 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: To evaluate the feasibility and assess safety parameters of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-mediated hyperthermia (HT; heating to 40–45 °C) in various pelvic targets in a porcine model in vivo.
Methods: Thirteen HT treatments were performed in six pigs with a commercial MRgHIFU system (Sonalleve V2, Profound Medical Inc., Mississauga, Canada) to muscle adjacent to the ventral/dorsal bladder wall and uterus to administer 42 °C (±1°) for 30 min (±5%) using an 18-mm target diameter and 100 W power. Feasibility was assessed using accuracy, uniformity, and MR-thermometry performance-based metrics. Safety parameters were assessed for tissues in the targets and beam-path by contrast-enhanced MRI, gross-pathology and histopathology.
Results: Across all HT sessions, the mean difference between average temperature (Tavg) and the target temperature within the target region-of-interest (tROI, the cross-section of the heated volume at focal depth) was 0.51 ± 0.33 °C. Within the tROI, the temperature standard deviation averaged 1.55 ± 0.31 °C, the average 30-min Tavg variation was 0.80 ± 0.17 °C, and the maximum difference between Tavg and the 10th- or 90th-percentile temperature averaged 2.01 ± 0.44 °C. The average time to reach ≥41 °C and cool to ≤40 °C within the tROI at the beginning and end of treatment was 47.25 ± 27.47 s and 66.37 ± 62.68 s, respectively. Compared to unheated controls, no abnormally-perfused tissue or permanent damage was evident in the MR images, gross pathology or histological analysis.
Conclusions: MRgHIFU-mediated HT is feasible and safety assessment is satisfactory for treating an array of clinically-mimicking pelvic geometries in a porcine model in vivo, implying the technique may have utility in treating pelvic targets in human patients.
Introduction
Mild hyperthermia (HT), which refers to increasing the temperature to 40–45 °C for an extended period of time up to 1.5 h, has been reported to be one of the most effective sensitizers for radiation therapy (RT) and/or chemotherapy [Citation1]. HT has been shown to inhibit the DNA repair, improve oxygenation, enhance the delivery of antineoplastic drugs [Citation2], and induce anti-tumor immunity [Citation3,Citation4]. Several technologies have been developed to administer HT for cancer treatment [Citation5–13]. In pelvic cancers, the addition of HT to RT can increase complete response rates (CRRs), partial response rates (PRRs) and improve overall survival [Citation3,Citation14,Citation15]. For example, a prospective, randomized, multicenter trial reported increased CRRs of 26% (p = .003), 22% (p = .01) and 6% (p> .05) in the treatment of cervical cancer (n = 114), bladder cancer (n = 101) and rectal cancer (n = 143) [Citation16], respectively. Such results showed the addition of HT to RT resulted in a significant enhancement of treatment efficacy. Other studies showing pelvic cancer treatment sites that could benefit from adding HT to an RT treatment regimen include bladder cancer [Citation16–18], rectal and colon cancer [Citation15,Citation19], uterine cancers (including both uterine cervix and uterine corpus)[Citation20–23] and prostate cancer [Citation24].
Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) can potentially be used to administer noninvasive, actively monitored, volumetric HT treatments using MR thermometry and feedback control [Citation25]. However, performing HT to pelvic targets within a heterogeneous space containing moving organs, moving fluids and a variety of tissues may be technically challenging due to the motion, the inhomogeneity in thermal property of tissues in the pelvic space, the required narrow temperature range and/or the extended duration of HT. Previous studies have demonstrated that a commercial, table-mounted MRgHIFU system (Sonalleve V2, Profound Medical Inc., Mississauga, Canada) has the ability to uniformly heat deep thigh muscle tissue and the muscle near the rectum in an in vivo porcine model [Citation26,Citation27]. However, these published studies focused only on extremities with limited motion (e.g., thigh muscle) or very specific geometries (e.g., muscle near the lower gastrointestinal tract), neither of which is fully representative of the spectrum of geometrical challenges that might be present in pelvic MRgHIFU treatment. Furthermore, the lack of histological analysis in previous studies limits the scope of the safety evaluations of HT induced by MRgHIFU techniques. As such, these studies were unable to provide evidence of any damage – or lack thereof – that HT may induce at the cellular level.
The purpose of this study was to evaluate the feasibility and safety parameters of using MRgHIFU to deliver HT to an array of different tissue geometries in the pelvis. These geometries were selected to represent a broader range of prospective HT treatment sites compared to previous studies. As such, this study aims to build on previously published work to enhance opportunities for the use of MRgHIFU-mediated HT in treating a wider array of pelvic malignancies than is done in current studies and/or practices. To achieve this, a set of representative pelvic targets were heated in an in vivo porcine model. Here, ‘representative’ is meant to reflect both the geometry of target locations and organs-at-risk (OARs) adjacent to treatment targets in or near the beam path that may be encountered in a variety of pelvic MRgHIFU HT treatments, as well as some challenges to successful therapy, such as organ motion during treatment (examples include peristalsis and bladder filling, among others). The accuracy, precision, temporal variation, heating uniformity, thermal dose, and heating time for each target were analyzed and compared in order to evaluate the feasibility of MRgHIFU-mediated HT in various pelvic geometries. Imaging evaluation, gross pathology and histological analysis were performed as a measurement of the safety profile of MRgHIFU-induced HT.
Materials and methods
Target site selection
Three targets were selected as representative treatment sites for pelvic HT therapy: the muscle adjacent to the ventral wall of the urinary bladder (MVB), the muscle adjacent to the dorsal wall of the urinary bladder (MDB), and the uterus. These sites were selected to mimic treatment geometries in terms of positions of the target relative to nearby OARs as well as prospective HIFU beam paths for an array of treatment sites including, but not limited to, those found in genitourinary cancers (e.g., bladder, ovarian, cervical, prostate, etc.), gastrointestinal cancers (e.g., anal, colorectal, etc.) and pelvic sarcomas. The selection of treatment sites was intended to be diverse while being within experimental constraints imposed by the size, weight, age and pelvic geometry of the pig model, as well as the limitations of the current configuration of the MRgHIFU system such as the maximum focal depth of 8 cm.
Each of these targets presents a unique set of conditions to consider. The MVB is a thin, relatively superficial muscle layer situated between adipose tissue and the urinary bladder, while the MDB, also a thin muscle layer, is deeper and requires traversing the bladder to reach it. As the urinary bladder is a fluid-filled organ with variable volume, there is potential for MR thermometry artifacts caused by the urine filling and organ motion, requiring additional considerations when planning accurate thermal treatment [Citation28]. The uterus presents a different tissue type than the muscle layers, one that not only may be in or near a number of HT targets in women, but also tissues whose rich blood flow could potentially convect heat more easily compared to muscle layers adjacent to the urinary bladder.
