Abstract
Purpose: To evaluate the safety, efficacy, and survival outcomes of computed tomography (CT)-guided thermal ablation for adrenal metastases from hepatocellular carcinoma (HCC).
Methods: This long-term retrospective study included 27 male patients (median age, 50 years; range, 34–77 years) with 29 adrenal metastatic tumors associated with HCC who underwent ablation between January 2004 and December 2015. The technical success rate, effectiveness rate, complications, and survival were recorded. Complications were assessed according to the Common Terminology Criteria for Adverse Events. Survival curves were estimated using the Kaplan–Meier method. A Cox regression model was used for the evaluation of factors predicting survival.
Results: A total of 33 ablation sessions were performed for the 29 tumors. No ablation-related death was observed, and the incidence of complications was 87.9%. Grade 1–2 complications occurred in 23 of the 33 sessions (69.7%), and grade 3 hypertension was the only major complication, occurring in eight sessions (24.2%). The technical success and effectiveness rates were 93.1% (27 of 29 tumors) and 92.6% (25 of 27 patients), respectively. The median progression-free survival and overall survival (OS) durations for the 27 patients were 6.9 months and 16.8 months, respectively. The median OS duration was longer for patients with adrenal oligometastases (21.8 months) than for those with (12.8 months) multiple metastases (p = .037). Adrenal oligometastases were the only significant predictor of OS (p = .043).
Conclusions: CT-guided ablation is a feasible and safe procedure for adrenal metastases from HCC, and it may be more beneficial for patients with adrenal oligometastases.
Introduction
Extrahepatic metastases (EHM) occur in 3–10% of all cases of hepatocellular carcinoma (HCC), with a 5-year survival rate of only 3% [Citation1,Citation2]. After the lungs, lymph nodes, and bone, the adrenal gland is the most frequent site of metastasis from HCC, with an incidence of 9.1%–16.9% [Citation3–5]. The development of EHM has been demonstrated as an independent predictor of a poor prognosis in patients with HCC [Citation6]; therefore, effective control of EHM is crucial for improving survival. At present, sorafenib and lenvatinib are considered first-line drugs for the treatment of metastatic HCC; however, the high heterogeneity in individual treatment responses limits their use worldwide [Citation7,Citation8]. Some studies showed that surgical resection could improve survival in selected patients with isolated metastasis [Citation9–11]. Nevertheless, complicated cases such as those involving extra-adrenal metastases are rarely eligible for surgical resection [Citation11].
Percutaneous computed tomography (CT)-guided thermal ablation is considered a curative treatment for small HCCs and has been shown to be clinically useful for the treatment of various tumors, such as those involving the lungs, kidneys, and prostate glands [Citation12–14]. Some studies also reported effective local control of EHM by ablation; however, these studies mainly focused on the lungs and lymph nodes, which are the most common sites of EHM [Citation15,Citation16]. To our knowledge, adrenal metastases have been documented only in case reports and a few studies with very small sample sizes (≤6) [Citation17–20]. Therefore, the value of CT-guided thermal ablation for adrenal metastasis from HCC has not been evaluated in large-scale studies with long-term follow-up periods.
Accordingly, the aim of this study was to explore the safety, efficacy, and survival outcomes of CT-guided thermal ablation in patients with adrenal metastases from HCC.
Methods
Patients
This retrospective study was approved by the institutional review board of the Sun Yat-Sen University Cancer Center and conducted in accordance with the principles of the Declarations of Helsinki. The medical records of patients with adrenal metastases from HCC who underwent CT-guided percutaneous thermal ablation between January 2004 and December 2015 were retrieved from a prospectively maintained electronic database. All patients had provided informed consent before receiving treatment.
