Abstract
Purpose: Osteomyelitis is one of the most serious complications linked to diabetes and increases the possibility of limb amputation considerably. There exists an important clinical need to improve management of osteomyelitis, especially for diabetic patients who are more susceptible to failures, relapses and chronicity of multiple bone infections. Magnetic resonance-guided focused ultrasound (MRgFUS) can offer a clinical management option for patients with osteomyelitis by providing a non-surgical and potentially rapid-recovery treatment option.
Material and Methods: A retrospective study with patients with confirmed osteomyelitis (n = 75) was performed at evaluating the feasibility to target bone infection sites with a clinically approved MRgFUS device (Sonalleve, Profound Medical, Mississauga, ON, Canada). The developed methodology allows using preexisting diagnostic magnetic resonance imaging (MRI) or computed tomography (CT) scans to evaluate the treatment feasibility directly using a MRgFUS treatment planning software.
Results: 74.7% of the cases included in our study passed the targetability criteria. Cases were deemed non-targetable if the target was less than 1 cm from the skin or close to a neuro-vascular bundle, metallic implants, or in the way of a defect in the overlying skin. For cases that passed the targetability criteria, an average among patients of 92.7 ± 5.2% of the gross treatment volume could be reached using treatment cells available at the Sonalleve system.
Conclusion: The retrospective study presented here is the first step to demonstrate the feasibility of utilizing MRgFUS for the thermal treatment of osteomyelitis.
1. Introduction
1.1. Osteomyelitis
The infection of the bone (osteomyelitis) is one of the most severe complications linked to diabetes, and it is a recognized factor that considerably increases the possibility of limb amputation [Citation1–4]. It is acknowledged that the diagnosis, treatment and management of osteomyelitis related to diabetes remains a very challenging problem [Citation2,Citation5–9]. Worldwide, and despite important progress, the incidence rate of amputations on diabetic patients is 10 to 20 times higher than the general population, representing 1.5–3.5 events per 1000 persons/year in patients diagnosed with diabetes [Citation10]. In Canada, diabetic patients are 23 times more likely to be hospitalized for a limb amputation than those without diabetes [Citation4]. Diabetes alone is associated with a 4.4-fold increase in risk for bloodstream infections compared to non-diabetic patients [Citation11]. For cases of hematogenous-induced infections in the vertebra, a cohort study with 70 patients reported 43% of comorbidity with diabetes [Citation12].
In the foot region, the damage to sensory nerves caused by diabetic peripheral sensorimotor neuropathy results in the lack of detection of minor injuries or ulcers that otherwise would be noticed. These injuries become the path for infiltration of pathogens into soft tissue that ends by penetrating the sub-lying bone [Citation1,Citation2,Citation7,Citation13]. The degree of severity of these infections varies from a superficial abscess, deep skin and soft tissue infections to osteomyelitis. The most common sites that develop initial injuries or ulcers are the calcaneum, head of the metatarsus and the toes [Citation14].
For osteomyelitis, the therapeutic roadmap is complex and requires a combination of surgical interventions, use of antibiotics and long-term follow-up. There is a clinical need to explore alternatives that will translate into better outcomes with a reduction of the risk of amputation [Citation1].
The eradication of the infection in the bone in diabetic patients is challenging because of an impaired response of the immune system. Staphylococcus aureus (S. aureus) is the most dominant pathogen causing bone infection, but it is often accompanied by several other micro-organisms [Citation1,Citation2]. S. aureus can elude the inflammatory cells and form biofilms that reduce the efficiency of the host immune response and also limit the effect of antibiotics. In addition, the advent of community-associated methicillin-resistant S. aureus (CA-MRSA) infections in the last years [Citation15] has increased the challenges in the management of diabetic foot infections. A recent study indicated that the highly virulent CA-MRSA strain USA300 was presented in >50% of patients with osteomyelitis [Citation16]. In vertebral osteomyelitis, S. aureus is also the most common bacteria responsible for infection [Citation17,Citation18], but recent evidence also shows an increase of infections caused by gram-negative bacilli [Citation19], which further complicates therapeutic management. MRSA-related vertebral osteomyelitis, which represents overall 40% of S. aureus-related infections are associated with higher rates of relapse and persistent bacteremia [Citation17].
1.1.1. Diagnosis
Important factors to consider in the diagnostic of osteomyelitis include symptomatic venous insufficiency, the presence of arterial occlusive disease, the presence of diabetes and characteristics of the wound [Citation20,Citation21]. Patients can show a very diversified range of symptoms from swelling (without skin lesion), bone tenderness to indolent fistula, open wounds and bone fracture [Citation1]. For vertebral infections, back pain is the most common symptom, with an 86% prevalence [Citation22]. Fever and high white cells count are often not present at early onset [Citation1,Citation22]. Acute osteomyelitis occurs over a period of a few days to weeks. Chronic osteomyelitis takes place over much longer periods of time from months to years, with the persistence of pathogens and low-grade inflammation. Identification of the main pathogen is critical but often challenging as the pathogen avoids positive culture from blood samples. Histopathology from direct bone biopsies (instead of superficial swapping) is preferred to establish the main pathogen [Citation1,Citation21,Citation23] when other symptoms of bone infection are present. For the diabetic foot infection, the PEDIS severity score [Citation23] has been proposed combining five indicators: perfusion, extent/size, depth/tissue loss, infection, and sensation. The lowest score (1) indicates lesions lacking the presence of purulence or signs of inflammation. The mild score (2) indicates a patient with no systemic illness and who shows at least two signs of inflammation (purulence, or erythema, pain, tenderness, warmth, or induration) but cellulitis/erythema extends <2 cm around the ulcer, and infection is limited to subcutaneous tissues. The moderate score (3) applies to a patient considered systemically well and metabolically stable but with more than one of the more advanced symptoms (cellulitis extending >2 cm, lymphangitic streaking, spread beneath the superficial fascia, deep-tissue abscess, gangrene, and involvement of muscle, tendon, joint or bone). The severe score (4) applies to a patient showing systemic toxicity or metabolic instability (fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, severe hyperglycemia, or azotemia).