Animal protocol
MRgHIFU induced HT was performed to the MVB, MDB and uterus in six female pigs (57.58 ± 11.17 kg). Pigs either received one (N = 1), two (N = 3) or three (N = 2) HT sessions on a given day. For multiple treatments on the same pig, a different location was selected for each treatment such that repeated heating to the same location was avoided. For multiple HT treatments on the same animal, the time interval between each HT session ranged from 17 to 67 min with a median of 24 min. Animal experiments were carried out with approval from the Institutional Animal Care and Use Committee and the Division of Comparative Medicine at Washington University School of Medicine in St. Louis. Before every experiment (or prior to the intubation) the animal was pre-sedated with an intramuscular injection of ketamine/xylazine cocktails including ketamine (2 mg/kg), xylazine (2 mg/kg) and telazol (4 mg/kg). Each animal was then intubated and ventilated. General anesthesia was maintained using isoflurane at a concentration of 1 -5%, with forced ventilation generally at a tidal volume of 10 ml/kg. In order to ensure acoustic propagation through the skin, the lower abdomen, the groin strain and the regions in proximity were carefully shaved with any remaining hair removed using depilatory cream (Nair, Church & Dwight Co., Princeton, NJ). A bardex lubricath foley catheter (SureStep™ Foley Tray System, Bard, Inc., Murray Hill, NJ) was used to drain the bladder prior to any MDB-targeting HT session, making the MDB more accessible when the MDB was deeper than the 8 cm Sonalleve treatment depth limit and the bladder was in the beam path. The bladder was not actively drained during any treatment session.
Experimental design
The Sonalleve V2 system was used for ultrasound exposures [Citation29,Citation30]. In HT treatment mode, the system is comprised a patient table locked into the bore of an MRI scanner (Ingenia 1.5T, Philips, Best, the Netherlands). The table contains a HIFU transducer immersed in an oil bath. The system is equipped with a 256-channel phase-array transducer with a radius of curvature of 70-mm. At a frequency of 1 MHz and pressure of −6 dB, the length and width of the focal point are 14.65 and 1.90 mm, respectively. The acoustic window on the patient table is sealed with a thin Mylar membrane and contains a 2-channel RF receive coil.
The anesthetized pigs were placed in a prone position on the MRgHIFU table. A 3-channel pelvic RF receive coil was secured over the targeted anatomy. To acoustically couple the animals’ skin with the ultrasound transducer, gel pads (Parker Laboratories Inc., Fairfield, NJ) shaved into a reciprocal shape with the morphology of the animals’ skin were used when necessary. A 2:1 homogenous mixture of ultrasound gel (Aquasonic, Parker Laboratories Inc., Fairfield, NJ) and degassed water was slowly placed between the animal’s skin, the acoustic window and the gel pad to create a relatively homogeneous acoustic path and minimize the potential for skin burns due to air bubbles in the beam path.
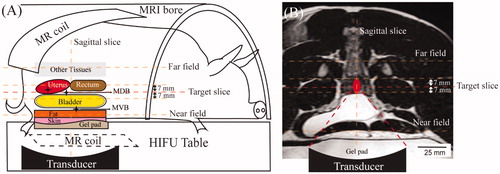
HT treatment planning was done using T2-weighted MRI [Citation27,Citation30] with the following parameters: echo time = 130 ms, repetition time = 1300 ms, number of signal averages = 1, spatial resolution (in-plane pixel size)=1.12 × 1.19 × 2.5 mm3, and flip angle = 90°. MRgHIFU-mediated HT was monitored with MRI thermometry using the proton resonance frequency shift (PRFS) method [Citation26,Citation30,Citation31]. For the temperature monitoring by MR thermometry, a dynamic, RF-spoiled, fast-field-echo echo-planar imaging (FFE-EPI) sequence was utilized to obtain magnitude and phase images to produce temperature maps. The FFE-EPI images used the following parameters: echo time = 19 ms, repetition time = 36 ms, flip angle = 20°, number of signal averages = 1, EPI factor = 11, voxel size = 2.5 × 2.5 × 7 mm3, and temporal resolution = 3.7 s. Six FFE-EPI planes (5 coronal, 1 sagittal) are used by the Sonalleve system for temperature monitoring, as illustrated in . In , the red dashed line represents the ‘target’ slice (the centermost portion of the focal region), and the other lines show the other monitoring planes: one slice each 7-mm distal and proximal to the target slice, a sagittal slice along the beam path perpendicular to the target slice, one near field slice located at the gel pad or the subcutaneous muscle layer and one far field placed beyond the focal plane. presents the location of the transducer, ultrasound beam path and temperature monitoring slices overlayed on the coronal MR images.
Figure 1. (A) Illustration of the experimental setup showing the animal’s position, MR thermometry slices, locations of MR coils, gel pad, and treatment organs. Stars indicate the three target areas: MVB (muscle adjacent to the ventral bladder wall), MDB (muscle adjacent to the dorsal bladder wall), and uterus. (B) Coronal slice of the MR images overlays with the schematic illustration of the beam path and the transducer in a HT session in the treatment of the uterus as an example.
In advance of performing HT in each pig, the MR thermometry sequence was run on an MRgHIFU commercially-available quality assurance phantom (Philips, Vantaa, Finland) for 5–10 min with the purpose to maintain the magnet hardware to a thermal-stable condition [Citation32]. All HT treatments were administered using a target region (cell) of 18-mm cross-sectional diameter – the smallest HT treatment cell size available for the Sonalleve system. This cell size of 18-mm was the only one used in this study as the tissue targets in the pigs were small (pig size was limited by size and weight constraints of what we could accommodate with our equipment and experimental setup) as well as the desire to limit heating to other non-target nearby structures in the pig pelvis. For all sonications, continuous ultrasound wave with a frequency of 1.0 MHz and acoustic power of 100 W was utilized. The output spatial-peak intensity of the system was 4321.34 W/cm2, calculated based on a validated method [Citation33]. The average derated maximum intensity in situ was 3572.78 W/cm2, estimated based on the tissue types in the beam path and the depth of the targets of all HT targets [Citation34]. The acoustic power of 100 W was selected based on its use in a previous publication [Citation27], as well as our intent to employ it in a subsequent clinical trial. The target temperature at the centermost slice was set at 42 °C with a sonication duration of 30 min (note: one HT session only lasted for 28 min due to MRI system failure). The target temperature and duration were chosen to represent clinical hyperthermia objectives.
