Abstract
Background: Treatment for locally recurrent breast cancer poses a significant challenge because the benefits in local control must be weighed against the increased risk of side effects of the treatment. Frequently, patients have been heavily pre-treated with radiation and several types of chemotherapy. Moreover, they often present with large volumes of bulky disease, further complicating management. Hyperthermia can be used to improve the efficacy of radiation, particularly in the setting of recurrent disease.
Methods: We reviewed our clinical and dosimetric experience of breast cancer patients who received hyperthermia and radiation for recurrent breast cancer from 2011 to 2017. Thirty-six patients were treated with hyperthermia and radiation. Median follow-up was 11 months. Thirty patients (83.3%) received prior radiotherapy. The most commonly used radiation fraction scheme was 32 Gy in 8 fractions. The median radiation dose at the time of recurrence was 35.5 Gy (range 20–64 Gy). Mild temperature hyperthermia was delivered two times per week.
Results: The median repeat radiation volume was 574 cc (range 11–3620 cc). Electrons, conventional photons, and IMRT radiation techniques were used. IMRT was used for large and complex treatment volumes and showed acceptable doses to organs at risk. The overall response rate was 61.1%. Complete response was observed in 17 patients (47.2%), partial response in 5 patients (13.9%), stable disease in 11 patients (30.6%), and progressive disease in 3 patients (8.3%). Twenty-six patients experienced acute grade 1 and 2 toxicities, primarily pain and erythema; and 26 experienced long-term grade 1 and 2 toxicities, mainly hyperpigmentation and lymphedema. Three patients developed new ulcerations that healed with conservative management. One patient developed pulmonary fibrosis resulting in mild dyspnea on exertion.
Conclusion: Hyperthermia and radiation provide good local control with a favorable side effect profile. Thermoradiotherapy may be offered to patients with recurrent breast cancer, including those with extensive volumes of disease.
Introduction
Breast cancer is the most prevalent type of cancer in women worldwide [Citation1]. Despite great strides made in detection amongst patients with early stage disease, roughly 30% of patients will relapse [Citation2]. Approximately 80% of these locoregionally recurrent breast cancers will occur within the 5 years following initial treatment [Citation2]. While management of recurrent breast cancer is a therapeutic challenge, in recent years, 5-year survival has steadily been increasing [Citation1–3]. This improvement in survival has been attributed to a multidisciplinary treatment approach, including combinations of surgery, radiation therapy, chemotherapy, immunotherapy and mild temperature hyperthermia [Citation4–8].
When used in conjunction with radiation, hyperthermia can increase the therapeutic index in part by interfering with DNA damage repair within the cancer cell population and improving the radiosensitivity of treatment-resistant cancer stem-like cells [Citation3,Citation9–13] and cells in S-phase [Citation14]. Hyperthermia enhances perfusion to increase drug delivery to the tumor and increases oxygenation to improve the efficacy of radiation [Citation15,Citation16]. Hyperthermia can also facilitate the activation and recruitment of immune effector cells to tumor regions [Citation14,Citation15]. Therefore, hyperthermia can create changes in tumor cells themselves and also modulate the tumor microenvironment to improve cancer control.
Recurrent breast cancer poses a significant clinical challenge because the benefits in local control must be weighed against the increased side effects from repeat irradiation. Many of these patients present with bulky, large volume disease, further complicating management. Hyperthermia has emerged as a useful adjunct treatment that can improve the radiosensitivity of cancer cells, thus achieving greater tumor control with lower doses of radiation compared to radiation alone [Citation17–20].
Here, we assess the effects of radiation and hyperthermia on recurrent breast cancer patients. We assess clinical outcomes including local control and side effects of treatment, and evaluate radiation techniques and radiation dose to organs at risk. These data will help guide treatment decisions on patients with not only limited but also extensive volumes of locoregional disease. We provide guidance on hyperthermia and re-irradiation fractionation strategies and radiation plan design for patients with various presentations.
Materials & methods
Patient data
Patient data were reviewed under a Cleveland Clinic approved IRB protocol. Thirty-six patients with recurrent breast cancer underwent concurrent hyperthermia treatment with radiation at the Cleveland Clinic from 2011–2017. Median follow-up of analysis was 11 months (range 1–123 months). Patient characteristics at initial presentation are summarized in Supplemental Table S1 and at time of recurrence are summarized in . The patients with inflammatory breast cancer had infiltrating ductal carcinoma with clinical presentations of rapid onset of breast erythema, edema or peau d’orange; erythema involving at least 1/3 of the breast or chest wall; and dermis and dermal lymphatic invasion on pathology. Patients were evaluated by medical oncologists, radiation oncologists and surgeons. Patients with locoregional disease progression were seen for palliative hyperthermia and radiation. Recurrences involved the chest wall, supraclavicular fossa and/or axilla. Clinical exam, CT and bone scan imaging were conducted to evaluate the extent of locoregional and distant disease.