1.1.2. Imaging of osteomyelitis
Assessment using medical imaging is recommended for the correct diagnosis and management of osteomyelitis [Citation1,Citation20,Citation23]. The radiographical evaluation includes signs for periosteal elevation, cortical disruption, medullary involvement and osteolysis [Citation21]. Traditional X-ray imaging shows evidence of abnormalities typically two weeks after infection onset [Citation24]. Computed tomography (CT) scans are often utilized for biopsy guidance and surgical procedures, especially for intervention in the vertebra [Citation21,Citation24]. Magnetic resonance imaging (MRI) is commonly used for diagnostic and extension assessment of the infection in the bone [Citation13,Citation24,Citation25] and is often preferred for early detection [Citation24,Citation26]. The changes in contrast in MRI can be observed as low-fat signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The difference of MRI contrast facilitates distinction between necrotic bone, granular tissue, sinus tracts and abscesses. The sensitivity for osteomyelitis using MRI is between 78% and 90% [Citation13,Citation26]. The specificity, while it is sometimes difficult to distinguish osteomyelitis from other causes of marrow edema, ranges from 60% to 90% [Citation26] and has a positive and negative predictive value as high as 93–100% [Citation27]. Ultrasound imaging is especially useful to assess surrounding soft tissue infection and also for the guidance of needle interventions [Citation24]. Imaging based on radiotracers, especially fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT), has gained acceptance in recent years for a better detection for chronic osteomyelitis with significantly higher sensitivity and specificity compared to other imaging modalities, including MRI [Citation6].
1.1.3. Treatment approaches
Administration of antibiotics (preferably intravenous) remains the first line of treatment for acute osteomyelitis and vertebral osteomyelitis. Bed-side debridement and surgical interventions, combined with antibiotics, are often the preferred treatment approach for chronic osteomyelitis and infections in the foot [Citation21]. The main purpose of debridement and surgery is to eliminate dead bone and repair vascular environment [Citation20,Citation23]. Depending on the extension of the damage, repair surgery may be necessary to compensate for large bone and soft tissue losses. For vertebral infections, surgical intervention is recommended only when there is a need to provide relief from the compression of the spinal cord or create a drainage path for the abscesses [Citation1]. Administration of antibiotics is often performed from 4 to 6 weeks up to 3 months, depending on the prevalence of symptoms. Intravenous administration is performed during the first 4–6 weeks. Nafcillin/Oxacillin and vancomycin are often used, respectively, for methicillin-sensitive S. aureus (MSSA)-related and MRSA-related infections [Citation1,Citation23]. For diverse streptococci, gram-negative bacilli, anaerobes and mixed-type infection, the first line of recommended antibiotic regime is based on, respectively, benzylpenicillin, quinolones, clindamycin and ampicillin [Citation1].
1.1.4. Outcomes
A retrospective meta-study with 1493 patients (from data collected from 16 different studies) indicated, for all types of osteomyelitis, a success rate (defined as the absence of immediate relapse) of 71.1% using antibiotics alone, 85.9% for surgical treatment alone and 84.7% for combined surgery and antibiotics regime [Citation21]1. However, the approach and success rate depend greatly on the site of infection (vertebral vs. foot), condition (acute vs. chronic), the prevalence of S. aureus and comorbidity with diabetes. On the latter, diabetes-related osteomyelitis in the foot region remains more challenging to manage because of the chronicity of the ulcers.
The outcome for patients treated for osteomyelitis depends greatly on the extension of bone damage. For diabetic foot infections, limb amputation is one of the most serious complications. However, there is no clear medical consensus in establishing the degree of the amputation. As noted in [Citation28,Citation29], reports differ significantly on the definition of “acceptable” outcome, which has been defined as total conservation of bone, loss of a section of the feet, total loss of feet or loss of limb below the knee. Recurrence is also challenging to define, especially if new infections may take a long time to occur, and they may happen at a different site. Despite this lack of consensus, it is clear that diabetic-related foot infections are prone to appear in the same patient multiple times, which complicates their management [Citation28]. MRSA is associated with more challenging management (requiring multiple surgical interventions), but overall, the outcome is not significantly different regarding limb amputation (defined as amputation not involving the ankle bone) compared to MSSA [Citation30].
For vertebral osteomyelitis, the situation is slightly different in the sense that true recurrence (reinfection of the original site or insufficient treatment) is associated with diabetes. One study with 215 patients showed that diabetes was directly associated with recurrence [Citation31]. Another study with 139 patients with MRSA-related hematogenous vertebral osteomyelitis showed that diabetes translated in persistent bacteremia (MRSA present more than 7 days after the beginning of treatment) and longer hospital stays [Citation17].
1.1.5. Adjuvant therapies
Experimental approaches for the treatment of advanced osteomyelitis have explored the use of localized delivery of antibiotics at the site of the infection. The most widespread approach is the placement of polymethyl methacrylate (PMMA) beads loaded with antibiotics [Citation32] in the treated area. The beads are then recovered in a second surgical intervention, usually two weeks after the first intervention. Despite the need for the second intervention, results of a retrospective [Citation33] and a randomized study [Citation34] indicated a moderate increase in the success rate on the reduction of infection recurrence compared to systemic antibiotic delivery. Another retrospective study compared the effect of vancomycin-loaded calcium sulfate (study group) and vancomycin-loaded PMMA (control group) in patients suffering from post-traumatic or postoperative osteomyelitis of the lower extremities, indicating a significantly higher infection eradication and lower re-operation rate in the study group [Citation35]. However, a multi-center randomized study [Citation36,Citation37] did not show a significant difference between systemic and localized delivery of antibiotics with beads in the management of osteomyelitis. More recently, the use of biomaterials, such as bioactive glass with antibiotic effects [Citation38] to prevent infection is gaining traction in bone reconstruction surgery [Citation39]. Bioactive glass is used as a material for bone reconstruction, and when used as an agent to locally deliver antibiotics, it eliminates the need for a second surgical intervention. Recent studies using bioactive glass for osteomyelitis treatment show early promising results, with success rates similar to PMMA-loaded beads [Citation40,Citation41]. A retrospective study compared the use of a gentamicin-impregnated collagen sponge versus PMMA loaded ones in 60 patients. It was indicated that the biodegradable material had a significant lower reoperative rate when compared to PMMA beads [Citation42]. A prospective longitudinal outcome study assessed the clinical applicability of bioactive glass and reported eradication of infection in 96% of all patients [Citation43]. A multinational retrospective study analyzed one hundred and sixteen patients with chronic osteomyelitis that were treated using bioactive glass as part of their treatment and showed a success rate of 90% [Citation44]. A recent study looked at 25 diabetic patients with an osteomyelitis infection in the foot treated with bioactive glass reported no sign of recurrence of infection after a mean follow up of 12 months [Citation45]. However, larger randomized studies are still needed to better establish the potential improvement in recurrence and the impact specific to diabetes-related infections.