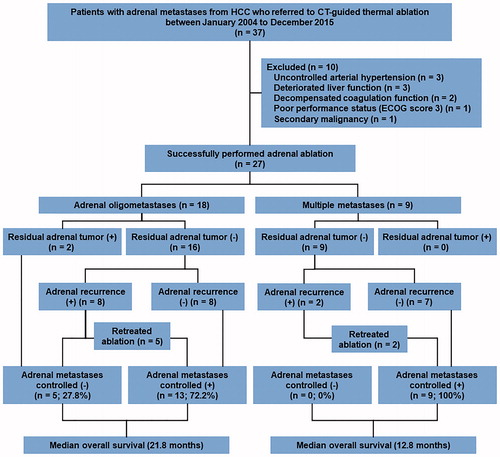
Adrenal metastases were identified by the presence of adrenal lesions with typical enhancement (early enhancement in the arterial phase and rapid wash-out in the portal and/or late phases) on routine radiographic images. The treatment modality for each patient was selected via consensus among members of a multidisciplinary team including hepatobiliary surgeons, medical oncologists, interventional radiologists, and diagnostic radiologists. The indications for local therapy were as follows: presence of adrenal metastases not invading the peripheral vessels, nerves, or organs; absence of intrahepatic tumors and/or extra-adrenal metastases or presence of intrahepatic tumors and/or extra-adrenal metastases that were well controlled by repeated locoregional treatment (ablation, arterial chemoembolization) and/or continuous systemic drugs (targeted inhibitors, chemotherapy); stable intrahepatic and/or extra-adrenal disease for at least 3 months before the initial adrenal ablation. If the adrenal lesion was curable, surgical resection was recommended as the first choice of treatment. For patients with contraindications for surgery and those who refused to undergo resection, adrenal thermal ablation was suggested as an alternative therapy. Accordingly, during the observation period, 37 patients with adrenal metastases from HCC had been referred for CT-guided thermal ablation. From these, 22 patients (59.5%) were not considered eligible for surgery because of a previous history of abdominal surgery (n = 11), an age of >70 years (n = 7), the presence of bilateral adrenal metastases (n = 3), and a history of contralateral adrenalectomy (n = 1). The remaining 15 patients (40.5%) opted for ablation. Nine of the 37 patients could not undergo ablation because of uncontrolled arterial hypertension (>160/100 mmHg; n = 3), poor liver function (Child–Pugh grade C; n = 3), decompensated coagulation function (platelet count <50 × 109/L; n = 2), and poor performance status (Eastern Cooperative Oncology Group score of 3; n = 1). One more patient was excluded because of a previous history of two malignancies. Eventually, the records of 27 patients (29 tumors) who underwent CT-guided thermal ablation were reviewed (.
Adrenal ablation
Radiofrequency ablation or microwave ablation was performed within 1 week after the diagnosis of adrenal metastases. Details of the ablation procedure are described in the Supplementary Material. Technical success was defined as completion of the thermal ablation procedure according to the planned protocol, with a complete response observed on images obtained 3–4 weeks after treatment. A complete response was defined as complete disappearance of any arterial enhancement in the adrenal metastatic lesions. A partial response was defined as a > 30% decrease in the cross-sectional diameter of viable (intratumoral arterial enhancement) areas in the adrenal lesions, considering the baseline diameter as a reference. Progressive disease was defined as a 20% increase in the diameter of viable intra-adrenal lesions, considering the baseline diameter as a reference. Patients with a partial response after the first ablation session received up to two additional sessions. The time interval between two sessions was restricted to 4–6 weeks.
Follow-up
The endpoint of follow-up was the time of death or the last visit of the patient until July 31, 2018. Contrast-enhanced abdominal CT or magnetic resonance imaging and chest CT were performed at 3–4 weeks after treatment. Subsequently, these procedures were performed every 10–12 weeks for up to 1 year and every 5–6 months thereafter. Ablation-related complications were assessed according to the Common Terminology Criteria for Adverse Events and classified as major and minor events. Major events (grades 3, 4, and 5) were defined as complications that led to substantial morbidities and disabilities, an increase in the level of care, and/or hospital admission or a substantial increase in the length of hospitalization. All other events were considered minor complications (grades 1 and 2).
Statistical analysis
Evaluations of technical success, effectiveness, and complications were based on per-lesion ablation. Adrenal tumor progression rates and survival outcomes were assessed on a per-patient basis. Survival was estimated using the Kaplan–Meier method and compared using log-rank tests. A Cox regression model was used for the evaluation of factors predicting survival. Statistically significant variables in the univariate analysis were included in the multivariate analysis. A p values of <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL). All data were recorded at Sun Yat-Sen University Cancer Center (number: RDDA2018000952).
Results
Patients
The patients’ demographic characteristics are summarized in . All 27 patients were male, and the median age was 50 years (range, 34–77 years). The median adrenal tumor diameter was 3.5 cm (range, 1.0–6.5 cm). At the time of the first adrenal ablation, 11 patients (40.7%) exhibited intrahepatic tumors with adrenal metastases and seven (25.9%) exhibited only adrenal metastases. Therefore, the disease was identified as HCC with adrenal oligometastases in these 18 (11 + 7) patients. Multiple EHM were present in the other 11 patients; five (18.5%) exhibited both adrenal and extra-adrenal metastases and four (14.8%) exhibited intrahepatic tumors with adrenal and extra-adrenal metastases.
