 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: This study investigates the feasibility of endobronchial ultrasound applicators for thermal ablation of lung tumors using acoustic and biothermal simulations.
Methods: Endobronchial ultrasound applicators with planar (10 mm width) or tubular transducers (6 mm outer diameter (OD)) encapsulated by expandable coupling balloons (10 mm OD) are considered for treating tumors from within major airways; smaller catheter-based applicators with tubular transducers (1.7–4 mm OD) and coupling balloons (2.5–5 mm OD) are considered within deep lung airways. Parametric studies were applied to evaluate transducer configurations, tumor size and location, effects of acoustic reflection and absorption at tumor-lung parenchyma interfaces, and the utility of lung flooding for enhancing accessibility. Patient-specific anatomical lung models, with various geometries and locations of tumors, were developed for further evaluation of device performance and treatment strategies. Temperature and thermal dose distributions were calculated and reported.
Results: Large endobronchial applicators with planar or tubular transducers (3–7 MHz, 5 min) can thermally ablate tumors attached to major bronchi at up to 3 cm depth, where reflection and attenuation of normal lung localize tumor heating; with lung flooding, endobronchial applicators can ablate ∼2 cm diameter tumors with up to ∼2 cm separation from the bronchial wall, without significant heating of intervening tissue. Smaller catheter-based tubular applicators can ablate tumors up to 2–3 cm in diameter from deep lung airways (5–9 MHz, 5 min).
Conclusion: Simulations demonstrate the feasibility of endobronchial ultrasound applicators to deliver thermal coagulation of 2–3 cm diameter tumors adjacent to or accessible from major and deep lung airways.
Introduction
Lung and bronchial cancers are the leading cause of cancer-related death worldwide, and in 2018 accounted for an estimated 234,030 new cases and 154,050 deaths in the United States [Citation1], and ∼2.09 million new cases and 1.76 million deaths worldwide [Citation2]. The standard treatment option for early-stage non-small cell lung cancer (NSCLC) and most types of lung metastases is surgical resection, however, approximately 15–30% of patients are ineligible for surgery due to factors such as aging, poor cardiopulmonary function, or medical comorbidities related with smoking [Citation3–5]. Image-guided stereotactic body radiation therapy and/or chemotherapy are also considered for non-surgical cases and treatment of advanced disease [Citation6–8]. As an alternative to the above treatment approaches, thermal ablation with recently introduced technology provides a minimally invasive approach for treating patients not suitable for surgical resection or traditional therapies [Citation9–11].
Percutaneous radiofrequency ablation (RFA) and microwave ablation (MWA) devices delivered under CT guidance are two widely developed technologies applied for high-temperature thermal ablation for lung cancer treatment, and have demonstrated potential for treating tumor sizes smaller than 3 cm diameter with reported advantages including improvements to local control and survival [Citation3,Citation12–14]. The percutaneous treatment of primary and metastatic disease may be contraindicated and/or less effective for large tumors located near major airways and blood vessels of the upper and central lung, with suitability often determined on a case-by-case basis [Citation13,Citation15–17]. Pneumothorax is a common complication of both RFA and MWA, and is often associated with percutaneous placement, needle trajectory, or adjustment of device positioning and orientation under CT imaging [Citation13,Citation18–20]. As an alternative to percutaneous needle-based ablation, endobronchial ultrasound imaging and fiberoptic bronchoscopy can be used to guide transbronchial insertion or intraluminal placement of RFA devices and cryotherapy applicators for treatment of tumors directly accessible from the bronchi [Citation21–23]. Endobronchial ablation with these approaches can provide debulking for locally advanced tumors adjacent to and obstructing major airways, with reduced interventions and possibly lower pneumothorax rates compared to percutaneous ablation [Citation24–26].
High-intensity ultrasound is a promising technology that applies minimally invasive approaches to treat tumors with precise spatial control of energy deposition and large ablation volumes possible. As one of the least invasive and most precise delivery platforms of ablation therapy, extracorporeal high-intensity focused ultrasound (HIFU) coupled with MRI or ultrasound real-time imaging and monitoring can deliver acoustic energy from outside the body to cause tumor coagulation in liver, kidney, pancreas, bone, or the brain, among others [Citation27–29]. Alternatively, endocavity and catheter-based ultrasound (CBUS) devices via percutaneous and intraluminal approaches within the body can deliver thermal therapy directly adjacent to or within target lumens and tissues not easily accessible by extracorporeal systems [Citation30]. With the guidance of MRI and ultrasound, endocavity HIFU and CBUS have been investigated for treatment of a variety of sites and indications, such as transurethral thermal treatment of prostate cancer and benign prostatic hypertrophy (BPH) [Citation31–33], and endoluminal thermal therapy of esophageal, biliary tract, and pancreatic cancer [Citation34–36].
Although extracorporeal HIFU has many advantages over other energy modalities, its applicability for treating lung tumors is complicated by limited acoustic access into the thoracic cavity, organ movement, and the high acoustic attenuation and reflections of acoustic energy due to the impedance mismatch of air-filled lung parenchyma with adjacent tissues [Citation37]. Recent in silico and in vivo investigations have demonstrated that lung flooding through infusing saline into air-filled lung may be used during HIFU procedures as a means to reduce local attenuation and reflection of acoustic energy within lung parenchyma, potentially allowing for more effective acoustic energy transmission and targeting of lung tumors [Citation37–40], although organ movement, focusing through the ribs, and need for reduction of typical HIFU treatment durations remain as challenges [Citation30,Citation38]. Given the limitations of other energy modalities and difficulties of treating lung with extracorporeal HIFU, there is motivation to explore endobronchial CBUS applicators as a more direct way to treat pulmonary tumors located adjacent to or accessible from the airways. Potential advantages of this minimally invasive approach include minimized requirement of lung flooding relative to extracorporeal HIFU and more precise and conformal ablative capability relative to other ablation modalities, which may reduce treatment durations and complications such as pneumothorax. Further, the reflections and acoustic attenuation of normal lung parenchyma may provide for preferential acoustic energy deposition and heating in tumors encasing or attached to a bronchial wall.
The purpose of our study is to apply acoustic and biothermal simulations to determine the feasibility and potential performance advantages of endobronchial ultrasound applicators to thermally coagulate pulmonary tumors adjacent to or accessible from lung airways. Parametric studies and 3D patient-specific lung model simulations were performed to investigate various applicator design features and anatomical conditions, including with or without lung flooding, with performance described by calculated temperature and thermal dose distributions.