1.2. Thermal therapy as an adjuvant modality for the treatment of infections
Exploration of thermal therapy as an adjuvant in the management of osteomyelitis has very recently attracted interest. A bibliographic search of the keywords osteomyelitis, thermal therapy, thermal effects using PubMed and Google Scholar shows practically no relevant results, except for a study using thermal ablation with laser as a surgical approach, not as a true adjuvant, for bone perforation in pediatric osteomyelitis [Citation46].
A search, including skin infections, provides evidence of the value and results of thermal therapy (especially mild-hyperthermia) on the management of infection [Citation47]. Localized mild-hypothermia (2 °C below baseline), which historically was administered during colorectal surgery, was found to correlate with three times higher incidence of surgically-related infections when compared to isothermic treatments [Citation48], and its use is now discontinued [Citation49]. The bioeffect associated was the reduction in oxygenation related to the vasocontraction induced by the local hypothermia, which then translated to a reduction of the immune response [Citation50]. In opposition, mild-hyperthermia (or at least keeping normothermic conditions) is being recognized to have a clear adjuvant value in reducing risks of surgery-related infections [Citation51]. A randomized study with 421 patients receiving clean surgery (breast, varicose vein, or hernia) reported a risk-reduction in post-surgery infections when using systemic and localized pre-operative mild-hyperthermia [Citation52]. The reduction ranged from 7.9% for systemic hyperthermia to 10.1% for local hyperthermia. However, the study did not report the exact values of target temperature, and the duration for systemic hyperthermia was significantly shorter than the local therapy. A preemptive increase in oxygenation in the wound area was assumed to be the bioeffect responsible for keeping immune response more active during the critical period of the surgical procedure [Citation53,Citation54]. A study with ten volunteers measured the change of oxygenation tension when local mild hyperthermia was applied using a thermal bandage, with temperature control set to either to 38 °C, 42 °C or 46 °C [Citation55] applied for 2 h, with measured subcutaneous temperature increases of +3 °C, +3.6 °C and +3.5○ respectively. Oxygenation tension increased by more than 50% when local hyperthermia was applied and remained increased even 1 h after heating ended. No significant difference was observed in the average of the oxygenation tension among the three regimes. The 46 °C regime showed more disperse results with a standard deviation of oxygenation tension of 120% compared to 50% when using 38 °C and 42 °C. Even if mild hyperthermia improves oxygenation levels favoring some of the anti-bacterial activity under the form of oxidative reactions, there is also the risk that a high temperature downplays the specific activity of neutrophil receptors involved in phagocyting bacteria [Citation56], which shows the complexity of the response to thermal stimulation. Another recent study investigated the use of localized mild heating (42–46 °C) via high-intensity focused ultrasound to improve] perfusion and antimicrobial efficacy in mouse staphylococcus abscess [Citation57] They discovered that adding a temperature increase to ciprofloxacin treatment caused a 2.5-fold in perfusion when compared to unheated lesions, significantly enhancing killing of S. aureus.
An in vivo study investigated the use of magnetic particles hyperthermia to destroy biofilms generated in peri-implant osteomyelitis [Citation58]. A metallic needle was implanted into the bone marrow cavity of rats and heated to 75 for up to 30 s at a time using an alternated magnetic field. Massive necrosis of tissue was observed in the rats treated with antibiotic and magnetic particles hyperthermia, which was not observed for the other treatment groups. They hypothesized that the magnetic particles were responsible for a better distribution of heating intensity. However, there are no results for a group treated with antibiotics and hyperthermia-induced by alternated magnetic field to sustain that hypothesis. Another study also employed high-frequency alternative magnetic fields (AMF) for the noninvasive treatment of biofilm specifically to prosthetic joint infections [Citation59]. They hypothesized that exposing 1 mm of the implanting surface to heat produced by AMF would be both bactericidal to pathogens and responsible for an increase in the sensitivity to microbial agents. The joint was exposed to a 5 min and 7 min AMF and in both cases, there was a significant reduction in colony forming units. Although AMF could heat uniformly the surface of implants, this technology still needs a safety tool to provide real time feedback, as the methodology presented in [Citation59] only uses acoustic emissions to detect boiling of tissue.
For the use of ablative-level thermal effects on infections, a previous in vivo study (murine model) from our group established the feasibility of treating MRSA-induced abscesses using thermal effects produced by high intensity focused ultrasound under MRI guidance [Citation60]. Two different ultrasound intensities were tested on MRSA (USA 400) subcutaneous abscesses [Citation61]. Bacteria growth and abscess healing were analyzed 1 and 4 days after treatment. Sixty (60) female BALB/c mice, aged 7–12 weeks were used in our preliminary study. From the 60 animals, fifty-five (55) successfully developed an abscess and were distributed to the following treatment groups: moderate temperature (MT) (n = 19), high temperature (HT) (n = 18) and control (n = 18). MT and HT groups reached, respectively, 52.3(±5.1) C and 63.8(±7.5) C. Average thermal dose for MT and HT were, respectively, 2.6 × 1010CEM43 and 6.2 × 1022CEM43. Each treatment group was subdivided into two sub-groups, corresponding to endpoints 1 and 4 days after the procedure.
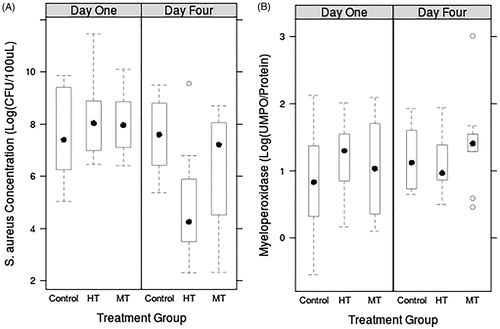
As shown in , a significant reduction in bacterial load was observed 4 days following HT treatment (p < 0.005). The figure also shows the concentration of Myeloperoxidase (MPO) as a marker of neutrophil recruitment. Compared to the concentration at day one, both HT (p < 0.003) and MT (p < 0.05) conditions showed a significant reduction in bacterial concentration at day four. No significant reduction in bacterial load was observed for any treatment group on day one. Neutrophil recruitment was comparable for all the groups. The macroscopic analysis showed small lesions in the muscle adjacent to the abscess. This study indicated that HT exposure of subcutaneous abscesses was capable of significantly reducing bacterial load four days post-treatment without significantly altering the activity of the innate immune system due to MRSA infection. The need to require such high thermal dose do produce a noticeable reduction of bacteria poses a problem since the required energy may not be compatible with surrounding structures preservation, especially increasing the risk of ischemia in a vasoconstriction environment.
