Abstract
Background: Magnetic resonance-guided focused ultrasound (MRgFUS) is used for non-surgical treatment of uterine fibroids, often in patients who have had prior myomectomy or Cesarean section. The presence of post-surgical MRI artifacts along the beam path are a common contraindication to MRgFUS treatment. While potential problems arising from superficial cutaneous scars can be circumvented through scar patching and other techniques, deeper artifacts are difficult to bypass. Consequently, many patients with deeper artifacts are often excluded from treatment because of the assumption that these artifacts could deflect the ultrasound beam resulting in off target heating or perturb accurate MR thermometry. We sought to determine if these deep artifacts affect MRgFUS treatment efficacy or safety.
Materials and Methods: A search of a MRgFUS center patient database yielded 19 patients with prior uterine surgery who had artifacts along the FUS beam path visible on MRI. Charts, operative reports (when available), screening MRI scans, and MRgFUS treatment scans were reviewed by an experienced MRgFUS treatment physician and artifacts were graded as mild, moderate, or severe.
Results: One-way ANOVA showed no significant correlation between artifact severity and percent non-perfused volume (%NPV) (p = .41) or between fibroid size and % NPV (p = .49). There were no adverse events in this patient population except for one case of endometritis that occurred months after the operation, unlikely to be related to the MRgFUS treatments.
Conclusion: Patients with uterine fibroids with post-operative susceptibility artifacts in the near-field can be successfully treated with MRgFUS.
Introduction
Uterine leiomyomas, commonly referred to as fibroids, are the most common pelvic tumor in women, with prevalence ranging from 20% to 51% of reproductive-age women in the US [Citation1,Citation2]. Common symptoms resulting from uterine leiomyomas include subfertility, dysmenorrhea, menorrhagia, urinary symptoms, fatigue, noncyclic pain and constipation [Citation2]. Current therapeutic options include medical management with hormones such as GnRH analogs, interventional options such as uterine artery embolization, invasive surgeries such as myomectomy and hysterectomy, or noninvasive magnetic resonance-guided focused ultrasound ablation (MRgFUS) [Citation2].
MRgFUS is an FDA approved treatment modality that utilizes focused pulses of ultrasound energy to ablate tissues within the body without the need for surgical intervention or skin incisions. MRgFUS is currently being used for the non-surgical treatment of uterine fibroids, providing a noninvasive, effective treatment alternative to myomectomy or hysterectomy that preserves fertility [Citation3,Citation4].
Scarring and artifacts from prior surgical operations are a common concern for MRgFUS providers treating uterine fibroids. There is an increased risk of skin burns along dermal scars, and metallic debris in the deeper soft tissues and along the uterine wall may alter the beam intensity and thus preclude ablation distal to the artifacts, may redirect the beam causing potential heating to adjacent structures such as bowel loops, and may impede accurate thermometry in the immediate surrounding tissues [Citation5–8]. Post-operative MRI scans in patients status post myomectomy show healing of the uterus and endometrial cavity at around 12 weeks [Citation9].
However, in some cases dark circular artifacts of unknown origin persist along the uterine wall and in the adjacent soft tissues for months to years after the surgery is completed. Proposed hypotheses for these artifacts include metallic shavings from surgical tools or residual metallic clips, or chronic blood products such as hemosiderin [Citation11] Interestingly, a few operative reports from patients with such deep artifacts described the use of resorbable sutures and no use of metallic clips, making the etiology of the MRI artifacts unknown. While potential problems arising from superficial dermal scars or artifacts can be largely circumvented through scar patching, positional changes, turning off individual beam elements, or bladder and bowel distention [Citation7,Citation10,Citation12], deeper artifacts can be difficult to bypass. Consequently, many patients with deep MRI artifacts along the beam path are excluded from treatment due to a lack of data on the potential risks associated with conducting MRgFUS procedures. To our knowledge, there are no published studies on whether artifacts from prior uterine surgeries have a significant effect on MRgFUS treatment efficacy or safety to date.”