Table 1. Patient characteristics at time of breast cancer recurrence (n = 36).
Radiation at time of recurrence
The most commonly used radiation fraction scheme (14 patients) was 32 Gy in 8 fractions delivered twice a week with each fraction scheduled 3–4 days apart. The median radiation dose was 35.5 Gy (range 20–64 Gy) and median fraction size was 3.0 Gy (range 1.2–4 Gy). Twenty patients received daily radiation, two patients received twice a day radiation. Re-irradiation treatments spanned a median of 25 days (range 4–47 days). Radiation plans and dosimetry were analyzed using Pinnacle (Philips) and MIM Software. The radiation planning techniques used and dose volume histogram parameters, including mean lung dose, percent of ipsilateral lung volume receiving >5 Gy (V5) and mean heart dose were assessed. Significance testing was performed by ANOVA analysis.
Hyperthermia delivery
Hyperthermia was given twice per week, lasting 60 min per session and performed 30–60 min before radiation if only one hyperthermia field was used. For patients with large volume treatments, hyperthermia was directed to the most bulky areas of disease and required two to three hyperthermia fields. For the patients who received hypofractionated radiation (e.g., 32 Gy in 8 fractions, with each fraction delivered twice a week for 4 weeks), hyperthermia was given to each field before and after each radiation session. Patients requiring three hyperthermia fields received treatment to two fields on a single day of the week and the third field on the second treatment day of the week, as the maximum number of hyperthermia fields treated in a given day was two. Alternatively, for patients who received daily radiation (e.g., 50 Gy in 25 fractions, with each fraction delivered daily), hyperthermia was given prior to radiation (e.g., Monday and Thursday for one field and Tuesday and Friday for the second field). For patients with ulcerated tumors, the ulcerated area was included because the ulceration was due to tumor erosion of the skin. The hyperthermia equipment included a microwave unit, water bolus and multi-applicators (BSD500, Pyrexar). Noninvasive thermisters were placed over the treatment area and an 915 MHz microwave unit was used to heat the target volume. Temperatures were measured using thermisters placed on the surface of the tumor. Six to 7 thermisters were used to measure temperature across the tumor. The median T90 (temperature exceeded by 90% of time points) was 40.2 °C.
Evaluation of overall response and side effects
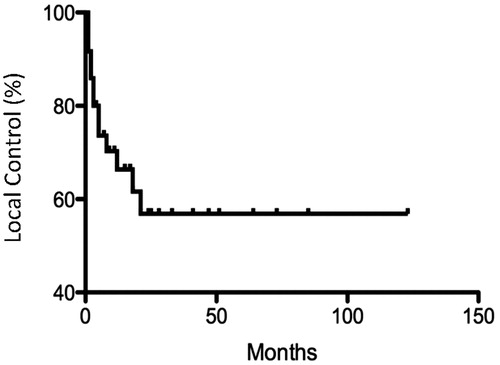
Response to hyperthermia and radiation was assessed by clinical inspection and CT imaging. Side effects and tumor regression were evaluated 4–6 weeks after completion of treatment and at routine follow-up thereafter. The CTCAE v4.0 grading scale was used for evaluation of clinical toxicity. Tumor control was determined by clinical exam and CT imaging, if available. Local control was defined as a complete response (CR) to treatment, that is, disappearance of all evidence of disease. Partial response (PR) was defined as a reduction in measurable disease. Stable disease (SD) was determined when there was neither a decrease in carcinoma to qualify for PR nor an increase in carcinoma to qualify for PD; progressive disease (PD) was indicated when there was an increase in measurable disease. Acute and long-term toxicity of treatment was assessed. Kaplan–Meier plots were generated for local control. Significance was assessed by log-rank test.
Results
Thirty six patients with recurrent breast cancer were treated with hyperthermia and radiation. The median time between primary diagnosis and recurrence was 5 years (range 0.6–24.6 years). At the time of initial diagnosis, most patients (38.9%) presented with stage III disease, 25% with stage II, 16.7% with stage I, and 1 patient presented with stage IV disease. Twenty-eight patients (77.8%) presented with grade 2 or 3 breast cancer. Twenty-five patients (83.3%) presented initially with invasive ductal carcinoma (Supplemental Table S1). All patients were previously heavily pretreated. Thirty-three patients (91.7%) received prior chemotherapy, 30 (83.3%) received prior radiation therapy to a median dose of 60.4 Gy (range 50.4–66.0 Gy). Twenty-nine (80.5%) patients had partial, complete or double mastectomy.