Table 1. Demographic characteristics of 27 patients and 29 adrenal metastatic tumors.
Four patients, including one with locally recurrent adrenal metastases, two with newly developed contralateral adrenal metastases, and one with locally recurrent and newly developed contralateral adrenal metastases, had previously undergone adrenalectomy. In addition, four patients had previously undergone systemic therapy, which included chemotherapy (n = 3) and sorafenib therapy (n = 1). All four patients underwent adrenal ablation after failure of the systemic therapy.
Ablation
At the time of the initial ablation, 25 patients (92.6%) exhibited a single adrenal lesion, whereas the remaining two patients exhibited one tumor in each adrenal gland. A single ablation session was required for 26 tumors. Two tumors required two sessions and one required three sessions. Therefore, a total of 33 ablation procedures were performed. The planned ablation procedure was completed in all tumors (100%). The complete response rate was 82.8% (24 of 29 tumors) after the first ablation. Residual tissue was detected for five lesions (five patients) with a diameter of >3 cm (median, 3.8 cm; range, 3.5–5.9 cm). Three of the five patients received additional ablation procedures (one session for two patients and two sessions for one patient) for the residual tumors. One patient declined ablation and opted for oral sorafenib therapy. In the remaining patient, extra-adrenal metastases were detected and systemic chemotherapy was subsequently performed. Therefore, the overall technical success rate was 93.1% (27 of 29 tumors), while the technical effectiveness rate was 92.6% (25 of 27 patients).
Adrenal lesion progression
The median follow-up duration after the initial ablation procedure was 19.3 months (range, 7.1–96.5 months). In addition to the two patients with residual tumors that were treated by systemic therapy, 40.7% patients exhibited adrenal tumor progression after ablation. These included eight patients with ipsilateral recurrence, two with newly developed contralateral adrenal metastases, and one with bilateral adrenal metastases. The median time to progression in these 11 patients was 7.5 months (range, 1.9–33.3 months). Seven patients were retreated with ablation after adrenal tumor progression. All tumors were successfully ablated and controlled until death or the last follow-up. One patient was treated with chemotherapy because of the presence of portal vein thrombosis, while one patient received sorafenib because of personal preference. The remaining two patients exhibited deterioration of liver function and received best supportive care (. Therefore, the lesions in 21 of the 27 patients (77.8%) were eventually controlled by CT-guided thermal ablation.
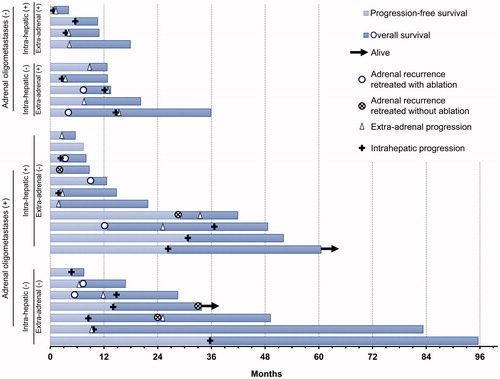
Figure 2. Outcome data for study population (two groups: adrenal oligometastases and multiple metastases). Swimmer’s plot showing disease progression reason, treatment for adrenal recurrence, time of survival and current status since the initial ablation of adrenal metastases.
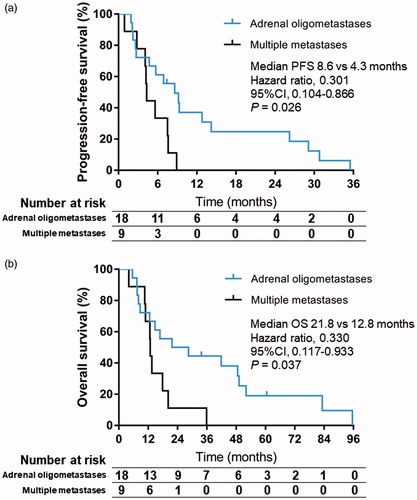
The median adrenal lesion-free survival duration for the 27 patients was 29.1 months (95% confidence interval [CI], 16.4–41.8 months), with no significant difference between patients with adrenal oligometastases and those with extra-adrenal metastases (p = .641). The median progression-free survival duration was 6.9 months (95%CI, 3.8–10.0 months; ), with a significant difference between patients with adrenal oligometastases (8.6 months) and those with multiple metastases (4.3 months, p = .026; hazard ratio, 0.301; 95%CI, 0.104–0.866; .