This study aims to determine whether post-surgical MRI artifacts affect the efficacy and/or safety of MRgFUS ablation of uterine leiomyomas.
Materials and methods
Aim
To determine if MRI artifact from prior surgery affects the efficacy and/or safety of MRgFUS ablation of uterine leiomyomas.
IRB exemption
The study protocol was reviewed by an independent IRB board and was determined to qualify for exemption under 45 CFR §46.101(b)(4) as the study was conducted using data that had already been collected for a prior investigation.
Patient sample
A retrospective study was conducted by performing a chart review using records from a MRgFUS treatment center. Records of MRgFUS treatments for uterine leiomyoma were searched for the key words ‘myomectomy,’ ‘C-section,’ and ‘abdominal scar.’ 56 out of 300 cases from treatments between May 2007 and March 2017 were identified and screened for clinical or radiological evidence of prior uterine surgery. As shown in , after screening for only cases with artifacts visible in the beam path and definitive evidence of prior uterine surgery 11 post-myomectomy and 8 post-C-section patients were identified. Their records were retrospectively reviewed to obtain time interval between surgery and MRgFUS treatment, non-perfused volume (NPV) from treatment, and any adverse events.
Evaluation of treatment efficacy
Percent non-perfused volume (%NPV) is a well documented measure of treatment efficacy. The NPV was calculated using the non perfused fibroid volume post treatment divided by the total fibroid volume pre treatment on T1 weighted FSE fat suppressed post contrast images. This metric reflects the technical success of the procedure, with 100% NPV being considered complete ablation.
Image evaluation
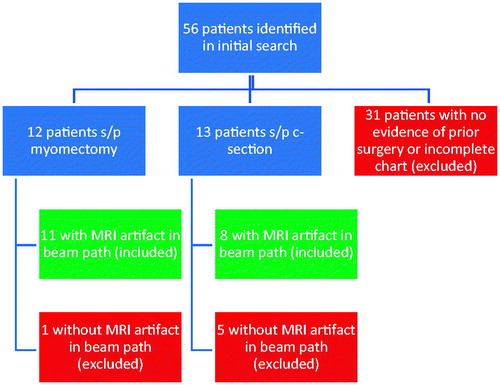
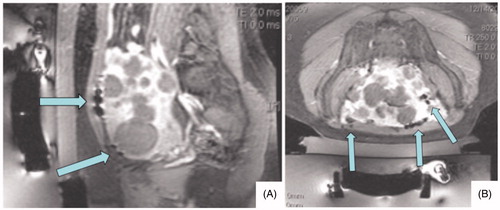
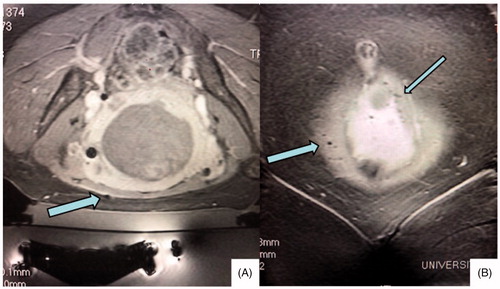
Planning images (T2 weighted images in 3 planes) and post-treatment images (T1 post contrast images with fat saturation) were evaluated in 3 planes and artifacts graded as mild, moderate or severe by a radiologist experienced with MRgFUS procedures. Grading was subjective and based upon the size (diameter) and number of unavoidable artifacts along the focused ultrasound beam path. Artifacts that were not in the beam path or able to be circumvented were not considered when assessing artifact severity. Artifact evaluation was conducted on a per patient rather than a per fibroid basis. depicts a patient with severe artifacts along the beam path that did not impede adequate ablation of fibroids distally. depicts a patient with mild artifact in the beam path (3 A). CT correlation (3B) in this patient shows no corresponding metallic object or artifact in the area of the MR artifact. The artifact is shown to persist on post-treatment MR imaging (3 C).
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine if there was any correlation between mean non-perfused volume and degree of artifact. P values <.05 were used as the threshold for statistical significance.
