang Y, Zhang C, He J, et al. The impact of gestational sac size on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment for caesarean scar pregnancy. Int J Hyperthermia. 2018;10:1–7.
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–445.
- Zou M, Chen L, Wu C, et al. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG. 2017;124:30–35.
- Qin J, Chen JY, Zhao WP, et al. Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet. 2012;117:273–277.
- Rabinovici J, David M, Fukunishi H, et al. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199–209.
- Hanstede MM, Tempany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case report. Fertil Steril. 2007;88:497.e5–7.
- Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol. 2014;26:151–161.
- Li JS, Wang Y, Chen JY, et al. Pregnancy outcomes in nulliparous women after ultrasound ablation of uterine fibroids: a single-central retrospective study. Sci Rep. 2017;7:3977.
- Godin P-A, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67:398–400.
- Zhang Y, Zhang C, He J, et al. The impact of gestational sac size on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment for caesarean scar pregnancy. Int J Hyperthermia. 2018;35:291–297.
- Zhu X, Deng X, Xiao S, et al. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int J Hyperthermia. 2016;32:144–150.
- Seow KM, Huang LW, Lin YH, et al. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23:247–253.
- Ben Nagi J, Helmy S, Ofili-Yebovi D, et al. Reproductive outcomes of women with a previous history of caesarean scar ectopic pregnancies. Hum Reprod. 2007;22:2012–2015.
- Chen L, Xiao S, Zhu X, et al. Analysis of the reproductive outcome of patients with cesarean scar pregnancy treated by high-intensity focused ultrasound and uterine artery embolization: a retrospective cohort study. J Minim Invasive Gynecol. 2018;S1553-4650:30448–30445.