Figure 3. Survival of the total 27 patients after thermal ablation for adrenal metastases from hepatocellular carcinoma. (A) Progression-free survival curve; (B) Overall survival curve.
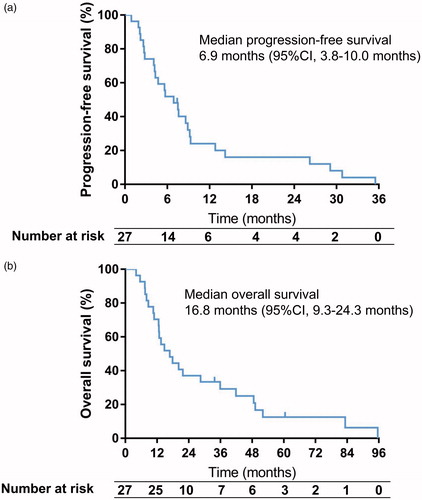
Figure 4. Progression-free and overall survival between patients with adrenal oligometastases versus multiple metastases after thermal ablation. Kaplan-Meier curve of progression-free survival (A) and overall survival (B) CI: Confidence Interval; OS: Overall Survival; PFS: Progression-free Survival.
HCC-free status was achieved for 4.7–35.5 months (median, 8.6 months) in seven patients without intrahepatic and/or extra-adrenal tumors after the initial ablation procedure. Three of these seven patients exhibited intrahepatic and/or extra-adrenal tumor progression, although there was no adrenal tumor recurrence until the last follow-up. The other four patients developed adrenal lesion recurrence. The median time to adrenal tumor progression was 15.5 months (range, 5.7–33.3 months), while the median time to intrahepatic and/or extra-adrenal tumor progression was 7.8 months (range, 5.7–14.2 months). None of the patients with intrahepatic and/or extra-adrenal tumors after the initial ablation procedure (20 of 27 patients) achieved HCC-free status until the last follow-up.
Survival
Twenty-five of the 27 patients (92.6%) died before the last follow-up visit. Causes of death included intrahepatic tumor progression (n = 10), extra-adrenal metastases (n = 10), liver failure (n = 4), and gastrointestinal bleeding (n = 1). No patient died of adrenal metastases. Two patients with adrenal oligometastases were alive until the last follow-up visit, with survival durations of 33.8 and 60.5 months, respectively. The 6-, 12-, and 24-month survival rates for the overall cohort were 88.9%, 66.7%, and 33.3%, respectively. The median OS duration after the initial ablation procedure was 16.8 months (95%CI, 9.3–24.3 months; . The 18 patients with adrenal oligometastases survived for a longer period than did the nine patients with multiple metastases (21.8 vs. 12.8 months, p = .037; hazard ratio, 0.330; 95%CI, 0.117–0.933; . Although patients with small adrenal metastases (t3 cm) tended to survive for a longer period than did those with large tumors (>3 cm), the difference was not statistically significant (p = .059).
An age of <50 years (p = .010), portal vein thrombosis (p = .006), extra-adrenal metastases (p = .043), and non-adrenal oligometastases (p = .043) were significant predictors of poor OS in univariate analysis (). However, multivariate analysis found adrenal oligometastases to be the only significant predictor of poor OS (p = .043; hazard ratio, 0.363; 95%CI, 0.136–0.970).
Table 2. Univariate analysis of prognostic factors that influenced overall survival.
Complications
The overall incidence of treatment-related complications in the 33 sessions was 87.9%. Procedure-related death or grade 4 adverse events were not observed during 30 days after ablation. Grade 3 intraprocedural hypertension (>180 mmHg) was the only major complication, occurring in eight of the 33 sessions (24.2%). In all patients, the blood pressure was controlled and gradually restored to the baseline range by a calcium channel blocker. The incidence of hypertension was higher for patients aged >65 years than for younger patients (66.7% [four of six patients] vs. 14.8% [four of 27 patients], p = .031). Minor complications occurred in 23 of the 33 sessions (69.7%). These included short-term grade 1 (eight patients) and grade 2 (eight patients) abdominal pain (16 sessions; 48.5%); minor hypertension (10 sessions; 30.3%) that did not require medication; and grade 1 fever, pneumothorax, hemothorax, and grade 2 fever (one session each; 3%).