Results
Patient characteristics
depicts the average age of the included patients, average time interval from last uterine surgery to MRgFUS treatment, average volume of fibroids treated, and total number of fibroids treated.
Table 1. Characteristics of included patients. 36 individual fibroids were treated in the 19 patients included in the analysis.
Treatment efficacy
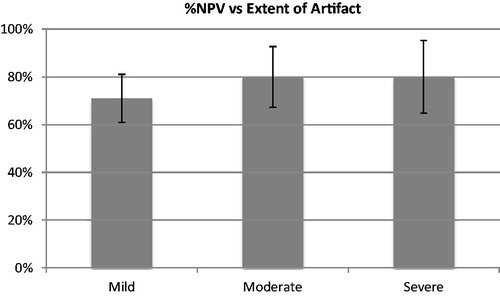
depicts treatment efficacy in patients with mild, moderate or severe artifact compared to the non-perfused volume (%NPV) of treated fibroids.
Table 2. Treatment efficacy for individual fibroids in patients with mild, moderate and severe artifact visible on T1 weighted post contrast fat saturation images.
As depicted in , one-way ANOVA showed no significant effect of artifact severity on %NPV (F = 0.68, p = .51). One-way ANOVA was also conducted in a similar manner on a subgroup of only myomectomy patients, again yielding no significant difference between mild, moderate or severe artifact groups (F = 0.92, p = .41). Patients in this study with C-sections exhibited only mild or moderate scarring. Thus, a t-test was conducted in the subgroup of only C-section patients, once again showing no significant effect of artifact severity on %NPV (p = .36). One-way ANOVA also yielded no significant relationship between fibroid size and % NPV (p = .49).
Adverse events
There were no adverse events such as skin burns or bowel injuries in the study group. Upon review of images, there was no evidence of off target heating or necrosis of adjacent tissues. The only reported adverse event was a patient with mild MRI artifact who developed endometritis many months after her procedure. To our knowledge at this time, there have been no reported instances of endometritis resulting from MRgFUS ablation of fibroids in either published literature or FDA pre-market approval studies. In addition, this patient’s artifacts were more superficially located in the abdominal wall and not adjacent to the endometrium. Thus, we find it unlikely that the MRgFUS procedure is related to this adverse event.
Discussion
Effects of artifact on MRgFUS efficacy and safety
This study reports that post-surgical artifacts from prior uterine surgery along the beam path do not have any impact on the efficacy or safety of MRgFUS ablation of uterine fibroids. Data analysis showed no significant difference in post-treatment %NPV of fibroids between groups with mild, moderate or severe artifacts. In addition, there was no significant correlation found between fibroid size and %NPV indicating that fibroids of any size can be efficaciously treated in the presence of artifact. None of the patients with artifact experienced any adverse events we believe to be attributable to the ablation procedure. As such, there is no evidence in this data set that these artifacts impede MRgFUS ablation. On retrospective evaluation of images, although parts of the thermal images were obscured by the artifact in the target zone, the FUS beam was able to successfully pass through the artifacts and induce thermal ablation of tissue in the targeted area of the fibroid.
Artifact etiology
Although the etiology of these artifacts has been assumed to likely be related to metallic debris remaining from prior surgeries, review of the operative reports of many of these patients and discussion of the cases with the surgeons directly revealed that all operations were performed with PDS absorbable sutures and without any metallic staples. depicts a patient with a mild artifact along the beam path in whom a CT pelvis without contrast found no corresponding CT metallic densities or artifact in the area, leading us to believe that the artifacts do not originate from microscopic or macroscopic metal left behind by surgical instruments. We contacted the manufacturer of the PDS sutures used, Ethicon, who stated: ‘Absorption of this suture is minimal until the 90th day postoperatively. Data from implantation studies in rats show that PDS suture is essentially absorbed between 182 and 238 days post implantation.’ Given the average time from last surgery to treatment in this study population was over 8 years, any PDS sutures used intraoperatively should have been fully resorbed by the time of the MRgFUS procedure.
