Abstract
Purpose: To evaluate the safety, functional and oncological outcomes associated with percutaneous cryoablation of stage T1b renal cell carcinoma (RCC).
Materials and methods: Institutional database was reviewed to identify patients treated by percutaneous CT-guidance cryoablation between 2013 and 2018 for biopsy-proven RCC tumors measuring 4.1–7.0 cm. The main outcome parameters analyzed were primary and secondary technique efficacy, progression-free survival (PFS), cancer-specific survival (CSS), loss of estimated glomerular filtration rate (eGFR) and complications. PFS and CSS were estimated by the Kaplan–Meier method. Complications were graded by the Clavien–Dindo system.
Results: Twenty-three consecutive patients were included (mean tumor diameter: 45.6 ± 6.2 mm; mean RENAL score: 8.1 ± 1.8). The technical success rate was 95.7%. Primary and secondary technique efficacy rates were 86.3 and 100%, respectively. Three patients found to have incomplete ablations at 3 months were successfully treated by repeat cryoablation. Median duration follow-up was 11 months (range: 3-33). Imaging showed PFS to be 85.7% at 6 months, 66.7% at 12 months and 66.7% at 24 months. One patient with a local recurrence at 12 months was treated by radical nephrectomy. One patient died from progression of disease within 12 months. One patient reported a complication grade ≥ II (4.3%). Mean eGFR loss was 4.4 ± 8.5 ml/min/1.73m2, which was significantly higher among those treated for central tumors (p < .05).
Conclusion: Cryoablation for stage T1b renal tumors is technically feasible, with favorable oncological and perioperative outcomes. Longer-term studies are needed to verify our findings.
Introduction
Partial nephrectomy (PN) has become the standard treatment for T1 tumors (≤7 cm), because of its favorable perioperative and renal outcomes compared with radical nephrectomy [Citation1,Citation2]. Percutaneous thermoablation (TA) (radiofrequency and cryoablation) have emerged as alternative therapies for T1a tumors in patients with high risks of morbidity. The recent consensus of the European Association of Urology (EAU) [Citation3], based on few early studies, including radiofrequency (RF) and cryoablation, is that percutaneous TA is an appropriate alternative therapy for tumors smaller than 3 cm (recommendation grade: C). According the EAU, mass biopsy should be performed before treatment and counseling about TA should include information about the likelihood of tumor persistence or recurrence after primary TA. Despite a higher local recurrence rate, intermediate metastasis-free and cancer-specific survival rates are comparable between the two approaches. RF carries a high risk of repeat ablation for tumors larger than 3 cm [Citation4,Citation5]. Cryoablation is a useful option for large tumors because of the ability to visualize the ablation zone during the procedure, and possibility of complementary treatment [Citation4,Citation6]. Aoun et al. [Citation7] demonstrated that tumor size (>3 cm) is not a risk factor for local tumor recurrence after percutaneous cryoablation. A recent study showed similar recurrence-free survival outcomes for PN and cryoablation for sporadic cT1b renal tumors (4–7 cm) [Citation8]. However, few studies of cryoablation for cT1b tumors are available [Citation9–11]. Atwell et al. evaluated percutaneous cryoablation of 46 T1b renal tumors, with 15.2% of major complications, and 96.4% progression-free survival at 1-, 3- and 5-year follow-up. Hebbadj et al. [Citation9] evaluated percutaneous cryoablation of 27 T1b renal tumors, with 95.7% of cancer specific survival at 12–24- and 36- month follow-up. A recent study of Gunn et al. [Citation10] evaluated 37 renal cell carcinoma (RCC), with 100, 86.1 and 62.6% of recurrence-free survival at 1–2- and 3-year follow-up. The present study evaluated the oncologic efficacy, safety, and functional outcome of percutaneous CT-guided cryoablation for renal cT1b tumors.
Materials and methods
Approval for this retrospective review study was obtained from the institutional review board.