HT was controlled by a temperature feedback algorithm developed by Tillander et al. [Citation27]. Briefly, the HT cells contain a predefined set of foci on concentric circles with diameters of 4, 8, 12 and 16-mm in the plane perpendicular to the beam path. The heating at each focus is controlled by adjusting the sonication power. The feedback algorithm also incorporates a maximum threshold temperature to prevent temperature overshoot. The threshold temperature was set to 44 °C at the near field slice in this study. During heating, if any points in the target plane show a temperature above the target temperature (here, 42 °C), or if any pixel in the beam path cross-section in the near field exceeded the maximum temperature threshold (here, 44 °C), the sonication power was to zero until the points fall back under the respective threshold; the heating then resumes at 100 W power. The near field slice was chosen for the maximum temperature check due to its proximity to the target (thus being representative of nearby non-target tissue). The use of the 44 °C threshold was a safety measure allowing for reasonably strict temperature control. It was motivated by the fact that temperatures ≥45 °C have been shown to cause thermal damage within minutes during HT treatments [Citation35] and Chu et al. [Citation26] found that when using a maximum temperature threshold of 45 °C with the Sonalleve, thermal-induced muscle coagulation occurred.
The temperature drift, due to magnetic field, phase changes or susceptibility changes, was corrected by the Sonalleve system through well-established methods described by Bing et al. [Citation32], which employs a second-order drift correction algorithm. In Bing et al. [Citation32] study, a temperature consistency compared to expected levels of 0.57 ± 0.58 °C and 0.54 ± 0.42 °C was reported in the heated and unheated region, respectively. To validate the functionality of this drift correction, our group performed a volunteer study using the Sonalleve [Citation30], in which temperatures were monitored in unheated subjects. In human leg muscles over the course of 60 min, measured body temperature changed by 0.49 ± 0.03 °C.
Analysis of HT feasibility
Thirteen HT sessions were conducted in vivo in the MVB (seven sessions), the MDB (three sessions) and the uterus (three sessions) in six different pigs. Temperature maps were processed using methods described by Kothapalli et al. [Citation30]. Data from all 13 sessions were analyzed using the temperatures acquired by MR thermometry. The accuracy of the MR temperature mapping has been assessed by comparison between fiberoptic temperature sensors and the Sonalleve-reported temperatures and deemed reasonable for clinical use (i.e., being within 1 °C of each other) [Citation27]. These results were verified on the system used in this study using a similar technique (data not shown). For the acquired FFE-EPI images processed offline, the IDL 6.1 (ITT Visual Information Solutions, Boulder, CO) was utilized, which implemented the magnetic field drift-correction algorithm described by Bing et al. [Citation32]. Temperature distributions within the target region-of-interest, or tROI (with an 18-mm diameter) in the centermost slice were evaluated and a number of factors tabulated: spatial mean temperature (Tavg), the maximum temperature of all the pixels within the tROI (Tmax), the minimum temperature of all the pixels within the tROI (Tmin), the temperature that only 10% of the pixels reached (T10), the temperature that 90% of the pixels reached (T90) and the spatial standard deviation of all the pixels within the tROI (σT). Each parameter was first tabulated in a given ‘dynamic’, i.e., one of the temperature maps acquired every 3.7 s, then averaged across all dynamics. For example, if Tavg,d is the average tROI temperature in a given dynamic, then Tavg is the average of all Tavg,d values for a specific dataset. Tavg values were also calculated for the cross-section of the near field slice along the beam path for safety monitoring purposes. A standardized thermal parameter, cumulative equivalent minutes at 43 °C of T90 (CEM43T90), that was found to be correlated to local benefit when HT was combined with RT in human patients [Citation36], was also calculated.
Four parameters were then used to quantify and assess the temperature in the tROI: temperature mapping accuracy, precision, temporal variation and heating uniformity. The accuracy was quantified as the difference between Tavg and the desired temperature of 42 °C. The precision, a measure of spatial temperature variability across the tROI, was assessed by σT. The temporal variation in a given dataset was calculated as the standard deviation of the Tavg,d values across all dynamics. The heating uniformity was calculated by the temporal average of the largest difference between the Tavg,d and either the T10,d or T90,d. These temperature metrics were selected due to their previous use in the literature [Citation26,Citation30] to allow for benchmarking of our data against other studies. Analyses were performed using GraphPad Prism version 8.0.1 (La Jolla, CA). The Mann–Whitney U test was used to determine whether there was a significant statistical difference (<.05) between different target sites.
For patient and treatment management purposes, it is important to assess both how quickly the temperature reaches the target therapeutic range after the initiation of sonication and how rapidly the temperature falls out of this temperature range after the sonication ceases. Thus, in order to compare the efficiency of the heating deposition or dissipation in the different target pelvic organs, the time required for the Tavg in the tROI to reach ≥41 °C after the start of sonication and cool down to ≤40 °C after the termination of sonication was also recorded. As more heat dissipation was expected when targeting relatively deeper organs, the one-tailed Mann–Whitney U test was used to assess significant statistical differences (<.05) between the times needed for heat deposition between different organs.
HT safety parameter measurement
Safety parameters of the HT treatments were measured by assessing thermal damage through three different techniques: contrast-enhanced MRI, gross pathology and hematoxylin and eosin (H&E) stain histological analysis. Negative controls for the HT were benchmarked using untreated tissue from similar structures as the target. In order to provide observable references during necropsy to triangulate the locations of the HT treatment targets, as well as provide a permanent tissue damage site for comparative evaluation of HT targets, ultrasound ablations to the skin layer, subcutaneous tissue, the skeletal muscle or the uterus were performed after the conclusion of HT. These ablation lesions were used as ‘positive controls’ for heating and damage assessment because they provided benchmarks for comparison of HT targets from the aspects of both temperature profile and histopathological changes. These positive controls, along with the unheated negative control tissues would allow for the clearest comparative illustration of the relative state of the HT target tissue against the two treatment extremes (permanent, destructive heat damage vs. no heating). The parameters of the ablation sonication were: 200-W power, 1.2-MHz frequency, 24-s duration and 8-mm diameter cell in the target coronal plane.
For the contrast-enhanced MRI assessment of potential thermal damage, two T1-weighted 3D high-resolution isotropic volume excitation (THRIVE) sequences [Citation27] were acquired before and after the injection of gadobenate dimeglumine (MultiHance; Bracco Imaging, Milan, Italy) about 1–2 min after the completion of the ultrasound ablation. In these images, thermal coagulation appeared as non-perfused areas. After completion of MRI, the animals were euthanized, and macroscopic dissection was performed close to the HT and ablation targets. The anatomical structure characters, the location of the ablated lesions, along with the MR images, were used as landmarks to identify the locations of the HT targets. Gross pathological analysis at the HT target sites was performed.