The median age of patients at the time of recurrence was 59.5 years (range 36–85) (). Twenty-seven patients (75%) recurred with grade 2 or 3 tumor. Twelve patients (33.3%) presented with right-sided recurrence, 15 (41.7%) with left-sided, and 9 (25%) had bilateral chest wall disease.
Twenty-two patients (61.1%) experienced a complete or partial response. Eleven patients (30.6%) had stable disease, and three patients (8.3%) developed progressive disease (). Kaplan–Meier analysis of local control (complete response) are shown in .
Table 2. Response rates of recurrent breast cancer patients treated with hypethermia and radiation (n = 36).
Table 3. Side effects of treatment.
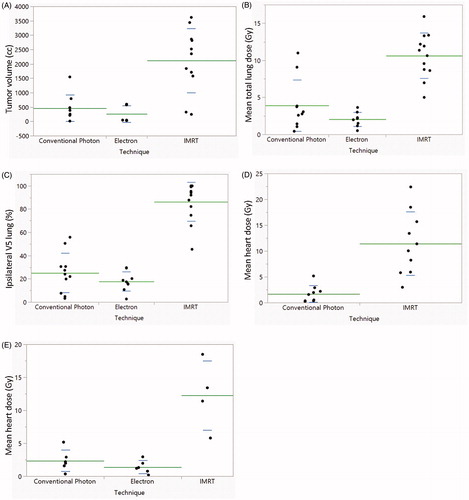
Twelve patients received intensity-modulated radiation therapy (IMRT), 13 patients received electron therapy, and 11 patients received conventional photons with opposed tangents with or without regional nodal irradiation. Patients who received IMRT had larger treatment volumes (median 2352.4 cc, range 247.0–3619.7 cc) compared with patients who received conventional photon radiation (median 260.6 cc, range 11.7–1548.0 cc) or electrons (median 47.0 cc, range 30.8–601.0 cc) (. Because patients receiving IMRT had large volumes of disease, their total mean lung dose was higher than those patients who received either electron or conventional photon techniques (p < .0001 for both) (. The difference in total mean lung dose between conventional photon and electron techniques was not significant (p = .16). Similarly, the percent of ipsilateral lung volume receiving greater than 5 Gy (V5) for patients receiving IMRT was higher than that of patients receiving electron therapy or conventional photon radiotherapy (p < .0001 for both) (. Mean heart dose irrespective of laterality of treatment was 1.71 Gy conventional photons (n = 9) and 11.44 Gy for IMRT (n = 10) (. For left-sided treatments, mean heart dose was 1.41 Gy for electrons (n = 6) (, 2.37 Gy for conventional photons (n = 6) and 12.28 Gy for IMRT (n = 4). For right-sided IMRT cases, mean liver dose was 12.72 Gy (n = 5).
Figure 2. Scatterplots of treatment volumes, lung and heart doses by treatment technique. (A) Planning treatment volumes, (B) mean lung dose, and (C) percentage of ipsilateral lung receiving greater than 5 Gy are shown for all patients. (D) Mean heart dose for all patients receiving conventional photon and IMRT plans. (E) Mean heart dose for patients receiving left-sided treatments only.
Acute toxicities included pain, erythema, edema, blisters, ulcerations, nausea, and scarring or fibrosis. The most frequent acute side effects were grade 1–2 pain and erythema (). Late toxicities included grade 1–2 hyperpigmentation or tanning, lymphedema, scarring or fibrosis, desquamation, pain and telangiectasias. One patient developed pulmonary fibrosis resulting in mild dyspnea on exertion. She received right chest wall re-irradiation with opposed tangents. Three patients developed shortness of breath in the setting of malignant pleural effusion. Six patients had ulcerations, half of whom presented with ulcerations at the time of presentation due to tumor growth. The 3 patients who developed skin ulceration after treatment healed with conservative measures. For these patients, the median time to ulcer development after completion of radiation was 69 days (range 45–217 days). All of these ulcers developed within the hyperthermia and radiation field.