Figure 1. MRSA (A) and MPO (B) concentrations in MRgHIFU-treated or control abscesses excised one to four days post-treatment.
Recent preliminary studies indicated that mild-hyperthermia has a very positive effect in combination with antibiotics [Citation62,Citation63]. An in vitro study [Citation62] showed that thermally activated liposomes loaded with ciprofloxacin produced a significant reduction (when compared to free ciprofloxacin) in the bacterial concentration of Pseudomonas aeruginosa when combined with mild-hyperthermia (41–42 C). Pseudomonas aeruginosa is associated with the formation of biofilms on metal implants. A recent in vitro study showed promising results for the combination of an elastin-like polypeptide liposome containing Ciprofloxacin or Cipro and heat (39 and 42 degrees Celsius), in the treatment of musculoskeletal bacterial wounds [Citation64]. Another in vitro study [Citation63] demonstrated a reduction of bacterial concentration of S. aureus when combining gentamicin with mild hyperthermia (up to 90 s at 44 °C). In [Citation65] the use of thermally activated liposomes was used in combination with alternating magnetic fields in a in vivo study to treat biofilm-infected implant, it was found a 3-log reduction in the bacterial load when AMF was applied. These results, if still early, are very promising and will motivate further studies investigating the feasibility of using thermal energy for the management of osteomyelitis in combination with thermally activated liposomes. Although AMF in combination with thermally activated liposomes has the potential to treat noninvasively biofilms on metallic implants, high intensity focused ultrasound has the ability of heating bacterial infections, noninvasively, precisely with real-time feedback, what represents a huge opportunity for research exploration
2. MRgFUS to deliver thermal energy for osteomyelitis treatment: Retrospective study
There exists an important clinical need to improve management of osteomyelitis, especially for diabetic patients who are more susceptible to failures, relapses and chronicity of multiple infections. Magnetic resonance-guided focused ultrasound (MRgFUS) can offer an attractive option for the management of patients with osteomyelitis by providing a non-surgical and potentially rapid-recovery treatment option. The ability of MRgFUS to treat structures located deeply inside the bone in a noninvasive manner, combined with the possibility to repeat treatments, if necessary, has the potential to reduce the need for surgical interventions. A minimally invasive technique will create a new opportunity to characterize the healing process under this new scenario. In addition, although Ultrasound imaging guided FUS (USgFUS) could provide real-time visualization of target motion, MRgFUS offers superior anatomic image quality and is capable of quantifying temperature change in real time [Citation66]. Furthermore, targeting bones using ultrasound imaging would require co-registration with another imaging modality since US clinical scanners accurately image the outer surface of bones but fail to show their inner structure [Citation67].
We evaluated the potential to target osteomyelitis using a minimally invasive approach as an adjuvant treatment, which can be combined with standard care, including administration of antibiotics, bed-side debridement and surgical interventions. Focused ultrasound (FUs) waves with controlled energy levels can be used to locally increase temperature, which can potentially increase the anti-bacterial effect of the immune response and antibiotics. Magnetic resonance imaging is the only imaging guidance modality approved for both targeting and monitoring the thermal energy delivery. Recent results from our group show that MRI can be used to monitor the delivery of mild-hyperthermia treatments in animals safely [Citation68,Citation69] and in patients [Citation70,Citation71], with the advantage of providing spatial control of the thermal energy delivery.
The hypothesis is that patients with osteomyelitis can be potentially treated with a clinically approved MRgFUS device. To test this hypothesis, the primary objective of the study was to establish the percentage of clinically relevant osteomyelitis sites that could be targeted using a clinically approved MRgFUS device. A retrospective imaging study was designed on patients who have been diagnosed with osteomyelitis and were non-contraindicated for MRI at our hospital (Thunder Bay Regional Health Sciences Center, Thunder Bay, ON, Canada). The study was approved by the hospital research ethics board. Inclusion and exclusion criteria are shown in . When the infection site was located less than 1 cm away from the skin, different patient positions were evaluated. If the ultrasound beam could not be placed in such a way to avoid the thin skin layer, the case was disqualified. This criterion has been previously used in targetability studies to avoid the risk of skin burn [Citation72]. Similar to other MRgFUS commercial protocols [Citation73], cases were disqualified if the clinician was incapable of bypassing essential structures such as irregularities in the skin, neuro-vascular bundles, major vessels, or metallic implants.
Table 1. Inclusion and exclusion criteria.
Seventy-eight (78) patients admitted between 2010 and 2016 who had a Most Responsible Diagnosis coded as osteomyelitis were pulled by the Health Records department and provided to the research team. All cases during this time period for which the diagnosis was confirmed by MR or CT images were included in this census study. Data collected included demographics, diagnosis information, medical history, treatment management, diagnostic MRI/CT images and MRI screening information to determine contraindications for MRI procedures
Images were loaded into a virtual MRgFUS Sonalleve platform (Profound Medical, Mississauga, ON, Canada) following a methodology developed in our institution. MRI/CT images were imported into the
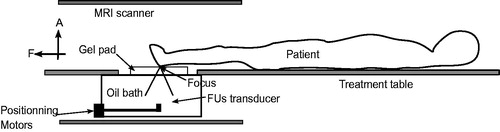
MRgFUS treatment planning platform to allow targeting analysis. shows a diagram of the MRgFUS device targeting the foot region with the patient in a feet first supine orientation. This position was used when patients had an infection in the calcaneus.
Figure 2. Patient positioning for treatment of osteomyelitis in the foot region using an MRgFUS system. “F” and “A” denote, respectively, foot and anterior directions.
shows the process followed by a case obtained from PACS into the Sonalleve virtual treatment planning. shows the process followed when multiple image series were used to do the virtual treatment planning.
Figure 3. Flowchart to import any diagnostic MRI/CT imaging set into treatment planning software for MRgFUS therapy.