Patient identification
Ninety-six patients with 102 biopsy-proven RCC that were treated with percutaneous cryoablation from January 2013 to August 2018 have been retrospectively reviewed. RCC smaller than 4.1 cm or larger than 7.0 cm were excluded. Patients were considered for cryoablation if they met at least one of the following criteria: unsuitability for surgery due to refusal of consent or comorbidities with surgical risks, poor renal function. Finally, 23 consecutive patients (6 women, 17 men, mean age: 74.9 ± 10.2 years) with 23 T1b renal RCC were treated with percutaneous CT-guided cryoablation, between July 2013 and August 2018, were included.
Each cryoablation was preceded by a biopsy performed during a separate dedicated visit. Two patients were metastatic at the time of diagnosis: one patient with an unusual single vertebral metastasis and the other with a single pulmonary metastatic nodule. All patients were previously referred to urological and interventional radiology consultations.
Procedures and follow-up
All the cryoablation procedures were performed by three radiologists, with 4 and 10 years of experience in ablation, under CT guidance. Moderate sedation or general anesthesia was induced according to the provisional duration of the procedure, the patient’s age, and any contraindications to general anesthesia. A CT scan, preceded in 17/23 (73.9%) cases by an injection of iodinated contrast medium, was performed immediately before the procedure to identify the tumor’s margins and any structures at risk. For this purpose, hydro-dissection was performed for 8/23 (34.8%) patients, by infusing 2% iodinated sterile saline water with a 22-gauge needle between the tumor and the structure at risk. Number and types of cryoprobes was selected in order to get an optimal spacing of 15 mm between cryoprobes. In cases of moderate sedation, skin and underlying tissue were anesthetized using 1% lidocaine. The cryoablation system (Galil Medical, Israel) was used in all cases. IceSphere probes (1.5 mm, Galil Medical Inc.) were used for 1/23patient. IceRod probes (1.5 mm, Galil Medical Inc.) were used for 22/23 patients. No angio-embolization or ureteral stent placement were used before the procedure.
For all patients, a conventional double 10-min freezing cycle, separated by a passive 10-min and an active 2-min thawing session, was performed. During the procedure, non-contrast CT imaging was performed each 3–5 min to monitor ice-ball growth, and to evaluate tumor margins and vulnerable structures at risk. If the ice ball did not cover the tumor after the first freezing cycle, the probe was repositioned. If vulnerable structures were identified near the ice ball, additional hydro-dissection was performed. A non-contrast CT was performed just after removing the probes, to identify any complications. All the patients were examined and left the hospital the day after the procedure if they had no complications. Median duration of hospitalization was 1 (1–6) day.
Patients underwent follow-up examinations at 1, 3, 6 and 12 months after the procedure, which included contrast-enhanced imaging (MRI or CT if MRI was contraindicated) and an interventional radiology consultation. After 12 months, follow-up visits were conducted by the urologist.
Data collection
For each patient, demographic data (sex, age, number of kidneys, performance status, Karnofsky index, American Society of Anesthesiologists [ASA] score, Charlson score), renal function (estimated Glomerular Filtration Rate [eGFR], estimated by Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]), tumor features (size, histology, Furhman grade, localization), procedure-related variables (type of anesthesia, dissection maneuvers, duration of procedure, number of cryoprobes and ablative cycles, technical success, complications, length of hospitalization) and follow-up data (technical efficacy, progression-free survival [PFS], cancer-specific survival [CSS], renal function) were collected. RENAL scores were calculated [Citation12]. Demographic and procedural data are shown in .
Table 1. Summary of features for patients and tumors treated with cryoablation.
Analysis
Technical success, primary technique efficacy and secondary technique efficacy were reported according to the SIR reporting standards. Technical success was defined as leaving tumor margins >5mm from at least a double-freezing cycle. Primary technique efficacy was defined as no contrast enhancement or tumor enlargement observed in imaging at the 3-month follow-up examination. If a residual tumor was observed at the 3-month follow-up, a repeat cryoablation was performed. Secondary technique efficacy was defined as a successful repeat cryoablation [Citation13]. LTP was defined as showing contrast enhancement at the ablation zone edge in contrast-enhanced MR/CT imaging ≥3 months. Major complications were defined as those classified grade ≥ II in the Clavien–Dindo system [Citation14].