Materials and methods
Endoluminal ultrasound applicator
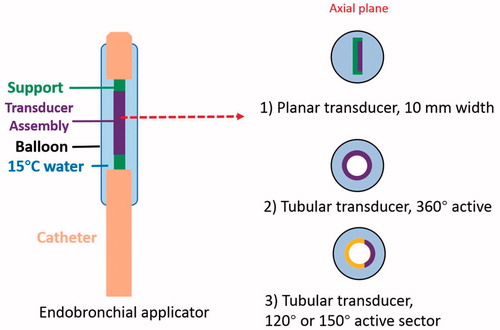
The generalized schema of the proposed endobronchial ultrasound applicators and treatment delivery () includes large diameter endoluminal applicators (∼10 mm outer diameter (OD)) with integrated planar or tubular ultrasound transducers for therapy delivery from within major bronchi, and smaller diameter applicators (∼2–5 mm OD) with tubular transducers for use within small bronchi of deep lung regions. The specific design features of the endobronchial ultrasound applicators modeled in this work are shown in , with designs varying according to intended positioning either within the primary and secondary bronchi of major lung airways or small bronchi within deep lung. Each prospective applicator contains a single planar ultrasound transducer (10 mm width, 20 mm length, 1–9 MHz) or tubular transducer (1.7–6 mm OD, 10–20 mm length depending on sites, 3–9 MHz, 120–360° active sector), with transducer assembly integrated within an inflatable endobronchial water cooling/coupling balloon (2.5–10 mm OD, 15–30 mm length depending on sites, inflated once deployed), positioned at the distal end of a flexible delivery catheter. Cooling water circulation (15 °C) within the integrated balloon can be applied to couple the ultrasound energy to target tissue adjacent to the airways, cool the transducer surface during operation, and possibly protect the bronchial wall from thermal damage.
Figure 1. Concept diagram of proposed treatment strategy for ablation of lung tumors adjacent to major and sub-segmental tertiary bronchi using endobronchial ultrasound applicators: larger diameter applicator configurations composed of planar or sectored tubular transducer segments for treatment of tumors at large airways, and smaller diameter applicators with tubular sources for targeting tumors adjacent to deep lung sites.
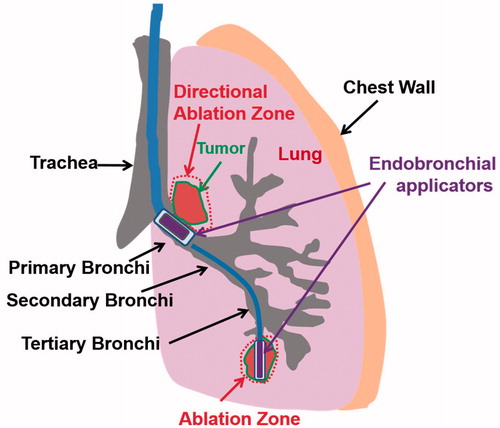
Figure 2. Design schema of endobronchial ultrasound applicators for thermal treatment of lung tumors adjacent to major bronchi and deep lung. Ultrasound transducer applicator configurations include (1) planar transducer, (2) fully active (360°) tubular transducer, and (3) angular sectored (120° or 150° active sector) tubular transducer, each integrated within an expandable balloon for coupling and water cooling.
Theory
The transient temperature distribution due to ultrasound energy deposition in tissue can be described by the Pennes bioheat equation [Citation41]:
(1)
(1)
where
is the tissue density (kg·m−3),
is the tissue-specific heat (J· kg−1K−1), T is the temperature (K), t is time (s), k is tissue thermal conductivity (W·m−1·K−1)),
is blood perfusion rate (kg·m−3 s−1)
is the blood-specific heat (J·kg−1K−1), and
is the blood temperature (310.15 K, or 37 °C).
is the power deposition due to ultrasound energy (W·m−3). The acoustic power deposition
of the ultrasound applicator can be approximated as follows:
(2)
(2)
where
is the acoustic amplitude absorption coefficient (Np·m−1) and
is the time-averaged acoustic intensity (W·m−2). Temperature calculations based upon EquationEquation (1)
(1)
(1) can be used to calculate iso-effect thermal dose distributions in terms of equivalent minutes of heating at 43 °C (EM43°C) [Citation42,Citation43], through summation of time intervals over a varying temperature exposure, as described:
(3)
(3)
where
is a proportionality constant that approximates the dependence of cell death rate on temperature, and
is the average temperature (°C) over the
th time interval
(minutes). This thermal dose concept, as derived from an Arrhenius model of cell damage accumulation, is a well-proven metric for predicting levels of thermal effects or damage on tissues and is widely used for hyperthermia and thermal ablation monitoring and assessment [Citation44,Citation45]. Damage thresholds vary for different tissue types, but in general, thermal dose values greater than 240–300 EM43°C can describe lethal thermal necrosis and values less than 10–30 EM43°C can describe reversible or no thermal damage [Citation44–46]. The acoustic and biothermal properties of the tissues employed in models are listed in [Citation47–51]. Due to lack of measurement data, we adopted the properties of cartilage tissue for bronchial wall, provided that greater than 50% of bronchial composition is cartilaginous tissue [Citation52]. For all thermal simulations, blood perfusion of the tissue was set as constant at lower temperature, as listed in [Citation53,Citation54], and reduced to zero upon reaching the temperature threshold of 52 °C for coagulative necrosis (closely equivalent to a thermal dose threshold of 300 EM43°C in this study) [Citation55]. Initial tissue temperature was set as 37 °C, and Dirichlet conditions of constant temperature (37 °C) were applied at the outer boundaries of the models. A convective boundary condition was applied on the outer applicator surface to represent water cooling within the endobronchial balloon:
(4)
(4)
where
is the outward normal vector, the convective heat transfer coefficient h is 1000 W·m−1K−1 and cooling water temperature
is set as 15 °C [Citation56].
Table 1. Thermal and acoustic properties of tissues used in the parametric study and patient specific simulations.
Parametric studies
Parametric studies using analytical approximations for acoustic intensity fields and simplified geometric models were performed to investigate a broad range of applicator design features including transducer geometry, operating frequency and driving surface intensity, as well as the influence of anatomical variation such as tumor size, tumor-airway separation distance, and impact of varying degrees of lung inflation or flooding on the thermal performance of the endobronchial applicators. 2D models using either a rectangular geometry for planar transducer applicators or axis-symmetrical cylindrical geometry for tubular transducer applicators models were created. Two types of heterogeneous tissue computational domains, as shown in , were adopted for this study, using the acoustic and thermal parameters of Model A of a tumor directly attached to the bronchial wall, and surrounded with either high or low inflated lung parenchyma (); Model B of a tumor at distance away from the bronchial wall, and surrounded with saline-infused flooded lung (). To reduce the computational cost and complexity in these parametric simulations, the acoustic energy produced by the transducers was approximated to follow fully-developed plane wave propagation with normal incidence angles at tissue interfaces [Citation57]. Lung parenchyma tissue was modeled as lower inflated lung and higher inflated lung representing different air inflation levels of lung tissues with different densities and speeds of sound, or as flooded lung, with corresponding acoustic properties applied (). Constant speed of sound and impedance of lung tissue with frequency is assumed. Detailed descriptions of the analytical expressions used for modeling the acoustic intensity distributions and energy deposition, incorporating reflections and transmission at the tumor-lung interface, are described in Section 1 and Equations (S1)–(S6) of the Supplemental materials.
Figure 3. 2D parametric simulation models with attaching (Model A) and adjacent but not attaching tumors (Model B) to the bronchial wall: transducer types (planar, 360° active tubular applicators), tumor sizes (0.5–3 cm, as denoted by yellow arrowed bar) and lung environments (lower and higher inflated lung, flooded lung) were varied to represent different treatment sites and conditions, as listed in .