After euthanasia, tissues (1) within the HT targets, (2) within ablation targets (for use as positive controls), (3) within ultrasound beam path and (4) far from the treatment locations (for use as non-heated negative controls) were identified and collected for the H&E analysis. The skin and muscle ablation lesions were used, along with imaging, as landmarks to locate the HT lesions during necropsy. Tissue types collected for HT-targets included ventral and dorsal bladder tissue adjacent to MVB and MDB (for safety evaluation of MVB and MDB), uterus and ventral abdominal wall musculature overlay with the urinary bladder. Tissues collected as positive controls included the ablated muscle, uterus, and skin/subcutaneous tissue. Tissue types collected within the ultrasound beam path included skin, subcutaneous fat, muscle proximal to the HT targets (i.e., representative of the near field), and muscle and colon tissue distal to the HT targets (i.e., representative of the far field). Tissues collected as negative controls included the skin, subcutaneous fat, uterus, bladder tissue, muscle tissue, and colon/rectum tissue outside the beam path and far from the sonication targets. Tissues collected for histopathology were fixed in 10% neutral buffered formalin. After fixation, the tissues were trimmed, processed according to standard procedures, paraffin-embedded and sectioned at 5-micron thickness. The sections were stained with H&E for standard histopathologic evaluation. For each lesion, 2–3 sections were examined. Tissue processing, staining and histopathologic examination were performed by the Division of Comparative Medicine Animal Diagnostic Laboratory.
Results
HT feasibility evaluation
Representative examples of HT treatment and delivery process for each target site (MVB, MDB and uterus) are shown in . tROIs are shown on the planning MRIs in . shows the voxel-by-voxel Tavg maps in the target slice. shows temperature as a function of time for Tavg, Tmax and Tmin corresponding to the treatment in . Note the stability of the temperature parameters throughout the entire 30-min HT session in ; this stability was also evident in all other analyzed treatments. A summary of the temporal-average evaluation statistics in the tROI for all HT sessions (Tavg, Tmax, Tmin, T10, T90, σT and CEM43T90) is presented in , as well as Tavg within the beam cross-section in the near field slice. Tavg in the target slice was 41.6 ± 0.4 °C, 41.6 ± 0.3 °C and 41.4 ± 0.2 °C for MVB, MDB and uterus, respectively, i.e., within 1.0 °C of the desired temperature (). In the near field, the Tavg was 38.1 ± 0.9 °C, 37.3 ± 0.4 °C, 37.5 ± 0.4 °C for MVB, MDB and uterus, respectively (). Tmax, T90 and T10 of the near field are also presented in . For the thermal ablation used for the positive control of the study, after ablation for 24 s, the maximum achieved temperature had an average of 95.22 °C ± 25.43 °C (range: 69.1–154.9 °C).
Figure 2. (A) Illustration of the target region-of-interest selection indicated by the red circle on the axial MRIs (target slice) for three different treatment locations. From left to right: muscle adjacent to the ventral wall of the urinary bladder (MVB), muscle adjacent to the dorsal wall of the urinary bladder (MDB), and the uterus. (B) The representative average temperature map of the axial plane (target slice) of the three pelvic targets. (C) The representative average temperature (Tavg), maximum temperature (Tmax), and minimum temperature (Tmin) during the 30-min HT session and the 5-min cooling periods.
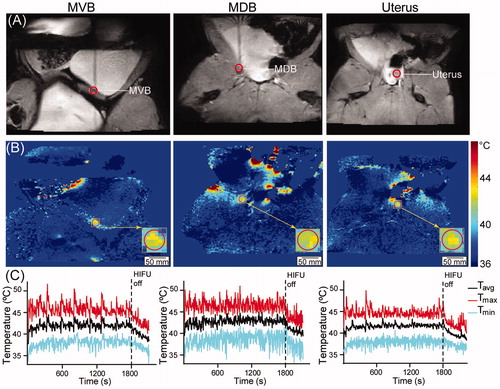
Table 1. Temperature statistics in the target region-of-interest (tROI) within the target slice and the beam cross-section in the near field.
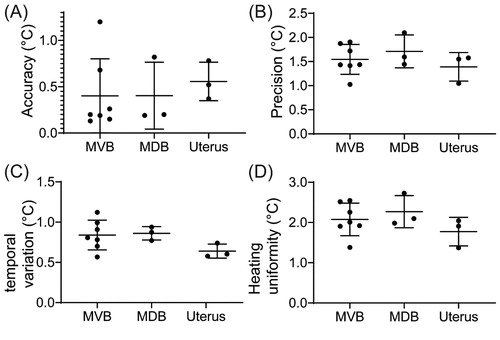
shows the calculated temperature accuracy (A), precision (B), temporal variation (C) and heating uniformity (D) within the target. Across all cases, an average accuracy of 0.51 ± 0.33 °C, a precision of 1.55 ± 0.31 °C, a temporal variation of 0.80 ± 0.17 °C and a heating uniformity of 2.01 ± 0.44 °C was achieved. also shows the breakdown of these parameters by treatment sites for the MVB, MDB and the uterus. No statistically significant differences were found in accuracy, precision, temporal variation or heating uniformity when comparing different treatment sites.
Figure 3. Temperature metrics for the three pelvic target region-of-interests in °C. (A) Temperature accuracy. (B) Temperature precision. (C) Temporal variation. (D) Heating uniformity.
The calculated CEM43T90 among different treatment sites is presented in . An average of 0.59 ± 0.31 min CEM43T90 was achieved across all cases. A comparison between the achieved CEM43T90 among three different organs is presented in . The average CEM43T90 values were 0.6 ± 0.3, 0.6 ± 0.4 and 0.5 ± 0.3 min to the MVB, the MDB and the uterus, respectively. No statistically significant differences were observed between the three groups.
Figure 4. (A) The CEM43T90 calculated based on the temperature in each voxel within the target region-of-interest (tROI). (B) Time (s) for the average temperature of the tROI to reach 41 °C after the start of the sonication. (C) Time (s) for the average temperature of the tROI to decrease to 40 °C after the termination of the sonication.
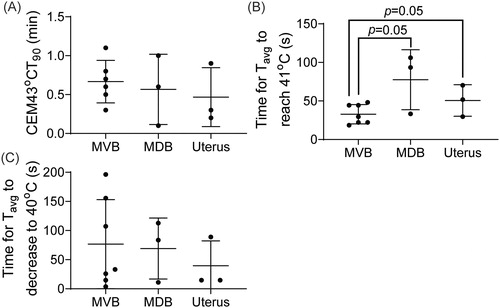
The durations needed for the average temperature within the tROI to increase to ≥41 °C and decrease to ≤40 °C (outside the HT range) are shown in , respectively. The Tavg,d reached ≥41 °C after the start of the sonication in 32.8 ± 12.6, 77.6 ± 38.9 and 50.6 ± 11.8 s for the MVB, MDB and uterus, respectively, with an overall average time of 47.3 ± 26.5 s. Compared to targeting the MVB, the time needed for Tavg.d to increase to ≥41 °C is statistically significantly longer when targeting the deeper MDB and uterus targets (p=.05, ). The average time for the Tavg,d to cool down to ≤40 °C was 76.7 ± 76.5, 69.1 ± 52.3 and 39.5 ± 42.8 s for MVB, MDB and uterus, respectively, with an overall average of 66.4 ± 62.7 s.