Continuous features were summarized as means, standard deviations and ranges. Patients’ clinical records were used to calculate CSS, and the Kaplan–Meier method to evaluate LTP and CSS, using PRISM 6 software package. The duration of CSS was defined from the date of cryoablation to the date of death or the date the patient was last known to be alive. p < .05 was considered significant.
Results
Mean follow-up was 13.9 ± 9.1 months and median follow-up was 11 months. Technical success was achieved for 22/23 patients (). A 55-year-old patient with a left-central, 46-mm, clear-cell carcinoma (CCC), which was metastatic at diagnosis, had a suboptimal cryoablation due to an insufficient number and misplacement of cryoprobes (). Primary and secondary technique effectiveness were achieved for 19/22 (86.3%) and 22/22 (100%) of the patients, respectively (). Rates for PFS were 6 months: 85.7%, 12 months: 66.7% and 24 months: 66.7% (). Rates for PFS including secondary technique efficacy were 6 months: 100%, 12 months: 81.8% and 24 months: 81.8% (). Two patients had local tumoral recurrences at 12 months, including a 77-year old man who had local and metastatic progression, and died from his cancer 8 months later, and an 84-year-old man whose local recurrence was treated by radical nephrectomy, and has shown no sign of local recurrence at 20 months later. Among all variables, only the presence of metastases at the time of cryoablation was associated with local tumoral progression at any time (p < .05; ). Rates for CSS were 6 months: 100%, 12 months: 100% and 24 months: 85.7% (Figure4(c)). One patient had died at 24 months after treatment, from metastatic urothelial cancer.
Figure 1. (A) Axial contrast-enhanced computed tomography (CT) images. Exophytic T1b renal tumor on middle third of the right native kidney (blue arrows). (B) Coronal computed tomography images during the ablation, using 5 cryoprobes, after hydro-displacement (white star). (C) Axial T2 at 1-month follow-up shows the ablation zone (white arrows). (D) Axial T1FS after injection shows no evidence of tumoral residue.
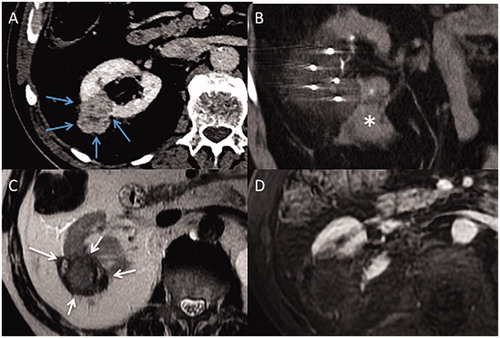
Figure 2. (A) Axial contrast-enhanced computed tomography (CT) image of a T1b renal tumor (maximum diameter: 45 cm) of lower pole of the right native kidney (white arrows) (B) Coronal contrast-enhanced computed image shows close contact between the tumor and renal sinus (grey arrows). (C) Incremental axial CT image during the procedure shows hydro-dissection to space out bowel and kidney (star). (D) Incremental coronal CT image shows a residual tumor at sinus contact (blue arrows), with margins <5 mm, and a 15 mm-gap between each four cryoprobes (double-headed arrows).
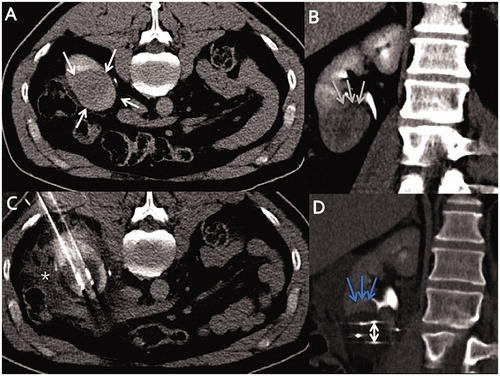
Figure 3. Axial contrast-enhanced computed tomography (CT) images. (A) T1b renal tumor (maximum diameter: 65 cm) on upper pole of the left atrophic native kidney (blue arrows), using seven cryoprobes under CT guidance. Incremental axial (B) and coronal (C) CT images during the procedure show 15 mm spacing between each probe (double-headed arrows). (D) Arterial axial contrast-enhanced CT image shows a LTP at 3 months (white arrows). (E) Axial contrast-enhanced CT shows no macroscopic residue at 3 months after a repeat cryoablation (black arrows).