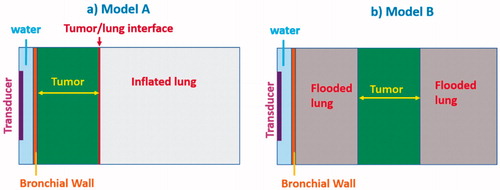
Models A and B were used to investigate distinct prospective cases, including targeting of tumors attached or adjacent to major bronchi (major airways) and tumors adjacent to small diameter tertiary bronchi (deep lung). Different applicator configurations, tumor sizes and distances from the bronchial wall were incorporated as summarized in . Specifically, bronchial wall thickness was set at 1.5 mm at major bronchi and 0.75 mm at tertiary bronchi, and lumen diameters were set as 10 mm at major airways and 2.5–5 mm at smaller airways, based on measurements extracted from multi-detector CT imaging [Citation58]. Tumor sizes in terms of radial depth extent were set as 1–3 cm at major airways and 0.5–1.5 cm at smaller airways with normal air-filled lung parenchyma (Model A, ), and 2 cm at major airways and 1 cm at smaller airways with saline flooding lung parenchyma (Model B, ). Acoustic power deposition profiles were calculated using Equations (S1)–(S6) in Supplemental materials, and imported into COMSOL Multiphysics 5.3 (COMSOL Inc., Burlington, MA) for thermal simulations. In the models described in , the absorption coefficient for all tissues is assumed to be equal to the corresponding attenuation coefficient, with all scattered energy locally absorbed. Some studies have suggested that the absorption coefficient () of the inflated lung parenchyma might be significantly less than the attenuation coefficient (
) due to its highly scattering properties [Citation59–61]. As such, additional parametric studies using the geometry of Model A were performed to investigate the influence of lower absorption coefficient in lung tissue, as described in section 2 of the supplemental materials. Acoustic surface intensities of the transducers were iteratively adjusted to achieve a maximum temperature of 75 °C within a 5 min sonication time. The resulting temperature profiles central to the transducer surface were analyzed to evaluate the performance of the endobronchial applicators. The minimum and maximum penetration depths were defined as the proximal and distal depths of the 52 °C temperature contour from the inner bronchial wall, corresponding to approximately 240 EM43°C thermal dose margins. The ablative margin was defined as the distance that the 52 °C temperature contour exceeded the target tumor boundary in the depth direction.
Table 2. Endobronchial ultrasound applicator configurations and tumor location with dimensions investigated within parametric studies, for tumors attached and unattached to the bronchial wall, and with and without lung flooding.
Patient-specific studies
To evaluate the feasibility and performance of the endobronchial ultrasound applicators for thermal therapy of lung and bronchial cancer in a more realistic and complex anatomical setting, a total of five distinct tumor models within an anatomical lung airway model were generated, as shown in . The lung airway structure with at least 2 mm lumen diameter of bronchi was segmented from the CT images of an adult patient with lung cancer using an airway segmentation algorithm developed by Nardelli et al. [Citation62], within the open source software platform 3D slicer (www.slicer.org). A total of five tumors between 1 and 3 cm diameter were segmented using iSeg (Zurich MedTech AG, Zurich, Switzerland) and were placed at different sites to represent a range of patient cases. The details of tumor locations, sizes and lung background conditions are summarized in . Using select applicator configurations from the parametric studies, as summarized in , acoustic simulations to calculate acoustic intensity distributions in the heterogeneous tissue computational domains were performed using a 3D full-wave FDTD acoustic solver (P-ACOUSTIC) within Sim4Life, based upon the linear acoustic pressure wave and with adaptive rectilinear meshes. The acoustic simulation voxel size varies with the frequencies of the endobronchial ultrasound applicator to /10 (
is the acoustic wavelength) for a convergent solution. An implicit time dependent FDTD thermal solver (P-THERMAL, T-CEM43) with maximum time step of 15 s was utilized to determine temporal 3 D temperature and thermal dose profiles. The maximum thermal simulation voxel size was set as 0.25 mm. Convergence tests were performed for each model to ensure all grid sizes were fine enough for stable acoustic and thermal solutions. The transient temperature and thermal dose distributions for applicator configurations within each patient model were analyzed to obtain maximum temperature penetration and sparing of the bronchial wall, lethal thermal damage volumes, and penetration depth.
Figure 4. Overview of 3D patient-specific lung bronchi/airway anatomy generated for Models 1–5, each incorporating a distinct tumor geometry/position and lung environment (higher inflated, lower inflated, and flooded lung).
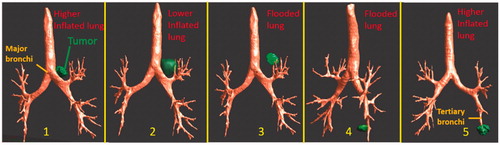
Table 3. Details of anatomical lung and tumor models and applicator configurations for patient-specific simulation studies.
Results
Parametric studies: tumors attaching to bronchi
The resultant minimum and maximum penetration depths of the 52 °C contour for the parametric studies of Model A are summarized in and for sites of major airways and deep lung, respectively. At major airways, an endobronchial planar applicator (10 mm × 20 mm, 1.2–3.4 W/cm2 acoustic surface intensities, , top row) can coagulate beyond the distal boundary of 3 cm tumors with an ablative margin of 4.1–6.8 mm at the frequency range of 3–5 MHz, but may result in sparing of the proximal tumor boundary and incomplete coagulation of the tumor. The proximal tumor sparing is more significant when treating smaller tumors (<2 cm in depth) at frequencies below 6 MHz. Complete coagulation along depth was achieved with appropriate selection of frequency ranges: 5 MHz for 3 cm tumor, 6–8 MHz for 2 cm tumor and 9 MHz for 1 cm tumor. In contrast, tubular applicators (6 mm transducer OD, 20 mm length, 10 mm balloon OD, 6.4–20.1 W/cm2 acoustic surface intensities, 3–9MHz, , bottom row) in major airways could coagulate smaller tumors (1 cm in radial depth) with approximately 2.5–5.5 mm ablative distal margin extending into lung beyond the tumor and proximal coverage extending 1.0–1.2 mm into the bronchial wall with 1.5 mm thickness, varying with frequency and lung inflation levels. For larger tumors of 2–3 cm, coagulation depth was limited to 10.4–15.4 mm deep from the inner bronchial wall for 4–9 MHz, resulting in sparing of the distal tumor and tumor–lung boundary. For deep lung cases accessible by smaller diameter tubular applicators (2–4 mm transducer OD, 10 mm length, 2.5–5 mm balloon OD, 8.6–29.3W/cm2 acoustic surface intensities, 4–9 MHz, ), applicators could ablate up to the 1.5 cm tumor at frequencies between 4 and 6 MHz, with 2.3–5.0 mm distal ablative margin and up to 0.5 mm sparing of proximal tumor boundary. At 7–9 MHz, ablation penetration to the distal tumor boundary was limited to smaller tumors (<1 cm in radial depth), with almost no sparing of bronchial wall. For both tumor sites with either a planar or tubular transducer, lung inflation level slightly affected the penetration depths at lower frequency range (4–6 MHz), however it had little influence at higher frequency range (7–9 MHz). Results for the parametric analysis of various values of the absorption coefficient ranging from to 0.1
in relation to the attenuation coefficient are described in supplemental materials (Section 2, Figure S1) in brief, as the absorption is decreased, heating of the proximal and distal tumor margins is increased.