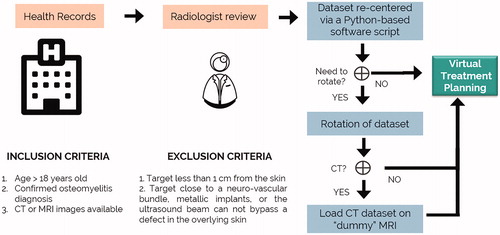
Figure 4. Flowchart to import multiple MRI/CT imaging series from the same patient into treatment planning software for MRgFUS therapy.
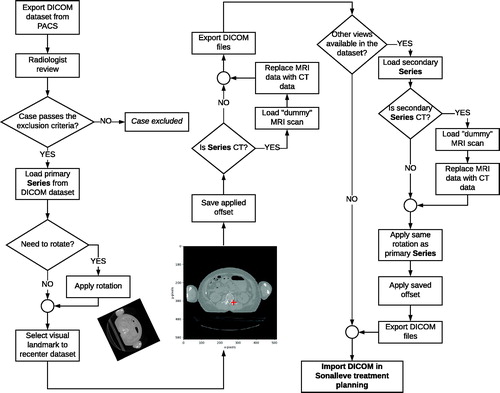
First, the radiologist reviewed the case to evaluate inclusion. Second, using a custom Python-based script, a visual landmark was placed over the infected bone in the primary series, this step generates an offset that will recenter the dataset. The Python-based script was also used to modify the image DICOM header file to incorporate data fields required by the Sonalleve software. This script is publicly available in a GitHub repository2 Third, if necessary, the primary series was rotated based on the optimal patient orientation determined by the radiologist. After, if the series is a CT, a “dummy” MRI scan was used to replace the MRI data with CT data, so that the treatment planning platform will open the case. Next, the offset generated after recentering the primary series was applied to the additional series. Finally, the set of series was imported into the Sonalleve platform to execute the virtual treatment planning.
This methodology was used for all available cases to plan the treatment of the infection site and thus facilitated establishing the percentage of clinically relevant osteomyelitis sites that could be targeted. From the original osteomyelitis DICOM images, we calculated the total lesion volume using a volumetric reconstruction from 2 D regions of interest (ROI) using a manual outline and Osirix [Citation74], the lesion volume was defined as the gross treatment volume shown defined as the bone inflammation visible on MRI or CT images. Only the infected bone was considered in the calculations since this study did not aim to target soft tissue abscess. Demographic data was organized with descriptive statistics. Multivariate analysis was performed to identify potential correlations between targetability and lesion volume, lesion location, age, sex, and diabetes as a comorbidity. Statistical analysis was performed for all the variables using the ANOVA test. From the initial sample size of 78 cases, three cases were eliminated due to poor image quality resulting in a total of 75 charts included in this study. This census size was deemed to be sufficient to produce significant results to demonstrate a successful outcome showing that a minimum of 30% of the cases are potential candidates for treatment with at least 50% of the target being reached. During the treatment planning treatment cells were placed with a maximum overlay of 30%.
3. Results
MRgFUS virtual treatment planning was performed on the images from 56 clinical cases out of the 75 that were deemed treatable (74.7%). For patients with diabetes as a co-morbidity (n = 34), 70.6% passed the exclusion criteria. shows the recruitment demographics. The cases covered included limb, pelvis and thorax locations. From the total number of cases excluded, 11/19 were due to proximity to skin and 8/19 because the target was close to neuro-vascular bundles, metallic implants, or the ultrasound beam could not bypass a defect in the overlying skin. For cases that passed the targetability criteria, an average among patients of 92.7 ± 5.2% of the infected bone could be reached utilizing treatment cells of 4, 8, 12, and 14 mm available at the Sonalleve system. The most common treatment cell used had 4 mm of diameter (59.1%), followed by 8 mm (33.5%), 12 mm (6.4%), and 14 mm (1%).
Table 2. Recruitment demographics.
It was found a statistical significance between age and cases that passed the targetability exclusion criteria (p = 0.002). This significance is explained by the age factor of osteomyelitis. Children are more likely to develop acute infections while adults tend to have a sub-acute and chronic infection that develops secondary to an open injury to bone and surrounding soft tissue making older patients less likely to benefit from our proposed treatment approach [Citation75]. No statistical significance was found between targetability and sex, lesion volume, location of the lesion, diabetes as a co-morbidity, or current osteomyelitis treatment. Further, it was found a trend between lesion total volume and targetability, as shown in . When the lesion was too small, placing cells in the infected region without compromising healthy bone was a challenge, causing a lower targetability. shows case 15, where the patient had an infection of 0.5454 ml in the right foot, and 0.50 ml was targetable (91.7%). As the lesion size increased, the targetability was higher, shows case 20, where a lesion of 13.5 ml in the knee had 99.61% of the volume covered by treatment cells. When the infected volume was more extensive than 100 ml, a decrease in targetability was observed. The rationale is that for large volumes, major structures start to get on the way of the ultrasound beam and full coverage is not always possible. shows case five, where a 123.6 ml infection located in the pelvis had a targetability of 86%.
Figure 5. Total lesion volume in ml versus average targetability. Although a trend is present, no statistical significance was found among those groups.
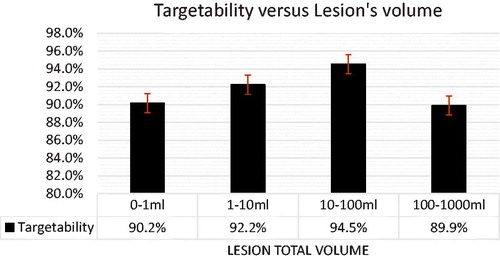
Figure 6. Case 15: MR image of a foot case with patient re-positioned to have the foot standing on the MRI table. The osteomyelitis site volume was 0.5454 ml, with a treated volume estimated at 0.50 ml by the planning software (91.7% coverage). The region in blue indicates the region attainable by the MRgFUS device. The focused ultrasound “acoustic beam” is delineated in yellow. The region in purple highlights the planning treatment volume.