HT safety parameter assessment
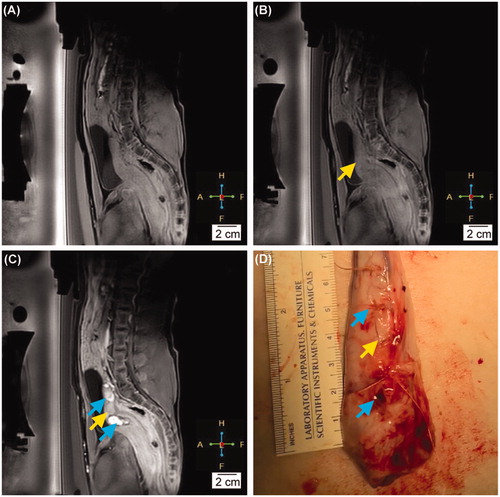
No abnormally-perfused tissue in the HT target region was observed on the post-treatment contrast-enhanced MRIs when compared to the pretreatment images (). This is starkly different than the ablated regions, seen as hyper-intense signals in the MRIs at the sites of the ablative lesions performed after HT sessions (). Similarly, for the gross pathology, no macroscopic lesion was observed at any location that received HT treatment or in the beam path. By contrast, several hemorrhagic regions showing severe edema were observed at the locations that received ablation (e.g., skin and subcutaneous tissue, muscle, and uterus), as shown in .
Figure 5. (A) The contrast-enhanced MRI before any intervention. (B) The contrast-enhanced MRI at the completion of the hyperthermia therapy. The yellow arrow indicates the location of the HT target. (C) The contrast-enhanced MRI right after ablation. The blue arrows indicate the location of the ablation lesions. (D) The gross pathology of the uterus after the completion of HT and ablation. The yellow arrow points to the location of the HT target. The blue arrow indicates the ablation lesions (thermal damage).
presents a classification of the histopathological findings based on the target locations in proximity to 13 targets receiving 30-min HT. Nine targets showed reversible changes expected of mild heat therapy as compared to the control, while four targets showed no notable lesions. None showed any permanent damage. Among the reversible changes, a minimal to mild congestion in the wall of the skeletal muscle adjacent to the urinary bladder wall or uterus was the most frequent phenomenon, occurring in five treatment sessions (). The second most common histological phenomenon, seen in four sessions, was an accumulation of white blood cells composed of mononuclear cells with fewer neutrophils and eosinophils, or a minimal perivascular inflammation in the interstitium of the muscularis of the urinary bladder or the uterus. In two HT targets, there were mild multifocal hemorrhages in the lamina propria or the interstitium of the muscularis of the urinary bladder wall or the uterus. These were presumed artifactual as they were also detected in the control tissue.
Figure 6. Representative H&E images of analyzed tissues. Columns left-to-right are tissues which received HT and/or were within ultrasound beam path, controls, and ablation targets. Rows show samples from different tissue sites: (A) muscle adjacent to the ventral wall of the urinary bladder (MVB), (B), uterus, and (C) skin and subcutaneous tissue. Yellow arrow shows congestion of the vasculature. Blue arrows show edema and the separation of the lamina propria from the epithelium.
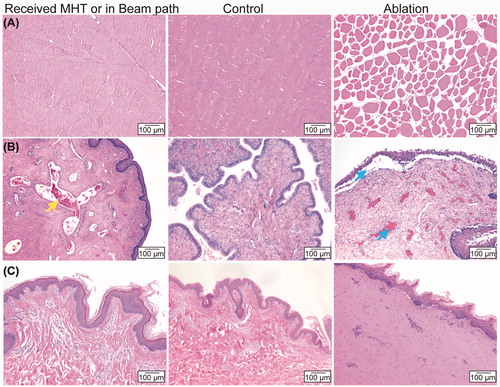
Table 2. Categorization of the post-HT histological analysis based on target locations.
Five samples of ventral abdominal wall musculature overlay with the HT targets in the urinary bladder (when heating MVB) were also analyzed, as shown in . Four showed no notable lesions and one lesion showed a mild separation of myofibers at the cutting edge, suggesting an artifact of tissue processing/sectioning. No notable HT-related lesions were detected for the skin and the subcutaneous fat layer along the ultrasound beam path, or for the colon and/or rectum tissue immediately distal to the target area. Ablation lesions as positive controls were characterized by an array of permanent damage types, including degeneration and necrosis of muscle fibers including hypereosinophilic, homogeneous, swollen fibers with nuclear pyknosis and fiber fragmentation in the skeletal muscle (), variable swelling and homogeneity of fibers and variable nuclear pyknosis in the subcutaneous tissue (). In uterus tissue, separation of the basal layer of the epithelium from the underlying lamina propria in the uterus was also possible, as were severe edema, hemorrhage and congestion (). These results suggest that 30-min HT treatments are able to be successfully performed without creating any detectable irreversible damage in the target tissue, the skin and subcutaneous tissue in the beam path, or the adjacent healthy tissue beyond the target.
Discussion
The results of our study demonstrate that MRgHIFU can feasibly deliver HT to a variety of pelvic target geometries in vivo in a porcine model with satisfactory safety characterization. For each of our HT targets, the average temperature accuracy was <0.8 °C with an overall average accuracy within 0.5 °C of the desired 42 °C target temperature. This agrees well with published guidelines requiring the temperature to be maintained within 1 °C of the target temperature [Citation30,Citation32,Citation37]. The average temperature variation was <1 °C for all three sites demonstrating that the average temperature was fairly stable throughout the 30-min treatment. Both the precision and uniformity measurements show that most voxels in the HT target remained within the hyperthermic temperature region throughout treatment. The lack of observed statistically significant differences in any temperature parameter between sites shows the stability of the therapeutic approach. These results thus display the feasibility of pelvic MRgHIFU HT treatments when using the 18-mm cell size. This could potentially be used to treat small clinical targets in a single treatment or larger targets by clustering multiple 18-mm cell treatments. The safety parameters of the MRgHIFU HT approach are shown to be satisfactory through the fact that the contrast-enhanced image assessments, gross pathology and histopathology showed either no damage or minimal, reversible tissue damage at the HT target sites and tissues in the beam path.