Figure 5. Parametric study: comparison of penetration depths (52 °C margin) of endobronchial ultrasound applicators in planar (10 mm width ×20 mm length transducer, 10 mm balloon OD, top row) or tubular configurations (10 mm balloon OD, bottom row) at 3–9 MHz for treatment of lung tumors varying from 10 mm (left) to 30 mm (right) depth attached to major airways. Two lung conditions (higher and lower inflated lung, represented in red and blue, respectively) were considered. The effective ablated zone extends between min-depth and max-depth, as depicted. BW: bronchial wall.
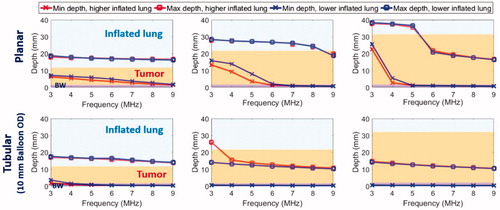
Figure 6. Parametric study: comparison of penetration depths (52 °C margin) of two sizes of endobronchial tubular ultrasound applicators (5 mm balloon OD, top row and 2.5 mm balloon OD, bottom row respectively) with frequencies of 4–9 MHz for treatment of deep lung tumors from smaller bronchial passages, with tumor varying from 5 mm (left) to 15 mm (right) radial depth. Two lung conditions (higher and lower inflated lung, represented in red and blue, respectively) were considered. The effective ablated zone extends between min-depth and max-depth, as depicted. BW: bronchial wall.
Parametric study: tumors unattached to bronchi
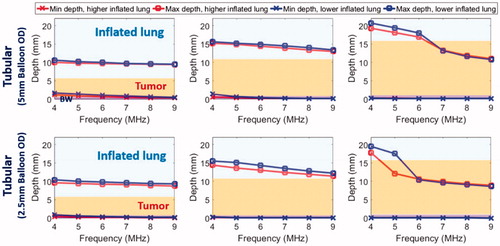
The parametric studies of Model B with tumors adjacent to but unattached with the bronchial wall, as shown in , were performed to include saline flooding of lung in both sites of major airways and deep lung, and the minimum and maximum penetration depths are summarized in . At major airways, an endobronchial planar applicator (10 mm × 20 mm, 3.6–9.7 W/cm2) at lower frequency (1–3 MHz) could produce a 2–2.5 cm long coagulation zone in depth which can cover the 2 cm tumor with up to 4 mm margin while sparing at least 2 cm of normal intervening flooded lung; in contrast, at higher frequency (>3 MHz), penetration to the outer boundary of the tumor was not achievable for tumors either 1 cm or 2 cm from the bronchial wall, and intervening flooded lung was not spared. In comparison, the tubular applicator (10 mm balloon OD, 7.8–17.5 W/cm2, 3–9 MHz, , top row) was not effective at targeting the whole tumor, with maximum penetration of 20.3 mm (8.8 mm within the 2 cm tumor) for tumors 1 cm from the bronchial wall, and almost no ablation coverage of tumors 2 cm from the wall.
Figure 7. Parametric study of lung flooding: penetration depths (52 °C margin) across frequency of ultrasound applicators with planar (10 mm × 20 mm, red line) or tubular transducers (10 mm balloon OD, blue line) for major airways placement, for treatment of a 2 cm lung tumor located 10 mm (top left) and 20 mm (top right) away from the outer bronchial wall (top row); comparison of deep lung endobronchial tubular ultrasound applicators (5 mm balloon OD, green line and 2.5 mm balloon OD, magenta line) for treatment of a 1 cm lung tumor located 5 mm (bottom left) and 10 mm (bottom right) away from the outer bronchial wall (bottom row). The ablation zone extends between min-depth and max-depth, as depicted. BW – bronchial wall. Min-depths of the tubular applicator with 5 mm balloon OD were overlapped by min-depth of the 2.5 mm balloon OD tubular applicator.
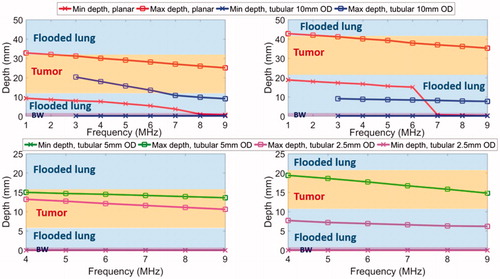
In deep lung, both endobronchial tubular ultrasound applicator (2–4 mm transducer OD, 10 mm length, 2.5–5 mm balloon OD, 14.7–38 W/cm2, 4–9 MHz, , bottom row) configurations demonstrated deeper penetration at lower frequency (4–5 MHz), but not enough to cover the distal tumor boundary. For tumors with 5 mm separation from the bronchial wall, the maximum penetrations at 4–9 MHz were 10.6–15.0 mm from the inner bronchial wall. Tumor coverage decreased when it was located further (∼10 mm) from the bronchial wall: at least 1.4–6 mm of the tumor depth was sub-ablative for the 5 mm balloon OD applicator, and no ablative coverage of the tumor was produced for the 2.5 mm balloon OD applicator.
Patient-specific simulations-tumors accessible from major airways
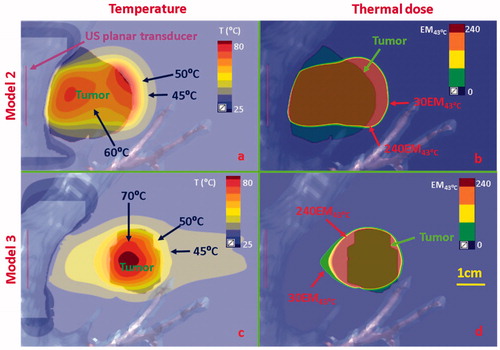
Acoustic and biothermal simulations on anatomical models with tumors located adjacent to major airways (Models 1–3, ) utilized optimal frequencies and applied power ranges identified in the parametric studies (). The temperature and thermal dose distributions at the end of 5 min ablations for two distinct tumor targets attached to the major bronchi, using a sectored tubular or planar transducer applicator, are shown in and Citation9(a,b), respectively. An endobronchial sectored tubular applicator (6 mm transducer OD, 20 mm length, 6 MHz, 9 W/cm2, 120° active zone) located within the major bronchi, shown in , demonstrated heating penetration above 52 °C along 1 cm (in depth) for a tumor attaching to the bronchial wall. Thermal dose contours of 240 EM43°C covered ∼87% of the target tumor volume within 5 min ablation, with up to 4 mm ablative margin in both coronal and axial planes. While not shown, it should be noted that transducer dimensions (length and/or active sector angle) could be adjusted to increase volumetric coverage. An endobronchial planar applicator (10 mm width × 20 mm length, 5 MHz, 4.5 W/cm2) shown in demonstrated deep ablative penetration of larger tumors (∼3 cm in depth) while providing some sparing of the bronchial wall (∼0.7 mm from the inner bronchial wall). The ablation margin was approximately 5 mm in depth beyond the tumor, demonstrating isolation of tumor heating with inflated lung conditions. When the tumor was not attached to bronchial wall (Model 3) as shown in , flooded lung was simulated as a means to increase acoustic energy propagation. The endobronchial planar applicator (10 mm × 20 mm, 3 MHz, 7.5 W/cm2) could ablate the target tumor (up to 5 mm ablation margin) located at least 2 cm away from the major bronchi while sparing the whole bronchial wall and preserving most normal (flooded) lung tissue between the outer bronchial wall and the tumor.