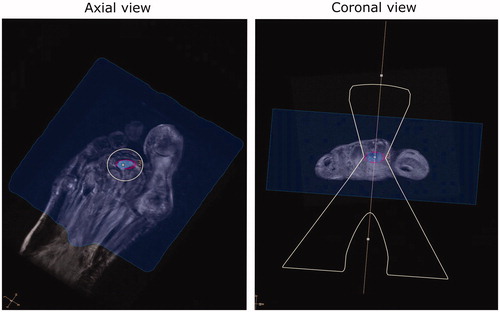
Figure 7. Case 20: MR image of a foot case with patient re-positioned from supine to prone. The osteomyelitis site volume was 13.4626 ml with a treated volume estimated at 13.41 ml by the planning software (99.61% coverage).
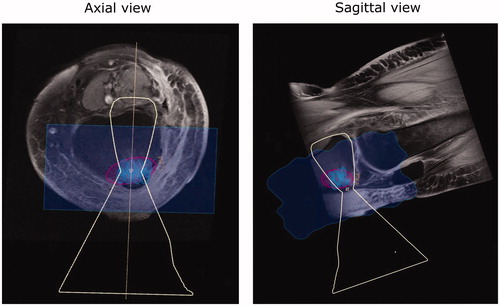
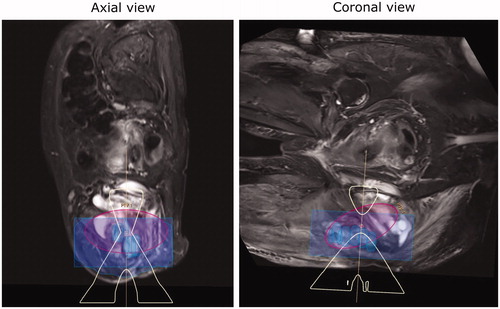
Figure 8. Case 5: MR image of a pelvis case with patient re-positioned from supine to decubitus. The osteomyelitis site volume was 123.58 m with a treated volume estimated at 106.25 ml by the planning software (86% coverage).
The average planning time for the virtual treatment was 49 min and 29 s, with a standard deviation of 33 min and 25 s due to high lesion size variability of the study, the values per lesion total volume are shown in .
Table 3. Virtual treatment planning time per lesion total volume groups.
During the virtual treatment planning, 63% of the cases had treatment cells containing a small part of non-lesion tissue. All the cases analyzed had some small portion of lesion tissue left out of the treatment planning, so we could avoid placing treatment cells that had more than ≈20% of non-lesion tissue. The study methodology provides a reduction of translation time by providing timely information at the onset of the design phase regarding the feasibility and the imaging characteristics of the targets for MRgFUS. This helps guide the design process, and it can also help re-purpose current devices by creating minimal modifications such that patients can benefit from earlier treatment.
4. Discussion: Implications of the study
Novel applications for MRgFUS usually result from discussions between clinicians and scientists regarding possible targets. Devices are then modified or designed for the specific target application, and extensive work on technological, pre-clinical and translational investigations begin to result in a device that is ready to be tested. Although productive and practical, this method can increase the time it takes to clinical application as multiple technological barriers can be encountered during clinical trials. Many obstacles can be overcome by first validating the targets on real images and establishing feasibility.
The planning tool provided the possibility of rotation of the image to evaluate a different angle to reach a target. This is a central feature that allows studying repositioning of the patient to reach a target. Being able to evaluate possible repositioning for targets before treatment from standard images is a strong feature of the tool, that also provides patient flow information for some targets to evaluate the feasibility of treatment. However, to evaluate the clinical feasibility of this tool in full is necessary to have a full overview of the patient’s case, since some positions might only be achievable if combined with clinical management of the pain.
A limitation of this study is that the evaluation of the reachable targets was done for a specific MRgFUS device, the Sonalleve platform. However, the tool that was designed for image processing is not proprietary for this system, and it is an open-source tool. It is then foreseeable to modify this tool to fit other MRgFUS systems. As per treatment time, cases with a lesion smaller than 1 ml would require <15 min for the whole procedure. Therefore, it would be up to the clinician to evaluate if subjecting the patient to a procedure that would require a higher preparation time than the therapy itself was ideal for that candidate. For cases where patients had an infection in a long bone, the estimated treatment time was as high as three hours; for those cases, it is possible that treatment would have to be performed during multiple sessions. A radiological study of the feasible targets is required to obtain information about the feasibility of thermometry on osteomyelitis targets. Thermometry is a reliable approved tool for monitoring temperature changes in water-based tissues [Citation76]. There are however some challenges when monitoring tissues with significant fat content, and different approaches have been proposed to provide thermal information in such structures as well as structures near or at the bone [Citation77–79]. In [Citation80], the temperature changes in the soft tissue surrounding the bone during the treatment of painful bone metastases was used to obtain an estimation of the bone temperature.
Since the feasibility of targeting clinically relevant osteomyelitis was demonstrated, future studies are required to evaluate thermometry imaging of targets to validate monitoring conditions for the control of the therapy. Although the previous study conducted by our group in the treatment of subcutaneous abscesses showed a reduction in bacterial load, in vivo studies with a more advanced model of osteomyelitis is necessary to confirm the loss of viability of bacterial cells in the bone. These results will be required to guide the clinical translation of the treatment.
5. Conclusions
The retrospective study presented here is the first step to demonstrate the feasibility of utilizing MRgFUS for the thermal treatment of osteomyelitis. A virtual treatment planning was performed in 74.7% of patients diagnosed with osteomyelitis at the Thunder Bay Regional Health Sciences Center that were able to take an MRI and had either CT or MRI images of the lesion site. For cases that passed the targetability criteria, i.e. the ultrasound beam could reach the lesion safely, an average among patients of 92.7 ± 5.15% of the infected bone could be reached using treatment cells available at the Sonalleve system. The background information on current practices for management of osteomyelitis, with an emphasis on the comorbidity with diabetes, and potential opportunities for therapies based on thermal effects was reviewed to support the rationale of the use of MRgFUS for this indication.
Acknowledgements
Authors acknowledge support by the Clinical Innovation grant of the Northern Ontario Academic Medicine Association. The authors would also like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant for their financial support.
Disclosure statement
The authors report no declaration of interest.
Notes
1 It is worth mentioning that bedside debridement is usually not considered a surgical intervention
2 https://github.com/ProteusMRIgHIFU/RecenterDICOM
References
- Lew D, Waldvogel F. Osteomyelitis. Lancet. 2004;364(9431):369–379.
- Lipsky B, Berendt A, Deery H, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39(7):885–910.
- Lavery L, Armstrong D, Murdoch D, et al. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007;44(4):562–565.