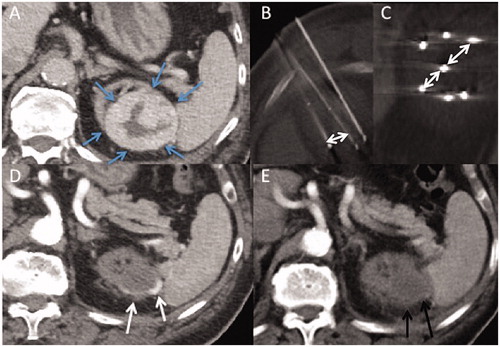
Figure 4. Kaplan–Meier survival curves for patients with stage T1b renal cell carcinoma treated with cryoablation. (A) Progression-free survival excluding secondary technique efficacy (B) Progression free-survival including secondary technique efficacy (C) Cancer-specific survival.
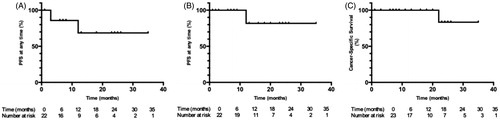
Table 2. Tested variables to predict LTP at any time.
Allowing hematuria <24 h and two asymptomatic hematomas, four patients (17.4%) suffered minor complications (Clavien–Dindo grade I), including three with hematuria without anemia for 2 weeks, and one who presented with hypothermia of 35° 1 h after the procedure. One patient (4.3%) suffered a complication grade II: a morphine overdose managed by naloxone in the recovery room.
Mean pre-operative eGFR was 68.1 ± 28.8 ml/min/1.72m2 and mean post-operative eGFR during the follow-up period was 63.7 ± 28.4 ml/min/1.72m2 (Δ − 4.4 ml/min/1.72m2; p > .05), i.e., 93.5% preserved renal function. Five patients developed a stage III chronic kidney disease. No patients required dialysis during follow-up. Notably, patients with central T1b RCC were more likely to lose renal function after cryoablation (Δ −14.6 ml/min/1.72m2, p < .05). Among the seven patients with single kidney, mean pre-operative eGFR was 53.7 ± 13.1 ml/min/1.72m2 and mean post-operative eGFR was 51.7 ± 15.6 ml/min/1.72m2 (Δ − 2.0 ml/min/1.72m2; p > .05).
Discussion
The EAU does not recommend cryoablation and RF for T1b renal RCC, owing to the high risk of recurrence after treatment. In our study, technical efficacy had not been achieved for three patients by their 3-month follow-up visits. They therefore each received a repeat cryoablation, which was without complication, with a 100% secondary technique efficacy without LTP afterward. Our series showed a high rate of PFS at 6 months (85.7%). The PFS rates declined at 12 months (66.7%) but only nine patients were followed up at this point. These PFS rates are comparable with those of Hebbadj et al. who evaluated 27 T1b renal RCC treated by percutaneous image-guided cryoablation, with PFS rates of 82.6%, 60.3% and 60.3% at 6-, 12- and 24-month follow-up respectively [Citation9]. The CSS rates were 100% at 6- and 12- month follow-up visits. Only one patient died from his cancer over the follow-up period. Few studies have compared surgery and TA for T1b RCC. Caputo et al. [Citation15] compared 161 surgeries and 31 thermoablations for T1b patients, among whom five patients experienced local recurrences after cryoablation (23%) and four after PN (3.2%). After 1:1 matching, cryoablation was inferior to PN for recurrence-free survival, but did not significantly differ in CSS and overall survival. Thompson et al. compared oncologic outcomes of 326 PN and 53 cryoablation procedures for T1b RCC [Citation8], and found local recurrence-free (PN: 96%, cryoablation: 97%) and distant metastasis-free survival (PN: 96%, cryoablation: 92%) did not significantly differ. However, these discordant studies, no prospective randomized studies with a large population have been performed, and oncological outcomes of these techniques have yet to be compared [Citation16].