Discussion
In this long-term retrospective study, we found that CT-guided thermal ablation was a feasible and safe procedure for the treatment of adrenal metastases from HCC. Survival analyses showed that patients with adrenal oligometastases exhibited better ablation outcomes than did those with multiple metastases.
For 27 patients who undergone CT-guided ablation for adrenal metastases from HCC between 2004 and 2015, the overall technical success and effectiveness rates were 93.1% (27 of 29 tumors) and 92.6% (25 of 27 patients), respectively. The tumor response rate was 92.6%, and the OS duration after the initial ablation procedure was 16.8 months. Yamakado and colleagues [Citation17] described the response rates and survival benefits for radiofrequency ablation combined with chemoembolization in six patients with adrenal metastases from HCC. The objective response rate (technical effectiveness) and median survival duration were 100% and 24.9 months, respectively [Citation17]. However, another recent study [Citation19] involving four cases observed a median survival duration of only 9.3 months, which is significantly different from that in abovementioned study. This discrepancy can be attributed to the intrahepatic tumor status, which is an important factor influencing the overall outcomes of patients with EHM [Citation21]. In the study by Yamakado et al., the median survival duration was significantly longer for patients with well-controlled liver disease than for those with uncontrolled intrahepatic lesions (61.6 vs. 13.8 months). This was further confirmed in the present study, where all patients were evaluated before the ablation procedure and included only if they exhibited stable intrahepatic and extra-adrenal lesions for at least 3 months before the procedure. In two other studies about adrenal metastases from all cancers, only one case involved HCC; therefore, the data were not representative or comparable [Citation18,Citation20].
Currently, oral multiple kinase inhibitors (sorafenib, lenvatinib, regorafenib, and cabozantinib) and systemic anti-programmed cell death protein-1 inhibitors (nivolumab and pembrolizumab) are recommended by popular guidelines as treatment options for HCC with metastases [Citation8,Citation22,Citation23]. However, subgroup analyses in several clinical trials showed lesser survival benefits for patients with EHM than for those without metastases [Citation24–29]. Sohn et al. reported that the tumor response rate for sorafenib treatment in patients with HCC and EHM was 5% (complete response: 0%, partial response: 5%), with a median survival duration of 11.9 months for patients with adrenal metastases [Citation30]. Previous studies have shown that local treatments for metastases achieve better control and outcomes, with survival durations of 12 to 21.4 months and 9.3 to 24.9 months after adrenalectomy and thermal ablation, respectively [Citation9,Citation10,Citation17,Citation19]. Our results support this theory by showing that local treatment may be effective in controlling limited metastases from HCC, and that patients with unresectable metastatic tumors are potential candidates for ablation therapy.
The term oligometastases was proposed to describe a special clinical form of metastases by Weichselbaum and Hellman in 1995 [Citation31]. This concept is commonly applied to cases where the number and sites of metastatic tumors are limited, consequently resulting in effective localized therapy [Citation32]. Although EHM has demonstrated as an independent poor prognostic factor in cases of HCC, outcomes may differ between patients with oligometastases and those with multiple metastases. Via subgroup analysis, our study found that patients with adrenal oligometastases survived for longer periods than did those with multiple metastases (p=.037); this indicates that patients with adrenal oligometastases from HCC may benefit more from CT-guided ablation. To our knowledge, no previous study has systematically compared the outcomes of CT-guided ablation between patients with adrenal oligometastases from HCC and those with multiple metastases from HCC. In the study by Yamakado et al., three cases of adrenal oligometastases exhibited survival durations of 12.6, 24.9, and 70.9 months, respectively. However, these data were only descriptive, and no statistical analyses were performed. Two other studies evaluating the outcomes of CT-guided ablation for oligometastases in the lungs and lymph nodes documented a 1-year survival rate of 58%–91%, which was consistent with the rate in the present study (66.7%) [Citation15,Citation16]. Although these studies did not include cases of adrenal oligometastases, the results can serve as meaningful references. Of note, HCC-free status was achieved for a median duration of 8.6 months (range, 4.7–35.5 months) in seven patients with adrenal metastases only. This indicates that patients without intrahepatic and/or extra-adrenal lesions can achieve a longer disease-free period after adrenal ablation.