- Public Health Agency of Canada. Report from the National Diabetes Surveillance System: diabetes in Canada. Ottawa (ON): The Agency; 2008.
- Treiman G, Oderich G, Ashrafi A, et al. Management of ischemic heel ulceration and gangrene: an evaluation of factors associated with successful healing. J Vasc Surg. 2000;31(6):1110–1118.
- Termaat M, Raijmakers P, Scholten H, et al. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(11):2464–2471.
- Berendt A, Peters E, Bakker K, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008;24(S1):S145–S161.
- Powlson A, Coll A. The treatment of diabetic foot infections. J Antimicrob Chemoth. 2010;65(Supplement 3):iii3–iii9.
- Valabhji J, Oliver N, Samarasinghe D, et al. Conservative management of diabetic forefoot ulceration complicated by underlying osteomyelitis: the benefits of magnetic resonance imaging. Diabet Med. 2009;26(11):1127–1134.
- World Health Organization. Global report on diabetes. World Health Organization. 2016.
- Stoeckle M, Kaech C, Trampuz A, et al. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med wkly. 2008;138(35–36):512.
- Bhavan KP, Marschall J, Olsen MA, et al. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis. 2010;10(1):158.
- Jeffcoate W, Lipsky B. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39(Supplement_2):S115–S122.
- Armstrong D, Lavery L, Harkless L. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859.
- DeLeo F, Otto M, Kreiswirth B, et al. Community-associated meticillin-resistant¡ i¿ Staphylococcus aureus¡/i¿. The Lancet. 2010;375(9725):1557–1568.
- Peyrani P, Allen M, Seligson D, et al. Clinical outcomes of osteomyelitis patients infected with methicillin-resistant Staphylococcus aureus USA-300 strains. Am J Orthopedics. 2012;41(3):117–122.
- Park KH, Chong YP, Kim SH, et al. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J Infect. 2013;67(6):556–564.
- Gupta A, Kowalski TJ, Osmon DR, et al. Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis. 2014;1(3):ofu107
- Graham SM, Fishlock A, Millner P, et al. The management gram-negative bacterial haematogenous vertebral osteomyelitis: a case series of diagnosis, treatment and therapeutic outcomes. Eur Spine J. 2013;22(8):1845–1853.
- American Society of Plastic Surgeons. Evidence-based clinical practice guideline: chronic wounds of the lower extremity. 2007. p. 1–21.
- Maffulli N, Papalia R, Zampogna B, et al. The management of osteomyelitis in the adult. the. Surgeon. 2016;14(6):345–360.
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362(11):1022–1029.
- Lipsky BA, Berendt AR, Cornia PB, et al. Infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–e173.
- Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg. 2009;23(02):080–089.
- Wang A, Weinstein D, Greenfield L, et al. MRI and diabetic foot infections. Magn Reson Imaging. 1990;8(6):805–809.
- Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84(9):1027–1033.
- Enderle M, Coerper S, Schweizer H, et al. Correlation of imaging techniques to histopathology in patients with diabetic foot syndrome and clinical suspicion of chronic osteomyelitis. The role of high-resolution ultrasound. Diabetes Care. 1999;22(2):294–299.
- Arag'on-S'anchez J. Evidences and controversies about recurrence of diabetic foot osteomyelitis a personal view and an illustrated guide for understanding. Int J Low Extr Wound. 2012;11(2):88–106.
- Arag'on-S'anchez J, L'Azaro-Mart'ınez J, Hernandez-Herrero C, et al. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabetic Medicine. 2012;29(6):813–818.
- Arag'on-S'anchez J, L'Azaro-Mart'ınez J, Quintana-Marrero Y, et al. Are diabetic foot ulcers complicated by MRSA osteomyelitis associated with worse prognosis? Outcomes of a surgical series. Diabetic Medicine. 2009;26(5):552–555.
- de Graeff JJ, Pereira NRP, Pereira P, et al. Prognostic factors for failure of antibiotic treatment in patients with osteomyelitis of the Spine. Spine. 2017;42(17):1339–1346.
- Walenkamp GH, Kleijn LL, de Leeuw M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop Scand. 1998;69(5):518–522.
- Patzakis MJ, Mazur K, Wilkins J, et al. Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin Ortho Res. 1993;295:112–118.
- Calhoun JH, Henry SL, Anger DM, et al. The treatment of infected nonunions with gentamicin-polymethylmethacrylate antibiotic beads. Clin Ortho Res. 1993;295:23–27.
- Luo S, Jiang T, Yang Y, et al. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskel Dis. 2016;17(1):502.
- Hake ME, Young H, Hak DJ, et al. Local antibiotic therapy strategies in orthopedic trauma: practical tips and tricks and review of the literature. Injury. 2015;46(8):1447–1456.
- Blaha JD, Calhoun JH, Nelson CL, et al. Comparison of the clinical efficacy and tolerance of gentamicin PMMA beads on surgical wire versus combined and systemic therapy for osteomyelitis. Clin Ortho Res. 1993;295:8–12.
- Hum J, Boccaccini AR. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: a review. J Mater Sci Mater Med. 2012;23(10):2317–2333.
- Rahaman MN, Bal BS, Huang W. Review: emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater Sci Eng C Mater Biol Appl. 2014;41:224–231.
- Drago L, Romano D, De Vecchi E, et al. Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: an in vitro and prospective clinical study. BMC Infect Dis. 2013;13(1):584.
- Romano C, Logoluso N, Meani E, et al. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis. Bone Joint J. 2014;96(6):845–850.
- Arief Atan A, Bajuri MY, Ali AM, et al. The effects of Gentamicin-Impregnated collagen sponge versus Gentamicin-Impregnated polymethylmethacrylate beads in patients with osteomyelitis. Asian J Pharm Clin Res. 2018;11(12):241–246.
- van Vugt T, Arts J, Geurts J. S53P4 Bioactive glass is clinically effective in one-stage surgery in treatment of chronic osteomyelitis. Orthop Proce. 2017;B-99(SUPP 2):99–99.
- Lindfors N, Geurts J, Drago L, et al. Antibacterial Bioactive Glass, S53P4, for Chronic Bone Infections – A Multinational Study. Cham (Switzerland): Springer International Publishing; 2017: 81–92. 2016156].