Two patients had a single metastasis location at diagnosis, and have been included in the analysis. Indeed, these metastatic sites were potentially accessible to a local tumoral treatment: stereotactic radiotherapy or thermoablation. Moreover, patients with oligometastatic renal RCC can achieve favorable clinical outcome, which justifies a target therapy on primitive renal tumor [Citation17,Citation18]. The association between preoperative metastasis and poor PFS suggests aggressive RCC.
The present study confirms the safety profile of cryoablation for renal T1b RCC. The low major complication rate (4.3%) is in line with Atwell et al. (15.2%) [Citation19] and Hebbadj et al. (11.1%) [Citation9] and explains the short in-hospital length (median: 1 day). The low hemorrhagic complication rate in our study (two asymptomatic hematomas) can partly be explained by clinicians’ choice to avoid treatment options that were likely to increase the risk of bleeding (such as coaxial needle use and immediate pre-ablation biopsy). Pre-operative angio-embolization [Citation20] and pathway embolization [Citation9], using hemostatic agents during needle removal, are often performed, without robust supporting evidence. Tumor size and aggressiveness, patient age, and number of cryoablation probes (but not preoperative embolization) have been identified as risk factors for bleeding [Citation21]. The absence of pre- and peri-procedure embolization in our series tends to reinforce these data. The absence of damage to the collecting system, including the treatment of central RCC, correlates with previous data [Citation22,Citation23]. Prophylactic use of ureteral stents does not seem justified in this setting. Surgery has a non-negligible hemorrhagic complication rate. A review of 886 robot-assisted PNs for 3-cm (mean size) RCC revealed 11% had major complications and 37 (4.2%) had bleeding that required transfusion [Citation24].
Loss of renal function (eGFR) is associated with cardiovascular events, hospitalization and risk of death [Citation25]. The present study is the third to evaluate functional outcome (Δ − 4.4 ml/min/1.73m2) after cryoablation for T1b RCC. Hasegawa et al. evaluated 23 patients treated by cryoablation for T1b RCC and found a mean eGFR loss of 7.6 ml/min/1.73m2 at 1 month after treatment [Citation4]. Gunn et al. [Citation10] evaluated 37 patients treated by cryoablation for T1b RCC and found no significant change in renal function after cryoablation (p > .05). Patients with central RCC had poorer functional outcomes after cryoablation (mean Δ −14.6 ml/min/1.73m2), possibly caused by functional impairment of healthy renal parenchyma and sinus within the ablation area. Although functional loss after cryoablation seems less than after surgical treatments [Citation26], a review of 17 studies found mean losses after cryoablation (Δ –4.9 ml/min/1.73m2) and PN (Δ –6.2 ml/min/1.73m2) did not significantly differ [Citation27].
Although technical efficacy was based on a 3-months follow-up imaging, a 1-month imaging was systematically performed. First, it allowed to ensure the absence of delayed complication described after cryoablation [Citation10] (abscess, pseudo-aneurysm, ureteral stenosis, fistula). Then, it allows early detection of residual unablated tumor, and, if there was any, to anticipate repeat cryoablation. MRI was performed as standard imaging, even if there is no recommendation on imaging modalities during the follow-up after renal cryoablations. Nevertheless, MRI seems to provide a better resolution in contrast between the ablation zone and the local tumoral recurrence, and gadolinium has lower nephrotoxic effects than iodinated contrast [Citation28].
Our study has some limitations owing to its retrospective nature with possible selection bias, the small patient cohort, and the limited follow-up time. A low number of patients have follow-up beyond 12 months, which limits the assessment of oncologic efficacy. Additionally, postoperative eGFR was measured at different points (1 or 3 months after the procedure), which makes the data heterogeneous.