Figure 8. Temperature and thermal dose distributions of patient-specific Model 1 on the coronal (a, b) and axial plane (c, d) after 300 s sonication with an endobronchial ultrasound tubular applicator (6 mm transducer OD × 10 mm length, 10 mm balloon OD, 6 MHz, 9 W/cm2, 120° active zone), as applied to deliver thermal coagulation to the tumor (∼1 cm in depth) attached to major airways. Temperature contours of 45 °C, 50 °C, and 60 °C are shown in (a) and (c), and thermal dose contours of 30 EM43˚C and 240 EM43˚C are shown in (b) and (d). US: ultrasound.
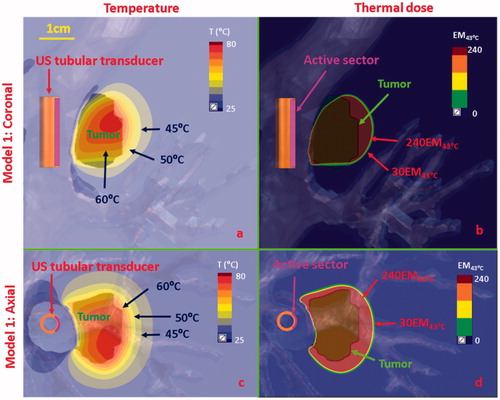
Figure 9. Temperature and thermal dose distributions along the coronal plane obtained after 300 s sonication using endobronchial planar applicators in (a, b) Model 2 representing treatment of a tumor directly attached to the bronchial wall with lower inflated lung, and (c, d) Model 3 representing a tumor separated by 2 cm from the bronchial wall with lung flooding. Two different applicator configurations were applied: 5 MHz, 4.5 W/cm2 for model 2; 3 MHz, 7.5 W/cm2 for Model 3. Temperature contours of 45 °C, 50 °C, 60 °C, and 70 °C are shown in (a) and (c), and thermal dose contours of 30EM43˚C and 240 EM43˚C are shown in (b) and (d). US: ultrasound.
Patient-specific simulations: tumors accessible from deep lung
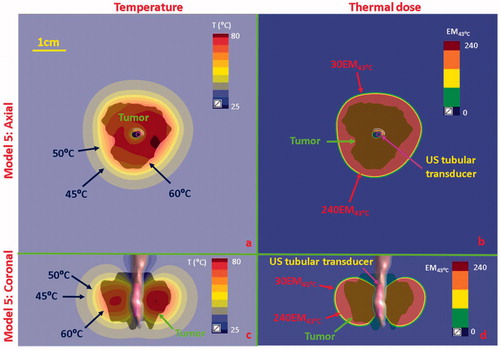
Simulations on two anatomical models with tumors located in deep lung adjacent to tertiary bronchi (Models 4 and 5, ) were performed for small diameter tubular applicators, and the temperature and thermal dose distributions calculated as shown in and Citation11. Two tubular applicators (2 mm transducer OD × 10 mm length, 7 MHz, 30 W/cm2, 150° active sector for Model 4 in and 1.7 mm transducer OD × 10 mm length, 7 MHz, 12.5 W/cm2, 360° active sector for Model 5 in ) were positioned within the tertiary bronchi, and the balloon OD was initially set to 2.5 mm and fitted to conform to the actual bronchi diameter as obtained directly from CT segmentation. For the case of Model 4 shown in , the 2 cm tumor was not attached to the small diameter tertiary bronchi, and thus flooded lung was simulated to provide acoustic energy access. In this scenario, the endobronchial sectored tubular applicator could penetrate 1.5 cm radial depth within the 2 cm tumor, thus only coagulating 54.5% of the tumor volume over 240 EM43°C with at least 0.6 cm distal tumor depth at sub-lethal thermal exposures (<50 °C and <30 EM43°C). For Model 5, where the ∼2 cm diameter tumor was fully attached and encasing the tertiary bronchi, as shown in , the selected endobronchial tubular applicator with 360° active zone demonstrated coagulation (>52 °C and >240 EM43°C) of 85.4% of the tumor volume coverage and 3–5 mm margin, as shown in the dose coverage of 240 EM43°C contour. The axial extent of the thermal damage closely followed the length of the ultrasound transducer, and although not shown it can be inferred that sequential repositioning of the applicator or selecting a longer transducer could improve the volume coverage.
Figure 10. Temperature and thermal dose distributions for patient-specific Model 4 along the coronal (a and b) and axial planes (c and d) after 300 s sonication with an endobronchial tubular applicator (2 mm transducer OD × 10 mm, 150° active zone, 7 MHz, 30 W/cm2) applied to deliver thermal coagulation to the tumor in conjunction with lung flooding. The 2 cm diameter tumor volume is adjacent to and separated by approximately 5 mm of lung from the tertiary bronchial airway in deep lung. Temperature contours of 45 °C, 50 °C, and 60 °C are shown in (a) and (c), and dose contours of 30EM43˚C and 240EM43˚C are shown in (b) and (d). US: ultrasound.
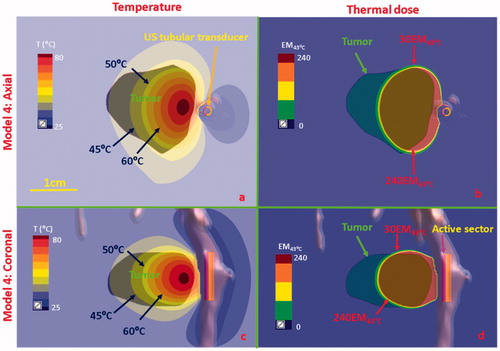
Figure 11. Temperature and thermal dose distributions for patient-specific Model 5 along the axial (a and b) and coronal planes (c and d) after 300 s sonication with an endobronchial tubular applicator (1.7 mm transducer OD × 10 mm, 360° active zone, 7 MHz, 12.5 W/cm2). The 2 cm diameter tumor volume is encompassing and adjacent to the tertiary bronchial airway, surrounding with higher inflated lung. Temperature contours of 45 °C, 50 °C, and 60 °C are shown in (a) and (c), and dose contours of 30EM43˚C and 240EM43˚C are shown in (b) and (d). US: ultrasound.