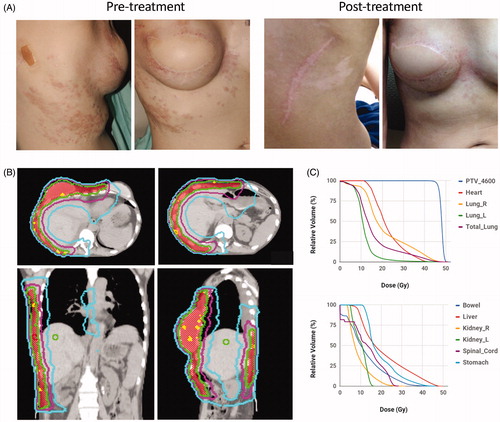
Typical results of patients treated with IMRT for extensive disease recurrence are shown ( and . One patient with triple negative breast cancer involving bilateral chest wall and extending to the left arm and back had a partial response (. She reported reduced bleeding, discharge and foul odor after treatment. Another patient with large volume recurrence of ER+, PR+, Her2+ breast cancer involving her right chest wall that wrapped around to her back had a complete response (. The radiation plans of these patients and dose-volume histograms are shown ( and ). Both of these patients went on to develop metastatic disease and ultimately died from disease progression.
Figure 3. Pre- and post-treatment images and radiation plan for a patient with extensive triple negative breast cancer recurrence treated with hyperthermia and IMRT. (A). Pretreatment (left) and 1 month post-treatment (right) images of a patient with recurrent cancer involving bilateral chest wall and left arm. (B) Radiation dose distributions on axial (left), coronal (middle), and sagittal (right) images for the patient. The PTV is shaded in yellow and treated to 32 Gy. Isodose lines are shown: 35.2 Gy (red), 32.0 Gy (green), 28.8 Gy (purple), 22.4 Gy (orange), 16.0 Gy (blue). (C) Dose volume histogram (DVH) for the PTV, lung, heart, spinal cord and liver are shown.
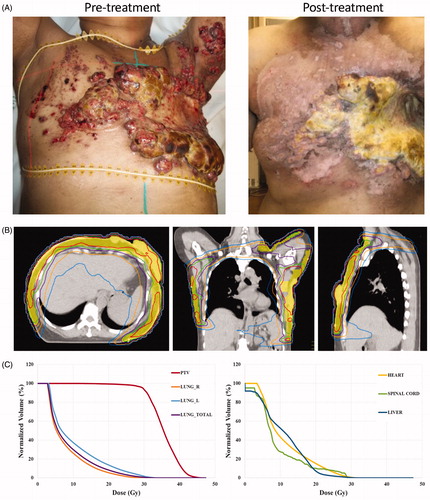
Figure 4. Pre- and post-treatment images and radiation plan for a patient with extensive ER + PR + Her2+ breast cancer recurrence treated with hyperthermia and IMRT. (A) Pretreatment (left) and 2 months post-treatment (right) images of a patient with recurrent Her2+ breast cancer. (B) Radiation dose distributions on axial (top), coronal (bottom left) and sagittal (bottom right) images for the patient. The PTV is shaded in red and treated to 46 Gy. Isodose lines are shown: 50 Gy (yellow), 46 Gy (green), 36 Gy (pink), 20 Gy (blue). (C) Dose volume histograms (DVH) for the PTV, heart, lung, small bowel, liver, kidney, spinal cord and stomach are shown.
Discussion
Our study demonstrates that patients, including those with large volumes of disease, can benefit from hyperthermia and radiation. Thermoradiotherapy can provide local control in many patients. The reduction in tumor burden decreases bleeding and foul odor from necrotic tumors to improve quality of life. A systematic review and meta-analysis conducted by Datta et al. across 34 studies showed a complete response rate of 60.2% in patients receiving combined hyperthermia and radiation compared to 38.1% in patients receiving radiation alone [Citation2]. A large observational study of recurrent breast cancer patients treated by hyperthermia and radiation reported a complete response rate of 70% with late toxicities of less than 1% after 5-years follow-up [Citation21]. While the overall response rate (61.1%) seen in our study was very good, we found a lower complete response rate compared to prior studies. This may be due to our inclusion of patients with large volumes of disease in whom only a partial response is expected. Of note, 10 (27.7%) of our patients had >1500 cc of disease, 6 of whom had >2300 cc of disease. Higher doses of radiation could be considered to improve control rates.
Not surprisingly, patients with extensive disease were typically treated with an IMRT technique. Patients with smaller volumes of disease were treated with electrons or conventional photons. Considering the large fields used for patients receiving IMRT, dose to organs at risk including lung and heart were higher than those for other radiation techniques. One patient who received radiation to her right chest wall developed shortness of breath secondary to pulmonary fibrosis. No patients developed acute cardiac complications. In general, toxicities of treatment were mild to moderate. Acute pain and erythema was reported in about 1/3 of patients. Common late toxicities were hyperpigmentation (8 patients) and lymphedema (6 patients). Six patients had ulcerations, three of whom developed new ulcerations that healed with conservative treatment.