In conclusion, this study confirms previous series showing the feasibility of percutaneous cryoablation for treating patients with T1b renal RCC unsuitable for surgery. This treatment requires close multidisciplinary collaboration before, during and after the procedure. Long-term studies with a larger population are needed to confirm these findings.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study. This study has obtained IRB approval from the Institute Paoli Calmettes and the need for informed consent was waived.
Disclosure of interest
No potential conflict of interest was reported by the authors.
References
- Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115(7):1465–1471.
- Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors – is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55–62.
- Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198(3):520–529.
- Hasegawa T, Yamanaka T, Gobara H, et al. Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: a multi-center study. Jpn J Radiol. 2018;36(9):551–558.
- Zagoria RJ, Traver MA, Werle DM, et al. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol. 2007;189(2):429–436.
- Okhunov Z, Chamberlin J, Moreira DM, et al. Salvage percutaneous cryoablation for locally recurrent renal-cell carcinoma after primary cryoablation. J Endourol. 2016;30(6):632–637.
- Aoun HD, Littrup PJ, Jaber M, et al. Percutaneous cryoablation of renal tumors: is it time for a new paradigm shift? J Vasc Interv Radiol. 2017;28(10):1363–1370.
- Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–259.
- Hebbadj S, Cazzato RL, Garnon J, et al. Safety considerations and local tumor control following percutaneous image-guided cryoablation of T1b renal tumors. Cardiovasc Intervent Radiol. 2018;41(3):449–458.
- Gunn AJ, Joe WB, Salei A, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: safety, technical results, and clinical outcomes. Cardiovasc Intervent Radiol. 2019;42(7):970–978.
- Breen DJ, King AJ, Patel N, et al. Image-guided cryoablation for sporadic renal cell carcinoma: three- and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology. 2018;289(2):554–561.
- Parsons RB, Canter D, Kutikov A, et al. RENAL nephrometry scoring system: the radiologist’s perspective. Am J Roentgenol. 2012;199(3):W355–W359.
- Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Caputo PA, Zargar H, Ramirez D, et al. Cryoablation versus partial nephrectomy for clinical T1b renal tumors: a matched group comparative analysis. Eur Urol. 2017;71(1):111–117.
- Petros FG, Matin SF. Cryoablation for cT1b renal tumors? Yet to be determined. Eur Urol. 2017;71(1):118–119.
- Gravis G, Faure M, Rybikowski S, et al. Radiation therapy following targeted therapy in oligometastatic renal cell carcinoma. Mol Clin Oncol. 2015;3(6):1248–1250.
- Lu X, Gu W, Zhang H, et al. Oligometastatic state predicts a favorable outcome for renal cell carcinoma patients with bone metastasis under the treatment of sunitinib. Oncotarget. 2016;7:26879–26887.
- Atwell TD, Vlaminck JJ, Boorjian SA, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol. 2015;26(6):792–799.
- Miller JM, Julien P, Wachsman A, et al. The role of embolization in reducing the complications of cryoablation in renal cell carcinoma. Clin Radiol. 2014;69(10):1045–1049.
- Kakarala B, Frangakis CE, Rodriguez R, et al. Hemorrhagic complications of percutaneous cryoablation for renal tumors: results from a 7-year prospective study. Cardiovasc Intervent Radiol. 2016;39(11):1604–1610.
- Rosenberg MD, Kim CY, Tsivian M, et al. Percutaneous cryoablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of hemorrhagic and collecting system complications. Am J Roentgenol. 2011;196(4):935–939.
- Warlick CA, Lima GC, Allaf ME, et al. Clinical sequelae of radiographic iceball involvement of collecting system during computed tomography-guided percutaneous renal tumor cryoablation. Urology. 2006;67(5):918–922.
- Tanagho YS, Kaouk JH, Allaf ME, et al. Perioperative complications of robot-assisted partial nephrectomy: analysis of 886 patients at 5 United States centers. Urology. 2013;81(3):573–579.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305.
- Rivero JR, De La Cerda J, Wang H, et al. Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol. 2018;29(1):18–29.
- Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196(4):989–999.
- Prince MR, Arnoldus C, Frisoli JK. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imag. 1996;6(1):162–166.