Figure 4. Average percent non-perfused volume (%NPV) of fibroids after treatment for patients with mild, moderate and severe artifact visible on T2 weighted pretreatment MRI.
Ethicon also stated that the sutures are available in dyed and clear variants, with dyed variants containing D&C Violet No. 2 which is a synthetic dye produced from petroleum and coal tar products. We believe it is possible that long after the sutures themselves have been absorbed post-surgically, carbon compounds present in this dye may persist and create an electrical field when exposed to the magnetic fields of an MRI machine, resulting in the characteristic artifacts seen in the study. Thus, these artifacts are likely electrical in nature and not metallic artifact as previously thought. Residual carbon products would not be visible on CT imaging and would not affect focused ultrasound beam characteristics, meaning that this hypothesis is consistent with the findings of this study. Further study with preclinical models on inducing electrical currents with these carbon compounds in an MRI scanner is needed to confirm this hypothesis.
Study weaknesses
Limitations should be acknowledged for this retrospective chart review. The sample size for this study is small and a larger chart review or prospective study would provide stronger evidence to support these conclusions. Artifact grading was performed by one radiologist in this study but may be subject to inter- or intraobserver variability. Future retrospective or prospective studies may further evaluate this subject by studying populations with and without artifact, monitoring symptomatic relief of patients following MRgFUS at various time intervals, standardizing artifact grading and utilizing larger data sets spanning multiple treatment centers. Grading of artifact on a per fibroid rather than per patient basis would also be a potential avenue for further investigation. Intra-operative MR thermometry images could be examined to determine whether there was heating or phase change (whether real or artifactual) near the intersection of the beam and artifact, as well as to determine whether the interaction of the beam and artifact resulted in any change to the max average temperature vs sonication energy in the target zone. Another limitation is the evaluation of artifacts on certain MRI sequences. When comparing MRI images taken from each patient, we found that post-surgical artifacts are more visible on post contrast T1 fat saturation images compared to pre-contrast T1 and T2 weighted images. Future studies are needed to evaluate the visualization of metallic versus electrical artifacts in the setting of MRgFUS.
The strength of this study is the novelty of the data, in that no previous studies have evaluated the effects of these artifacts on MRgFUS and the sample size is sufficient to provide preliminary conclusions to guide future research.
Geolocation information
Study conducted at University MRI: 26.3852882°N, 80.0978759°W
Data availability
The data that support the findings of this study are available from the corresponding author, VS, upon reasonable request.
Precis Statement
This study aims to determine if non-surgical treatment of fibroids using focused ultrasound is safe and effective in patients with history of previous uterine surgery and artifacts along the beam path.
Disclosure statement
The authors report no conflict of interest.
References
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet. 2015;131(2):117–122.
- Gorny KR, Woodrum DA, Brown DL, et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol. 2011;22(6):857–864.
- LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol. 2010;194(1):274–280.
- Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183(6):1713–1719.
- Zaher S, Gedroyc WM, Regan L. Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):98–102.
- Yoon SW, Lee C, Cha SH, et al. Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol. 2008;18(12):2997–3006.
- Hassanuddin A, Choi JH, Seo DW, et al. Factors affecting tumor ablation during high intensity focused ultrasound treatment. Gut Liver. 2014;8(4):433–437. PubMed PMID: 25071910.
- Tsuji S, Takahashi K, Imaoka I, et al. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Keserci B, Duc NM. Volumetric magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids through abdominal scars: the impact of a scar patch on therapeutic efficacy and adverse effects. J Ther Ultrasound. 2017;5(1):22.
- Kim YS, Bae DS, Park MJ, et al. Techniques to expand patient selection for MRI-guided high-intensity focused ultrasound ablation of uterine fibroids. AJR Am J Roentgenol. 2014;202(2):443–451.
- Haramati N, Penrod B, Staron RB, et al. Surgical sutures: MR artifacts and sequence dependence. J Magn Reson Imaging. 1994;4(2):209–211.