Overall, the assessment results agree well with previous studies that analyzed only specific homogeneous target geometries [Citation26,Citation27], although in certain cases there were some differences. For example, Chu et al. measured a temporal variation of 0.3 ± 0.2 °C in HT sessions of the pig leg muscle [Citation26], somewhat lower than our result. We also had some outliers, such as one MVB heating session that had an accuracy of 1.2 °C. While this could be due to statistical variation, there are a couple of other potential explanations. First, unlike the previous studies in which large areas of homogenous muscle were targeted, our target sites occupied smaller volumes abutting other tissues and organs (e.g., bladder or rectum), despite the fact that we used the same 18-mm diameter for tROI as in these other studies. Here, as there were different types of tissues within the tROI whose varying thermal properties could impact the measured thermometry parameters; this could give rise to some of the observed differences. This impact could be exacerbated by the fairly course spatial resolution (2.5 × 2.5 × 7 mm3) of the temperature monitoring. More tissue heterogeneity both throughout the volume and per voxel could give rise to higher measured thermometry parameters compared to previous studies with more homogenous targets.
Furthermore, motion from breathing, bladder filling and/or digestive peristalsis can create MRI thermometry artifacts that may result in higher temporal variability in the tROI [Citation27,Citation38–40], especially given that the excursion times for these tissues is on the same order of magnitude as the temporal resolution of the MR thermometry images (3.7 s). In comparison to prior studies, although the temporal resolution was the same, the HT targets herein were in closer proximity to sources of artefactual motion (bladder filling, rectum peristalsis, etc.), potentially influencing MRI thermometry. Finally, another difference with previous studies [Citation26,Citation27] was the target temperature and threshold of the maximum temperature set in the near field. Chu et al. [Citation26] used a target temperature of 42.5 °C and a threshold of 43 °C, while we had a target temperature of 42 °C and used a slightly higher threshold (44 °C). Our selection of target temperature was to be in line with our desired clinical applications in human patients following the conclusion of this study. The higher threshold selection (and thus broader target-to-threshold range) was to allow the system more flexibility to manage with the higher tissue heterogeneity in our targets as well as noise/artifacts due to greater proximity to sources of potential motion. These differences in target temperature and threshold selection could contribute to some of the differences seen in the temperature statistics.
CEM43T90, a metric of thermal dose and has been shown to be clinically meaningful, was calculated for each treatment. For example, increased CEM43T90 was reported to associate with a better local relapse-free survival in patients with cervical carcinoma [Citation41], locally advanced cervical cancer [Citation42] and locally advanced breast cancer [Citation43]. The overall average CEM43T90 of 0.6 min reported herein is lower than the minimum thermal dose requirements proposed in recent guidelines for superficial hyperthermia of tumors [Citation44], and lower than results described from study by Chu et al. [Citation26]. Clinically, higher CEM43T90 might be achieved by increasing the target temperature and/or total treatment time, both of which can be adjusted using a clinical MRgHIFU system capable of HT, such as the Sonalleve. This is shown in the Chu et al. study where they used a target temperature of 42.5 °C and treated for 44–60 min. With that said, the absence of observed statistically significant differences between treatment sites in this study speaks to the stability and reproducibility of the MRgHIFU HT technique analyzed here.
It is important to gauge the time for Tavg to increase to ≥41 °C (i.e., within 1 °C of the target temperature) after the start of the sonication and the time for the average temperature to decrease to ≤40 °C (i.e., fall below the hyperthermic range) after the termination of the sonication. Longer durations for the temperature build-up in vivo lengthen the overall treatment time. While times on the order of a few minutes may have minimal impact, substantially longer treatments increase the chance for discomfort (due to having to hold the same position for a protracted period of time) and/or motion which can lead to temperature monitoring artifacts and/or loss of good patient-transducer coupling. Assessing the ramp-up times is thus important to gauge if they might be problematic; excessively long treatment times might even require some additional intervention (such as sedation) to ensure successful treatment. Here, the ramp-up time for the Tavg to increase to ≥41 °C was statistically significantly shorter in the shallow MVB targets compared to the deeper uterus. However, these differences were not deemed clinically significant because all measured times were ∼2 min maximum; reasonably short for patients to maintain still with good transducer contact.
Assessment of cool-down times following HT is potentially important as long cool-down durations could have implications for patient management. If a treatment regimen requires multiple HT targets in a single session, or if treatment is interrupted, it would be useful to understand how long temperatures in the heated tissues take to return from the hyperthermic region to ensure accurate and safe MR-guided HT when treatment is resumed and minimize the chance for nearby healthy tissues to be overheated. Protracted cool down times could result in longer times for the patient to wait between HT applications and/or spur the use of intervention such as flushing of organs like the bladder with cool water and/or ice packs placed on the skin to allow for a more efficient continuation of treatment. Such interventions might also be needed to aid in patient comfort/relief due to the heating process, especially with a longer cool down time. The relatively short cool down times (3–4 min or less) noticed in this study across all treatment sites implies that minimal management may be needed when using the 18-mm cell. Further investigation is needed using larger treatment cells with (presumably) longer associated cooling down periods, to determine if more active interventions might be warranted.
In this study, no abnormally-perfused area was detected on contrast-enhanced MRI or under gross observation at the HT target site, in agreement with two previous studies that performed HT in porcine leg model [Citation26,Citation27]. The use of ablation sites was a novel approach in this study to provide a system for localization of the HT targets as well as positive controls, both of which are absent from the previous studies. The hyper-intensive lesion shown in the contrast-enhanced MR were consistent with results from a prior publication [Citation45], and matched the severe hemorrhage and fluid-filled cavities observed in the macroscopic and histological results. With that said, some studies found post-ablation targets to be seen as non-perfused areas in the MR images, as opposed to the hyper-intense regions seen here [Citation46–48]. However, the power of 200 W and the sonication duration of 20 s that we employed were much lower than the power of 305–402 W [Citation46–48] and sonication duration of 90—3864 s [Citation46–48] used in these studies, implying that the immediate effect on the tissue could be different. Furthermore, the ablative target of normal uterus here is generally more vascularized than uterus fibroid targets of these studies [Citation47]. This implies that hemorrhage and/or fluid-filled cavities are a more likely outcome in for our ablation targets compared to uterine fibroids. Ultimately, the differences seen in the observable, permanent damage of the ablation sites in this study compared to the HT targets and unheated tissues, reinforce the utility of the MRgHIFU induced HT treatment.