Discussion
This study investigated prospective design features and delivery strategies for endobronchial ultrasound applicators through acoustic and biothermal simulations on 2D parametric and 3D anatomical models, as a minimally invasive method for thermal ablation of pulmonary tumors accessible from central major or deep lung bronchial passages. Simulations demonstrate that through appropriate selection of the transducer configurations and sonication settings, tumors that are attached to and accessible via major airways can be ablated at up to 3 cm depth from the bronchial wall, while tumors adjacent to or encapsulating smaller deep lung airways can be ablated at up to 1.5 cm radial depth. Simulations also show that lung flooding, as proposed for use with extracorporeal HIFU, can provide improved acoustic energy propagation from endobronchial ultrasound devices to enhance ablation of tumors closely adjacent to but separated from accessible lung airways.
For cases of tumors attached to the airway, the acoustic impedance mismatch and high attenuation of the normal lung parenchyma at the distal tumor boundary can help to isolate ultrasound energy within the tumor and enhance heating at the tissue interface. Normal inflated lung tissue has a complex structure with inhomogeneous acoustic properties and variable air content or inflation levels during respiration [Citation61,Citation63,Citation64], which affects and limits ultrasound propagation. A previous study demonstrated acoustic impedance of lung ranged from 0.1 Mrayl of fully inflated lung to 1.4 Mrayl of deflated or fully collapsed lung, with some studies also demonstrating frequency-dependent lung speed of sound and soft tissue-lung reflection coefficients [Citation49,Citation61,Citation65,Citation66]. To simplify the modeling in this study, speed of sound and impedance were not varied with frequency, and two inflation levels of normal lung were considered to bracket the expected range of variation in acoustic properties. For both lung inflation levels, significantly higher acoustic attenuation values () and substantial impedance mismatching (0.37–0.85 Mrayl in the inflated lungs, versus 1.82 Mrayl in the target tumor) were used compared to the values used for tumor and other adjacent soft tissues. Thus, for cases of lung tumors directly attached or enveloping the bronchial wall, a large portion of the acoustic energy (∼13–43% acoustic intensity, at normal incidence) was reflected from the tumor-inflated lung interface, while transmitted energy into the lung was steeply attenuated with depth, thus, constraining energy deposition and heating to within the tumor and a narrow boundary within inflated lung. Further, the high absorption coefficient used for lung results in high preferential heating at the distal tumor-lung interface, which for specific combinations of transducer geometry, frequency, and tumor size, can be so dominant that in tandem with the maximum temperature set-point employed herein (∼75 °C), corresponding simulations resulted in sub-ablative coverage of the proximal tumor region ( and Citation6). The present study also investigated the potential impact of lower absorption compared to attenuation through parametric studies described in supplemental materials, with results shown in Figure S1 demonstrating that as absorption decreases (coefficients ranging from to 0.1
), the effective extent of ablation is shifted toward the proximal boundary of the tumor, and can result in more uniform heating and ablation coverage through the tumor depth, while possibly decreasing the distal ablative margin. Although not explicitly shown in the present study, more thorough tumor heating and larger margins than the 3–5 mm attained here can be achieved by using greater applied powers/intensities and higher maximum temperature set-points, and/or longer treatment durations.
This study also investigated the potential impact of utilizing lung flooding to enhance acoustic energy access for thermal ablation of lung lesions in close proximity but not directly attaching to lung airways. Saline-infused lung flooding, which was recently investigated for lung sonography enhancement and extracorporeal HIFU lung tumor ablation [Citation37,Citation38,Citation40], has been demonstrated to reduce the acoustic impedance mismatch between normal lung and tumor, and significantly reduce the attenuation of normal lung tissue. Parametric studies and patient-specific simulations of endobronchial applicators herein have demonstrated that lung flooding may enhance ultrasound energy propagation through lung parenchyma to allow localized heating within a target tumor up to 2 cm separation distance from major airways, without excessive thermal damage to normal flooded lung tissue (, and Citation9(c,d)), and with a similar margin of 3–5 mm compared to extracorporeal HIFU reported in previous studies [Citation39]. Simulations demonstrate selective heating of tumor targets over intervening flooded lung tissue using planar transducer configurations, which is a result of the higher (∼3.5×) attenuation/absorption coefficients in tumor over flooded lung. However, as shown in parametric studies () and Model 4 (), the utility of lung flooding might be of limited utility for applicators with tubular transducer configurations, as the radial intensity falloff inherent to tubular transducer sources, not the tissue attenuation, limits energy penetration (<1.5 cm) and increases heating of normal tissues between the bronchial wall and the tumor.
This study provides preliminary guidance for continued development of endobronchial ultrasound devices for treatment of central and peripheral lung tumors. Simulation results indicate that larger endobronchial applicators with selected planar transducer configurations (10 mm × 20 mm, 3–7MHz) and coupled encapsulating balloons may be suitable for coagulating large tumors extending 2–3 cm in depth from and attaching to the major bronchi ( and Citation9(a,b)), or for targeting closely adjacent tumors up to 2 cm in size and less than ∼2 cm separation from the major bronchi through lung flooding ( and Citation9(c,d)). For larger partially circumferential tumors, the device may need to be rotated and/or translated sequentially to fully cover the entire tumor volume. Sectored tubular transducers (10 mm balloon OD, 7–9 MHz) may be suitable for treating smaller tumors (<2 cm in depth) attached to and partially encapsulating major bronchi efficiently ( and Citation8), with a pre-set appropriate active sector angle and minimal repositioning. The proposed endobronchial ultrasound approach, with positioning within and integrated cooling of the inner bronchus, could provide better protection of central airways and major vessels compared to contemporary percutaneous RF or microwave ablation approaches, which require physical penetration through the lung parenchyma and have associated risk of thermal damage on the central airways, bronchial disruption or pneumothorax [Citation19,Citation20]. Simulations also indicate that compact applicator configurations with miniature tubular transducers (1.7–4 mm OD) and expandable balloons (2.5–5 mm OD) may be capable of coagulating tumors at up to 1.5 cm radial depth attaching to the tertiary bronchi ( and Citation11), or adjacent tumors at up to 1.5 cm radial depth and up to 0.5 mm separation from the tertiary bronchi with lung flooding ( and Citation10). These ultrasound applicator constructs, which could incorporate flexible catheter assemblies as small as 1–4 mm OD [Citation30,Citation67,Citation68], could be combined with endobronchial navigational techniques, such as fluoroscopy, optical sensing, or electromagnetic navigation bronchoscopy [Citation22,Citation69–72], to navigate for placement adjacent to peripheral lesions that are <3 cm in diameter and not easily accessible to percutaneous devices [Citation73,Citation74].