Our study highlights the challenges of treating patients with recurrent breast cancer and proposes a radiation treatment algorithm stratified by extent of disease. For extensive recurrences requiring complex radiation field designs such as tumor recurrences extending to the back or arm, IMRT should be considered. Our data suggest that IMRT plans can offer effective tumor control that can improve quality of life. Notably, we have found that even large IMRT volumes can be achieved with acceptable dose to organs at risk. Smaller simpler tumor recurrences lend themselves to electron plans that result in lower lung and heart doses. For intermediate volumes of disease, 3 D conformational radiation techniques can be considered.
Many of these patients have received numerous other treatments including radiation and multiple types of chemotherapy. This prior treatment not only increases the risk of side effects of additional treatment, but also selects for treatment-resistant cancer cells, namely cancer stem-like cells [Citation22]. Hyperthermia has been shown to sensitize cancer stem-like cells to radiation to improve tumor control [Citation10–13]. While hyperthermia can improve tumor control in both treatment naive and heavily treated patients, its benefits are best seen in patients who have had prior radiation. In a meta-analysis of recurrent breast cancer patients treated with hyperthermia and radiation, the complete response rate was 66% for patients who were previously irradiated [Citation2]. Similarly, in a phase III clinical trial of superficial cancers, about 60% of whom had breast cancer, previously irradiated patients had a response rate of 68.2% after receiving combined hyperthermia and radiation compared to 23.5% for patients receiving radiation alone [Citation23]. These data suggest that the relative benefits of hyperthermia are best seen in pre-irradiated cancer patients.
Conclusion
Our study analyzes 36 patients with recurrent breast cancer, with particular emphasis on clinical and dosimetric considerations of radiation and hyperthermia treatment. Based on our results, the extent of tumor recurrence and in turn, radiation field size, can help determine the most appropriate technique of radiation treatment design. Our study demonstrates that even patients that have failed multiple prior treatments and present with extensive tumor recurrence can benefit from hyperthermia and radiation. Referral of patients for evaluation of hyperthermia and radiation sooner, when there is a smaller burden of disease, may result in improved tumor control and reduced toxicity.
Acknowledgement
The authors thank the Cleveland Clinic Center for Hyperthermia
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(Suppl 3):43–46.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Kouloulias V, Triantopoulou S, Uzunoglou N, et al. Hyperthermia is now included in the NCCN clinical practice guidelines for breast cancer recurrences: an analysis of existing data. Breast Care. 2015;10(2):109–116.
- Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344(apr26 1):e2718.
- Cheng L. Hazard of recurrence among women after primary breast cancer treatment-a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Prevent Biomarkers. 2012;21:800–809.
- Camphausen KA, Lawrence RC. Principles of radiation therapy. In: Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, editors. Cancer management: a multidisciplinary approach. UBM Medica; 2008.
- Vernon CC. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731–744.
- Kowal CD, Bertino JR. Possible benefits of hyperthermia to chemotherapy. Cancer Res. 1979;39:2285–2289.
- der Zee VJ. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184.
- Huang H, Yu K, Mohammadi A, et al. It’s getting hot in here: targeting cancer stem-like cells with hyperthermia. J Stem Cell Transplant Biol. 2017;2:113–121.
- Oei AL, Vriend LEM, Krawczyk PM, et al. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;33(4):419–427.
- Atkinson RL, Zhang M, Diagaradjane P, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. 2010;2(55):55ra79.
- Man J, Shoemake JD, Ma T, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769.
- Hall EJ, Giaccia AJ. Hyperthermia. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199.
- Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425.
- Burger AEE, Pain SJ, Peley G. Treatment of recurrent breast cancer following breast conserving surgery. Breast J. 2013;19(3):310–318.
- Zagar TM, Higgins KA, Miles EF, et al. Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol. 2010;97(3):535–540.
- Zagar TM, Oleson JR, Vujaskovic Z, et al. Hyperthermia for locally advanced breast cancer. Int J Hyperthermia. 2010;26(7):618–624.
- Li G, Mitsumori M, Ogura M, et al. Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma. Int J Clin Oncol. 2004;9(3):179–183.
- Linthorst M, Baaijens M, Wiggenraad R, et al. Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: results in 248 patients. Radiother Oncol. 2015;117(2):217–222.
- Luo M, Clouthier SG, Deol Y, et al. Breast cancer stem cells: current advances and clinical implications. Methods Mol Biol. 2015;1293:1–49.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085.