Histopathologic examination showed no remarkable lesions in the HT target tissues, in the overlaying skin, or the subcutaneous tissue along the beam path. Instead, minimal to mild congestion was observed in some of these targets. Our findings are consistent with other reports that HT exposure may cause vascular congestion, dilation and potentially hemorrhage [Citation49–51]. In two HT targets, there was mild, multifocal hemorrhage in the wall of the urinary bladder and the uterus. However, several mild hemorrhages were also noted in the corresponding control samples. Thus, these findings were likely related to tissue collection. The second most common histological feature was an accumulation of white blood cells in the interstitium of the muscularis of the bladder or the uterus tissue. Such phenomena agree with previous reports that HT is able to initiate an immune response of both the innate and adaptive immune systems through the release of extracellular heat shock proteins (HSP) [Citation52–57]. This transient damage, characteristic of HT -level heat exposure, was different from the irreversible damage lesions seen in the histopathology of the ablated tissue samples.
A few limitations of this study deserve mention. First, this study did not analyze a comprehensive set of pelvic clinical targets and/or geometries and only used female pigs. Pig size and allowed weight on the tabletop limited the accessible targets, especially when coupled with the maximum HT target depth of 8 cm in the current implementation of the Sonalleve clinical MRgHIFU system. The pigs would have had to be older or larger to accommodate male-specific organs (e.g., prostate) of targetable size (depth in these instances would also have raised targetability concerns). However, a review of our clinical cases (not shown) implies that the geometries analyzed herein have applicability to other pelvic targets (e.g., cervix, prostate, colorectal, pelvic sarcomas, among others). Second, we did not do anything to manage motion-related MRI thermometry artifacts due to, e.g., respiration, peristalsis and bladder filling, other than securing the pigs tightly to the HIFU tabletop with the vendor-provided MRI pelvis coil and using sand bags to limit the extent of the respiratory excursion. This was thus reasonably representative of treating a patient with minimal intervention. Minimizing intervention is desirable as it can limit risk to the patient and potentially enhance comfort which in turn helps patients maintain good coupling to the transducer. The one caveat to this was that the pigs were anesthetized, so the breathing motion was more regular than it might be in a conscious human subject. Concerns about artifacts due to peristalsis could be managed with interventions such as inserting a saline-filled rectal balloon as was done by Chu et al. [Citation26]. Ultimately, in this study, peristaltic motion resulted in some minor artifacts in the far field, but, in general, did not inhibit HT, although the lack of intervention could have contributed to the somewhat improved thermal parameters seen in the Chu et al. study compared to here.
Another aspect is that, to ensure consistency between each of the HT sessions used in this study, only a single set of settings of the Sonalleve system was used in this study. The treatment cell size, sonication power, heating time, target temperature and near field temperature threshold used for feedback are all user-adjustable parameters. Here, parameters were selected (i.e., 42 °C target temperature, 100 W power, etc.), to reflect what our group intends to employ in a subsequent clinical trial, except for the use of the 18-mm diameter treatment cell (the smallest available on the Sonalleve system). This was motivated by target size limitations within the pigs; given the size/age of pigs we could reasonably treat, the tissue targets were too small to accommodate larger cell sizes. Different clinical and experimental targets may differ in geometry (size, shape, depth, etc.) and desired heating characteristics (target temperature, heating time, etc.) thus the preferred operating parameters will need to be determined on a study-by-study (or case-by-case) basis. An investigation into techniques to optimize the operating parameters of the system is outside the scope of this analysis, although the consistency of our results across different sites and depths argues for some degree of treatment robustness to operating parameter selection. As to the choice of cell size, further study is needed to investigate the impact of using the larger cell sizes if they are deemed to be clinically relevant for the desired targets, although some limited work has been done on the feasibility of larger cell sizes in homogenous in vivo targets [Citation27].
It is also important to note that the pig model used herein does not perfectly correlate with human tumor targets. For example, the 6–8 mm thickness of abdominal subcutaneous fat in the pigs is likely thinner than in most human patients, and the distribution of fat between tissue layers may be different between pigs and humans. This may present issues in human treatments, especially as the MR thermometry method used herein does not model temperature change well in adipose tissue, although neither the subcutaneous fat nor the intra-tissue fat layers caused significant challenges in the porcine model used in this study. Furthermore, the targets here are likely smaller than human tumor targets of interest. In order to cover larger targets, treatments would need to be comprised of larger cell sizes and/or the use of multiple clustered hyperthermia cells sequentially delivered within the same treatment session or spread across multiple treatment sessions. The depth and/or limited access due to bone/air proximity of portions of targets, among other aspects, may pose further issues in treating human patients. Ultimately, future work in both clinical trials and device development is likely needed to elucidate and/or address all of these challenges.
Nevertheless, the results of this study support proceeding into human clinical trials. The variety of target geometries, along with the accuracy and consistency of the achieved dose distributions speak strongly to the potential of MRgHIFU as a tool for applying HT to an array of pelvic targets. This work corroborates that the described MRgHIFU technique would be a feasible and viable approach for HT treatments of human pelvic malignancies with acceptable safety parameters. However, additional work would be needed to optimize application techniques on a site-specific basis.
Conclusion
In this study, we demonstrated the feasibility of using MRgHIFU to heat a set of clinically mimicking pelvic targets including the muscle adjacent to the ventral and dorsal bladder wall, and the uterus with satisfactory safety parameters. The temperature was maintained within the desired range with reasonable accuracy, precision, temperature variation, and heating uniformity for up to 30 min. Safety parameters of this HT treatment modality were measured for all targets by contrast-enhanced MRI, macroscopic tissue observations, and by histological analysis. The results support the assertion that the technique is feasible for use in treating an array of pelvic malignancies in human patients.
Disclosure statement
Ari Partanen is a paid consultant for Profound Medical Inc. The other authors have no relevant conflicts of interest to declare. This study was partially supported by grants from the Foundation for Barnes-Jewish Hospital and National Institutes of Health (NIH) grants R01MH116981 and R01EB027223.
References
- Chicheł A, Skowronek J, Kanikowski M. Thermal boost combined with interstitial brachytherapy in breast conserving therapy - assessment of early toxicity. Rep Pract Oncol Radiother. 2011;16:87–94.
- Rao W, Deng ZS, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev Biomed Eng. 2010;38:101–116.
- Longo TA, Gopalakrishna A, Tsivian M, et al. A systematic review of regional hyperthermia therapy in bladder cancer. Int J Hyperth. 2016;32:381–389.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30:531–539.
- Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21:731–744.
- Stauffer PR, van Rhoon GC. Overview of bladder heating technology: matching capabilities with clinical requirements. Int J Hyperth. 2016;32:407–416.
- Hedayatnasab Z, Abnisa F, Daud W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater Des. 2017;123:174–196.
- Habash RWY. Therapeutic hyperthermia. In: Romanovsky AJ, editor. Handbook of clinical neurology. Amsterdam (Netherlands): Elsevier; 2018. p. 853–868.