While this preliminary work forms the basis of determining feasibility and indications of the potential advantages of this endobronchial ultrasound ablation approach, several simplifications and approximations were incorporated into the simulations. To minimize the computational cost and complexity, simplified plane-wave propagation expressions and tissue model geometries and interfaces were employed in the parametric study as described Equations (S1)–(S6) in Supplemental materials. Despite these simplifications, the parametric simulations generated similar maximum temperatures (75 °C versus 75–80 °C) and ablative margins (both with 3–5 mm) with the patient anatomic simulations, which incorporated more sophisticated acoustic modeling methods and complex tumor and tissue geometries. Additionally, the patient-specific models developed herein only incorporated lung bronchi with diameter >2 mm, and did not include major blood vessels and vasculature, which would act as local heat sinks to reduce the target temperatures and modulate temperature distributions, depending on their proximity to the applicator and target tumor [Citation75,Citation76]. Considerations of extended clinically relevant structures (e.g., ribs, blood vessels, major nerves or heart) or additional patient-specific anatomical geometries may provide further insights into device design and treatment strategies. Furthermore, only solid tumors with uniform acoustic and thermal properties were modeled to broadly represent nodular NSCLC or metastatic disease. In practice, the tumor may be more diffuse or infiltrating, and tumor composition and heterogeneity may be significantly different depending on the exact type of disease or metastasis present. For example, necrotic tumors may have lower attenuation and blood perfusion, whereas tumors such as pulmonary metastases of osteosarcomas may have areas of higher attenuation around calcifications and heterogeneous blood flow, variations which would significantly affect acoustic propagation and thermal distributions. Slight variation in tumor tissue properties from the nominal values employed herein are not expected to significantly alter results for tumors attached to the airways, due to the thermal dominance of the tumor-inflated lung boundary, but may be more impactful in flooded lung conditions where preferential tumor heating relies on its higher attenuation and absorption relative to flooded lung. In consideration of future work to enhance modeling accuracy, additional measurements of the acoustic and thermal properties of human lung and tumors/metastases could be quantified and incorporated, including dynamic changes to thermal properties [Citation77], as well as to speed of sound and density [Citation49,Citation66]. Future work may also focus on specific device development, applying more detailed theoretical analyses of design and treatment delivery strategies, along with experimental device fabrication and testing on the bench and studies in lung tissue in vivo.
Toward completion of device development and implementation, we envision deep lung catheter-based ultrasound devices (∼2 mm OD) deployed through the working channel of a flexible bronchoscope, and positioned at the target site under real-time steering and guidance using CT-based electromagnetic navigation methods typical of radial endobronchial ultrasonography in peripheral lung [Citation78]. The ultrasound ablation can be performed once the water-coupling balloon is inflated, and at completion the device can be deflated and withdrawn. Similarly, flexible or rigid video bronchoscopes with larger bores can facilitate placement of larger ultrasound devices (5–10 mm OD) in the larger upper airways, following similar techniques currently applied for interventions ablating obstructing tumors in the trachea and bronchus. These procedures could be via trans-nasal or oral bronchoscopic introduction, while under procedural sedation or general anesthesia, following approaches common to interventional pulmonology [Citation78]. Post-ablation monitoring and evaluation could be performed using CT, PET, and/or MRI based imaging techniques [Citation79].
Conclusion
In summary, theoretical simulations were performed to evaluate the feasibility and prospective performance characteristics of endobronchial ultrasound for thermal treatment of lung tumors directly attached to or closely adjacent to bronchi. Various sizes of endobronchial ultrasound applicators were proposed, with larger device configurations integrating planar or tubular transducers and 10–12 mm OD expandable cooling balloons demonstrated as capable of thermally coagulating ∼3 cm diameter tumors adjacent to major airways. Smaller diameter endobronchial devices (2.5–5 mm balloon OD), have potential to thermally coagulate up to 2–3 cm diameter tumors within deep lung with 3–5 mm margin. Lung flooding was also shown to enhance acoustic access to and enable ablation of tumors in close proximity (up to 2 cm away) but not attached to the airways.
Supplemental Material
Download PDF (223.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68(6):394–424.
- Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue – is microwave not just for popcorn anymore? Radiology. 2009;251(3):617–618.
- Powell JW, Dexter E, Scalzetti EM, et al. Treatment advances for medically inoperable non-small-cell lung cancer: emphasis on prospective trials. Lancet Oncol. 2009;10(9):885–894.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205.
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311.
- Prezzano KM, Ma SJ, Hermann GM, et al. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol. 2019;10(1):14.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non–small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682.
- Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260(3):633–655.
- Palussière J, Catena V, Buy X. Percutaneous thermal ablation of lung tumors – Radiofrequency, microwave and cryotherapy: where are we going? Diagn Interven Imaging. 2017;98(9):619–625.
- Hartley-Blossom Z, Healey T. Percutaneous thermal ablation for lung cancer: an update. Surg Technol Int. 2019;34:359–364.
- Healey TT, Dupuy DE. Radiofrequency ablation: a safe and effective treatment in nonoperative patients with early-stage lung cancer. Cancer J. 2011;17(1):33–37.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879.
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705–711.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261(2):643–651.
- Smith S, Jennings P. Lung radiofrequency and microwave ablation: a review of indications, techniques and post-procedural imaging appearances. Br J Radiol. 2015;88(1046):20140598.
- Smith S, Jennings P. Thoracic intervention and surgery to cure lung cancer: image-guided thermal ablation in primary lung cancer. J R Soc Med. 2019;112(6):218–225.
- Gillams A, Lees W. Analysis of the factors associated with radiofrequency ablation-induced pneumothorax. Clin Radiol. 2007;62(7):639–644.
- Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med. 2012;117(2):201–213.
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241(1):275–283.
- Sherwood JT, Brock MV. Lung cancer: new surgical approaches. Respirology. 2007;12(3):326–332.
- Eberhardt R, Kahn N, Herth FJ. Heat and destroy': bronchoscopic-guided therapy of peripheral lung lesions. Respiration. 2010;79(4):265–273.
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest. 2010;137(4):890–897.
- Harris K, Puchalski J, Sterman D. Recent advances in bronchoscopic treatment of peripheral lung cancers. Chest. 2017;151(3):674–685.
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest. 2005;127(6):2007–2014.
- Beeson J. Palliation of tracheobronchial carcinoma: the role of cryosurgery. J Perioper Practice. 2007;17(7):332–339.
- Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treatment Rev. 2012;38(5):346–353.
- Saccomandi P, Lapergola A, Longo F, et al. Thermal ablation of pancreatic cancer: a systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35(1):398–418.
- Ellens NP, Partanen A. Preclinical MRI-guided focused ultrasound: a review of systems and current practices. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2017;64(1):291–305.
- Salgaonkar VA, Diederich CJ. Catheter-based ultrasound technology for image-guided thermal therapy: current technology and applications. Int J Hyperthermia. 2015;31(2):203–215.
- Sommer G, Pauly KB, Holbrook A, et al. Applicators for magnetic resonance-guided ultrasonic ablation of benign prostatic hyperplasia. Invest Radiol. 2013;48(6):387–394.
- Chopra R, Colquhoun A, Burtnyk M, et al. MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: initial feasibility in humans. Radiology. 2012;265(1):303–313.
- Chaussy CG, Thüroff S. High-intensity focused ultrasound for the treatment of prostate cancer: a review. J Endourol. 2017;31(S1):S-30–S-37.
- Melodelima D, Prat F, Fritsch J, et al. Treatment of esophageal tumors using high intensity intraluminal ultrasound: first clinical results. J Transl Med. 2008;6(1):28.