The safety of thermal ablation for adrenal lesions, which has been proven in previous studies [Citation17–20], was also confirmed in the present study, where ablation-related death and grade 4 adverse events were not observed. The rate of grade 3 intraprocedural hypertension was 24.2%, which is consistent with previous data [Citation17,Citation19].
This study had some limitations. First, it was a retrospective, single-center study. Second, the sample size was small, although it was considerably larger than those in previous studies. Third, we did not include a control group for comparing the outcomes of ablation with those of systemic therapies for adrenal metastases from HCC.
In conclusion, the findings of this study suggest that CT-guided thermal ablation is a feasible and safe procedure for adrenal metastases from HCC. Moreover, this procedure may be more beneficial for patients with adrenal oligometastases than for those with multiple metastases.
Authors’ contributions
Ming Zhao, Ning Lyu and Yanan Kong conceived and designed the study. Ming Zhao, Ning Lyu, Tao Pan, Luwen Mu, Xuqi Sun, Shaolong Li, Haijing Deng and Jinfa Lai collected the data. Ning Lyu, Yanan Kong and Ming Zhao analyzed and interpreted the data. All authors were involved in the drafting, review, and approval of the report and the decision to submit for publication.
| Abbreviations | ||
| CI | = | confidence interval |
| CT | = | computed tomography |
| HCC | = | hepatocellular carcinoma |
| OS | = | overall survival |
Supplemental Material
Download PDF (328.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171.
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166.
- Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–1787.
- Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28(9):1256–1263.
- Aino H, Sumie S, Niizeki T, et al. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol Clin Oncol. 2014;2(3):393–398.
- Hsu CY, Liu PH, Ho SY, et al. Metastasis in patients with hepatocellular carcinoma: prevalence, determinants, prognostic impact and ability to improve the Barcelona Clinic Liver Cancer system. Liver Int. 2018;38(10):1803–1811.
- Sanoff HK, Chang Y, Lund JL, et al. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21(9):1113–1120.
- Raoul JL, Kudo M, Finn RS, et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: sorafenib and beyond. Cancer Treat Rev. 2018;68:16–24.
- Momoi H, Shimahara Y, Terajima H, et al. Management of adrenal metastasis from hepatocellular carcinoma. Surg Today. 2002;32(12):1035–1041.
- Park JS, Yoon DS, Kim KS, et al. What is the best treatment modality for adrenal metastasis from hepatocellular carcinoma? J Surg Oncol. 2007;96(1):32–36.
- Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21(2):95–101.
- Breen DJ, Lencioni R. Image-guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol. 2015;12(3):175–186.
- Mouli SK, Kurilova I, Sofocleous CT, et al. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol. 2017;209(4):740–751.
- Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;71(1):17–34.
- Mu L, Sun L, Pan T, et al. Percutaneous CT-guided radiofrequency ablation for patients with extrahepatic oligometastases of hepatocellular carcinoma: long-term results. Int J Hyperthermia. 2018;34(1):59–67.
- Pan T, Xie QK, Lv N, et al. Percutaneous CT-guided radiofrequency ablation for lymph node oligometastases from hepatocellular carcinoma: a propensity score-matching analysis. Radiology. 2017;282(1):259–270.
- Yamakado K, Anai H, Takaki H, et al. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol. 2009;192(6):W300–5.
- Wolf FJ, Dupuy DE, Machan JT, et al. Adrenal neoplasms: effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81(8):1717–1723.
- Hasegawa T, Yamakado K, Nakatsuka A, et al. Unresectable adrenal metastases: clinical outcomes of radiofrequency ablation. Radiology. 2015;277(2):584–593.
- Doreille A, N’Kontchou G, Halimi A, et al. Percutaneous treatment of extrahepatic recurrence of hepatocellular carcinoma. Diagn Interv Imaging. 2016;97(11):1117–1123.
- Jung SM, Jang JW, You CR, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27(4):684–689.
- European Association for the Study of the Liver, European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750.
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57 (4):821–829.
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173.
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63.
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952.
- Sohn W, Paik YH, Cho JY, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol. 2015;62(5):1112–1121.
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378–382.
- Johnson B, Jin Z, Haddock MG, et al. A curative-intent trimodality approach for isolated abdominal nodal metastases in metastatic colorectal cancer: update of a single-institutional experience. Oncologist. 2018;23(6):679–685.