- Mellal I, Oukaira A, Kengene E, et al. Thermal therapy modalities for cancer treatment: a review and future perspectives. Int J Appl Sci. 2017;4:14.
- Gupta R, Sharma D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem Neurosci. 2019;10:1157–1172.
- Habash RWY, Bansal R, Krewski D, et al. Thermal therapy, part 2: hyperthermia techniques. Crit Rev Biomed Eng. 2006;34:491–542.
- Cabuy E. Hyperthermia in cancer treatment hyperthermia in cancer treatment. Natl Cancer Inst. 2016;1:1–48.
- Johannsen M, Thiesen B, Wust P, et al. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperth. 2010;26:790–795.
- Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No.: CD006377.
- De Haas‐Kock DFM, Buijsen J, Pijls-Johannesma M, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev. 2009;CD006269.
- van der Zee J, González González D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch deep hyperthermia group. Lancet. 2000;355:1119–1125.
- Wittlinger M, Rödel CM, Weiss C, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol. 2009;93:358–363.
- Uchibayashi T, Yamamoto H, Kunimi K, et al. Radiofrequency capacitive hyperthermia combined with irradiation of chemotherapy for patients with invasive bladder cancers. Int Urol Nephrol. 1995;27:735–741.
- You QS, Wang RZ, Suen GQ, et al. Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: long-term results of 44 patients. Int J Hyperth. 1993;9:19–24.
- Fuwa N, Morita K, Kimura C, et al. Clinical experience of 8MHz RF hyperthermia with radiotherapy of cancer of the uterine cervix. Gan No Rinsho. 1987;33:799–806.
- Overgaard J. Hyperthermia as an adjuvant to radiotherapy. Review of the randomized multicenter studies of the European society for hyperthermic oncology. Strahlenther Onkol. 1987;163:453.
- Harima Y, Nagata K, Harima K, et al. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. 2001. Int J Hyperthermia. 2009;25:338–343.
- Hornback NB, Shupe RE, Shidnia H, et al. Advanced stage IIIB cancer of the cervix treatment by hyperthermia and radiation. Gynecol Oncol. 1986;23:160–167.
- Hurwitz MD, Hansen JL, Prokopios-Davos S, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer. Cancer. 2011;117:510–516.
- Enholm JK, Köhler MO, Quesson B, et al. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng. 2010;57:103–113.
- Chu W, Staruch RM, Pichardo S, et al. Magnetic resonance-guided high-intensity focused ultrasound hyperthermia for recurrent rectal cancer: MR thermometry evaluation and preclinical validation. Int J Radiat Oncol Biol Phys. 2016;95:1259–1267.
- Tillander M, Hokland S, Koskela J, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43:1539.
- Schooneveldt G, Bakker A, Balidemaj E, et al. Thermal dosimetry for bladder hyperthermia treatment. An overview. Int J Hyperth. 2016;32:417–433.
- Kothapalli S, Partanen A, Zhu L, et al. A convenient, reliable, and fast acoustic pressure field measurement method for magnetic resonance-guided high-intensity focused ultrasound systems with phased array transducers. J Ther Ultrasound. 2018;6:1–8.
- V. V. N. Kothapalli S, Altman MB, Zhu L, et al. Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia therapy: a healthy volunteer study. Int J Hyperth. 2018;34:1381–1389.
- Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–823.
- Bing C, Staruch RM, Tillander M, et al. Drift correction for accurate PRF-shift MR thermometry during mild hyperthermia treatments with MR-HIFU. Int J Hyperth. 2016;32:673–687.
- Civale J, Rivens I, Shaw A, et al. Focused ultrasound transducer spatial peak intensity estimation: a comparison of methods. Phys Med Biol. 2018;63:055015.
- Delchar TA. Physics in medical diagnosis. Vol. 11. Berlin (Germany): Springer; 1997. p. 185–186.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperth. 2011;27:320–343.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085.
- Craciunescu OI, Stauffer PR, Soher BJ, et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys. 2009;36:4848–4858.
- Winter L, Oberacker E, Paul K, et al. Magnetic resonance thermometry: methodology, pitfalls and practical solutions. Int J Hyperth. 2016;32:63–75.
- Lam MK, Huisman M, Nijenhuis RJ, et al. Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases. J Ther Ultrasound. 2015;3:5.
- Chu W, Staruch R, Pichardo S, et al. MR-HIFU mild hyperthermia for locally recurrent rectal cancer: temperature mapping and heating quality in first patient. Proceedings of the 12th International congress of hyperthermic oncology; New Orleans, LA. 2016. p. 144.
- Ohguri T, Harima Y, Imada H, et al. Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: data from a multicentre randomised clinical trial. Int J Hyperth. 2018;34:461–468.
- Franckena M, Fatehi D, Bruijne M. D, et al. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer. 2009;45:1969–1978.
- Craciunescu OI, Blackwell KL, Jones EL, et al. DCE-MRI parameters have potential to predict response of locally advanced breast cancer patients to neoadjuvant chemotherapy and hyperthermia: a pilot study. Int J Hyperth. 2009;25:405–415.
- Trefná HD, Crezee H, Schmidt M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int J Hyperth. 2017;33:471–482.
- Daub CA, Sepmeyer JA, Hathuc V, et al. Endometrial ablation: normal imaging appearance and delayed complications. Am J Roentgenol. 2015;205:W451–W460.
- Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol. 2012;81:3624–3630.
- Zhang W, He M, Huang G, et al. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int J Hyperth. 2016;32:623–629.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperth. 2016;32:496–503.
- Emami B, Nussbaum GH, Hahn N, et al. Histopathological study on the effects of hyperthermia on microvasculature. Int J Radiat Oncol. 1981;7:343–348.
- Song CW. Effect of hyperthermia on vascular functions of normal tissues and experimental tumors. J Natl Cancer Inst. 1978;60:711–713.
- Brady LW, Heilmann HP, Seegenschmiedt MH, et al. Molecular and cellular mechanisms of hyperthermia. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy. Vol. 173. Berlin: Springer; 2012. p. 295–308.
- Jolesch A, Elmer K, Bendz H, et al. Hsp70, a messenger from hyperthermia for the immune system. Eur J Cell Biol. 2012;91:48–52.
- Milani V, Noessner E, Ghose S, et al. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperth. 2002;18:563–575.
- Payne J, Nair MPN, Ambrus JL, et al. Mild hyperthermia modulates biological activities of interferons. Int J Hyperth. 2000;16:492–507.
- Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperth. 2005;21:713–716.
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperth. 2009;25:610–616.
- Zhang H, Mehta K, Cohen P, et al. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett. 2008;271:191–204.