- Lafon C, Theillere Y, Prat F, et al. Development of an interstitial ultrasound applicator for endoscopic procedures: animal experimentation. Ultrasound Med Biol. 2000;26(4):669–675.
- Adams MS, Salgaonkar VA, Plata‐Camargo J, et al. Endoluminal ultrasound applicators for MR‐guided thermal ablation of pancreatic tumors: preliminary design and evaluation in a porcine pancreas model. Med Phys. 2016;43(7):4184–4197.
- Lesser TG, Schubert H, Bischoff S, et al. Lung flooding enables efficient lung sonography and tumour imaging in human ex vivo and porcine in vivo lung cancer model. Eur J Med Res. 2013;18(1):23.
- Wolfram F, Boltze C, Schubert H, et al. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res. 2014;19(1):1.
- Wolfram F, Lesser TG. A simulation study of the HIFU ablation process on lung tumours, showing consequences of atypical acoustic properties in flooded lung. Z Med Phys. 2019; 29(1):49–58.
- Wolfram F, Reichenbach JR, Lesser TG. An ex vivo human lung model for ultrasound-guided high-intensity focused ultrasound therapy using lung flooding. Ultrasound Med Biol. 2014;40(3):496–503.
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia. 2009;25(1):3–20.
- Dewhirst MW, Viglianti B, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343.
- Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia. 2005;21(8):745–753.
- Duck FA. Physical properties of tissues: a comprehensive reference book. London: Academic Press; 2013.
- Mcintosh RL, Anderson V. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys Rev Lett.. 2010;05 (03):129–151.
- Dunn F. Attenuation and speed of ultrasound in lung: dependence upon frequency and inflation. J Acoust Soc Am. 1986;80(4):1248–1250.
- Holmes KR, Chen MM. Local thermal conductivity of Para-7 fibrosarcoma in hamster. Adv Bioengin. 1979;4:147–149.
- Giering K, Lamprecht I, Minet O, et al. Determination of the specific heat capacity of healthy and tumorous human tissue. Thermochim Acta. 1995;251:199–205.
- Matsuba K, Thurlbeck WM. A morphometric study of bronchial and bronchiolar walls in children. Am Rev Respir Dis. 1972;105(6):908–913.
- Wu C-H, Lindsey DC, Traber DL, et al. Measurement of bronchial blood flow with radioactive microspheres in awake sheep. J Appl Physiol. 1988;65(3):1131–1139.
- Williams L, Leggett R. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas. 1989;10(3):187.
- Prakash P, Salgaonkar VA, Clif Burdette E, et al. Multiple applicator hepatic ablation with interstitial ultrasound devices: theoretical and experimental investigation. Med Phys. 2012;39(12):7338–7349.
- Ross AB, Diederich CJ, Nau WH, et al. Curvilinear transurethral ultrasound applicator for selective prostate thermal therapy. Med Phys. 2005;32(6Part1):1555–1565.
- Prakash P, Salgaonkar VA, Diederich CJ. Modelling of endoluminal and interstitial ultrasound hyperthermia and thermal ablation: applications for device design, feedback control and treatment planning. Int J Hyperthermia. 2013;29(4):296–307.
- Montaudon M, Desbarats P, Berger P, et al. Assessment of bronchial wall thickness and lumen diameter in human adults using multi‐detector computed tomography: comparison with theoretical models. J Anatomy. 2007;211(5):579–588.
- Hartman C, Child S, Penney DP, et al. Ultrasonic heating of lung tissue. J Acoust Soc Am. 1992;91(1):513–516.
- Moros EG, Fan X, Straube WL. Ultrasound power deposition model for the chest wall. Ultrasound Med Biol. 1999;25(8):1275–1287.
- Mikhak Z, Pedersen PC. Acoustic attenuation properties of the lung: an open question. Ultrasound Med Biol. 2002;28(9):1209–1216.
- Nardelli P, Khan KA, Corvò A, et al. Optimizing parameters of an open-source airway segmentation algorithm using different CT images. BioMed Eng OnLine.. 2015;14(1):62.
- Bauld T, Schwan H. Attenuation and reflection of ultrasound in canine lung tissue. The J Acoust Soc Am. 1974;56(5):1630–1637.
- Pedersen PC, Ozcan HS. Ultrasound properties of lung tissue and their measurements. Ultrasound Med Biol. 1986;12(6):483–499.
- Oelze ML, Miller RJ, Blue JP, Jr, et al. Estimation of the acoustic impedance of lung versus level of inflation for different species and ages of animals. J Acoust Soc Am. 2008;124(4):2340–2352.
- Oelze ML, Miller RJ, Blue JP, Jr, et al. Impedance measurements of ex vivo rat lung at different volumes of inflation. J Acoust Soc Am. 2003;114(6):3384–3393.
- Lesh MD, Guerra P, Roithinger FX, et al. Novel catheter technology for ablative cure of atrial fibrillation. J Intervent Cardiac Electrophysiol. 2000;4(1suppl):127–139.
- Torii S, Mori H, Jinnouchi H, et al. Renal denervation with ultrasound therapy (paradise device) is an effective therapy for systemic hypertension. J Thorac Dis. 2018;10(S26):S3060.
- Spliethoff JW, Evers DJ, Klomp HM, et al. Improved identification of peripheral lung tumors by using diffuse reflectance and fluorescence spectroscopy. Lung Cancer. 2013;80(2):165–171.
- Tan K, Shishkov M, Chee A, et al. Flexible transbronchial optical frequency domain imaging smart needle for biopsy guidance. Biomed Opt Express. 2012;3(8):1947–1954.
- Xie F, Zheng X, Xiao B, et al. Navigation bronchoscopy-guided radiofrequency ablation for nonsurgical peripheral pulmonary tumors. Respiration. 2017;94(3):293–298.
- Santos RS, Gupta A, Ebright MI, et al. Electromagnetic navigation to aid radiofrequency ablation and biopsy of lung tumors. Annals Thoracic Surg. 2010;89(1):265–268.
- Horeweg N, Van Der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187(8):848–854.
- Gilbert C, Akulian J, Ortiz R, et al. Novel bronchoscopic strategies for the diagnosis of peripheral lung lesions: present techniques and future directions. Respirology. 2014;19(5):636–644.
- Steinke K, Haghighi KS, Wulf S, et al. Effect of vessel diameter on the creation of ovine lung radiofrequency lesions in vivo: preliminary results. J Surg Res. 2005;124(1):85–91.
- Iguchi T, Hiraki T, Gobara H, et al. Percutaneous radiofrequency ablation of lung tumors close to the heart or aorta: evaluation of safety and effectiveness. J Vasc Intervent Radiol. 2007;18(6):733–740.
- Prakash P, Diederich CJ. Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: implications for treatment planning, monitoring and control. Int J Hyperthermia. 2012;28(1):69–86.
- Vieira T, Stern J-B, Girard P, et al. Endobronchial treatment of peripheral tumors: ongoing development and perspectives. J Thorac Dis. 2018;10(S10):S1163.
- Rasmussen F, Madsen H. Imaging follow-up of RF ablation of lung tumours. Cancer Imaging. 2011;11(1A):S123.