- Giglio RD, Stefani I, Mondello T, et al. BIOACTIVE GLASS S53P4: a new opportunity for the treatment in the diabetic foot osteomyelitis. Eur J Intern Med. 2018;54:e15–e16.
- Privalov VA, Krochek IV, Lappa AV. Diode laser osteoperforation and its application to osteomyelitis treatment. In European Conference on Biomedical Optics, International Society for Optics and Photonics 2001:180–185.
- Doherty CB, Doherty SD, Rosen T. Thermotherapy in dermatologic infections. J Am Acad Dermatol. 2010;62(6):909–927.
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334(19):1209–1216.
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–134.
- Sheffield CW, Sessler DI, Hopf HW, et al. Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension. Wound Repair Regen. 1996;4(3):339–345.
- Kumar S, Wong PF, Melling AC, et al. Effects of perioperative hypothermia and warming in surgical practice. Int Wound J. 2005;2(3):193–204.
- Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–880.
- Rabkin JM, Hunt TK. Local heat increases blood flow and oxygen tension in wounds. Arch Surg. 1987;122(2):221–225.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997–1004.
- Ikeda T, Tayefeh F, Sessler DI, et al. Local radiant heating increases subcutaneous oxygen tension. Am J Surg. 1998;175(1):33–37. :
- Frohlich D, Wittmann S, Rothe G, et al. Mild hyperthermi downregulates receptor-dependent neutrophil function. Anesth Analg. 2004;99:284.
- Wardlow R, Sahoo K, Dugat D, et al. High Intensity Focused Ultrasound (HIFU) heating improves perfusion and antimicrobial efficacy in mouse staphylococcus abscess. Ultrasound Med Biol. 2018;44(4):909–914.
- Fang CH, Tsai PI, Huang SW, et al. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Inf D. 2017;17(1):516.
- Chopra R, Shaikh S, Chatzinoff Y, et al. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci Rep. 2017;7(1):7520.
- Rieck B, Bates D, Zhang K, et al. Focused ultrasound treatment of abscesses induced by methicillin-resistant Staphylococcus aureus: Feasibility study in a mouse model. Med Phys. 2014;41(6Part1):063301.
- Simor A, Gilbert N, Gravel D, et al. Methicillin-Resistant staphylococcus aureus colonization or infection in Canada: national surveillance and changing epidemiology, 1995–2007. Infect Control Hosp Epidemiol. 2010;31(4):348–356.
- Munaweera I, Shaikh S, Chatzinoff Y, et al. Temperature-sensitive liposomal ciprofloxacin for the treatment of biofilm on prosthetic joint implants using alternating magnetic fields. In 34th Annual Society for Thermal Medicine Meeting, Society for Thermal Medicine. 2017;42.
- Vogel K, Pullagura A, Levi-Polyachenko N. Laser induced precision heating for improved bacterial destruction with gentamicin. In 34th Annual Society for Thermal Medicine Meeting, Society for Thermal Medicine. 2017;48.
- Nigatu AS, Ashar H, Sethuraman SN, et al. Elastin-like polypeptide incorporated thermally sensitive liposome improve antibiotic therapy against musculoskeletal bacterial pathogens. Int J Hyperthermia. 2018;34(2):201–208.
- Munaweera I, Shaikh S, Maples D, et al. Temperature-sensitive liposomal cipro oxacin for the treatment of bio lm on infected metal implants using alternating magnetic fields. Int J Hyperthermia. 2018;34(2):189–200.
- Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Inter Rad. 2014;37(6):1611–1617.
- Renaud G, Kruizinga P, Cassereau D, et al. In vivo ultrasound imaging of the bone cortex. Phys Med Biol. 2018;63(12):125010.
- Pichardo S, K¨Ohler M, Lee J, et al. In vivo optimisation study for multi-baseline MR-based thermometry in the context of hyperthermia using MR-guided high intensity focused ultrasound for head and neck applications. Int J Hyperther. 2014;30(8):579–592.
- Chu W, Staruch RM, Pichardo S, et al. Magnetic resonance guided high-intensity focused ultrasound hyperthermia for recurrent rectal cancer: MR thermometry evaluation and preclinical validation. Int J Radiat Oncol. 2016;95(4):1259–1267.
- Chu W, Staruch R, Pichardo S, et al. MR-HIFU mild hyperthermia for locally recurrent rectal cancer: Temperature mapping and heating quality in first patient. In 12th International Congress of Hyperthermic Oncology, Society for Thermal Medicine 2016:53.
- Chu W, Pichardo S, Huang Y, et al. Phase I trial of MR-HIFU mild hyperthermia with radiation and chemotherapy for recurrent rectal cancer: second patient. In 34th Annual Society for Thermal Medicine Meeting, Society for Thermal Medicine 2017:53.
- Shim J, Staruch RM, Koral K, et al. Pediatric sarcomas are targetable by MR-Guided High Intensity Focused Ultrasound (MR-HIFU): anatomical distribution and radiological characteristics. Pediatr Blood Cancer. 2016;63(10):1753–1760. [https://onlinelibrary.wiley.com/doi/abs/10.1002/pbc.26079].
- Food U, Administration D: PMA P110039: FDA Summary of Safety and Effectiveness Data 2012.
- Rosset A, Spadola L, Ratib O. OsiriX: An Open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–216.
- Carek P, Dickerson L, Sack J. Diagnosis and management of osteomyelitis. Am Fam Physician. 2001;63(12):2413–2420. [http://europepmc.org/abstract/MED/11430456].
- Hynynen K. MRIgHIFU: A tool for image-guided therapeutics. J Magn Reson Imaging. 2011;34(3):482–493.
- Waspe AC, Mougenot C, Pichardo S, et al. Simultaneous PRF and T1-mapping based MR thermometry for monitoring high-intensity focused ultrasound ablation of primary bone tumors. In Accepted to be presented in the 21st Meeting of the annual meeting of International Society for Magnetic Resonance in Medicine 2013.
- Hey S, de Smet M, Stehning C, et al. Simultaneous T1 measurements and proton resonance frequency shift based thermometry using variable flip angles. Magn Reson Med. 2012;67(2):457–463.
- Todd N, Diakite M, Payne A, et al. Hybrid proton resonance frequency/T1 technique for simultaneous temperature monitoring in adipose and aqueous tissues. Magn Reson Med. 2013;69:62–70.
- Huisman M, Lam MK, Bartels LW, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultras. 2014;2:1–10.
