?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Radiation skin injury (RSI) causes changes in skin temperature, but detailed information on the thermographic responses is currently lacking. We investigated thermographic patterns after radiotherapy. We hypothesized that skin temperature may be used as a diagnostic and early predictor of RSI severity.
Method: All breast cancer patients received radiotherapy after unilateral postmastectomy. The contralateral supraclavicular area served as control, and the frontal thermal image of torso was taken by a thermal infrared imager weekly. We defined areas of interest on bilateral symmetrical supraclavicular area, and analyzed the difference of average and maximum skin temperature (DSTaverage and DSTmax) between them. The extent of the weekly variation in DST (DSTW) was calculated using a mathematical formula to represent a trend of skin temperature change. RSI and symptoms related to RSI were scored from baseline to 2 weeks after the end of radiotherapy.
Results: Forty-one patients were enrolled in this study. In comparison to the baseline, the DSTaverage and DSTmax increased significantly over time during radiotherapy (p < .05). The onset of DST increase was accompanied by the onset of radiation dermatitis, and the maximal DST also appeared at the peak of Radiation Therapy Oncology Group (RTOG) and symptom scores. Radiation dose, DSTaverage, burning-feeling and pulling were the independent variables affecting RTOG score according to multivariate analysis (p < .001, p < .034, p < .001, p < .001). Patients with DSTWaverage >1.223 or DSTWmax >1.114 in second week showed a late higher dermatitis score (RTOG score ≥2).
Conclusion: This study confirmed that RSI was associated with thermographic response. Our results suggested that the follow-up observations of skin temperature during radiotherapy could provide the objective evaluation criteria and prediction methods for RSI.
Introduction
As the largest organ of the body and one of the fastest self-renewing tissues, the skin plays a vital role in maintaining homeostasis and regulating temperature [Citation1]. Radiation skin injury (RSI) is a common adverse reaction. Approximately 87–95% of irradiated patients are characterized by swelling, redness, pigmentation, ulceration, fibrosis, pain, burning and itching of the skin [Citation2–4]. RSI has an impact on discomfort and the quality of life of patients [Citation5,Citation6], and may require interference with radiation schedules and complex surgical reconstruction, especially when combined with molecular targeted therapy [Citation7–9]. However, evaluation of RSI is not straightforward. A gold standard for clinically rating the severity of radiation skin injury does not exist. There are currently several scoring systems including the Radiation Therapy Oncology Group/The European Organization for Research and Treatment of Cancer (RTOG/EORTC), the Danish, the European, and the Biomed 2 side-effect scales [Citation10]. They are all defined by the subjective evaluation of clinical oncologists, which are usually biased. There is a need for a rapid, reliable, objective and noninvasive clinical tool to score RSI and even predict the development of skin damage early.
Skin temperature changes due to laser or thermal injury have been measured in many studies with temperature and time as predictors of skin damage. The most common method of measuring skin temperature is the infrared thermography. Previous studies have also shown that radiation leads to the development of cutaneous vasculature and generation of an inflammatory response, which will increase skin temperature [Citation11]. Consequently, the changes in the difference of skin temperature (DST) may be used as an objective, quantitative, and functional surrogate measure to determine and predict RSI. The main purposes of this study were threefold: (1) to describe the thermographic response after radiation; (2) to investigate whether there were significant differences in DST among different time periods and different RTOG scores in the course and (3) to test if thermographic response could be used to predict the development of RSI in the incipient stage. We assumed that RSI can be scored and predicted by skin temperature changes after selecting the appropriate controls.
Materials and methods
Patients
This prospective study was approved by the local institutional review and ethical committees under the registration number NCT04047823 (www.clinicaltrials.gov). Informed consent was obtained from all patients. Female patients with a pathologically proven breast cancer who underwent three-dimensional conformal radiotherapy or intensity modulated radiation therapy (3DCRT or IMRT) after modified radical mastectomy were eligible for the study. All patients received chemotherapy either before or after radiation therapy. Systemic chemotherapy before radiotherapy was completed at least 2 weeks before enrollment. Patients were allowed to have concurrent endocrine therapy and biologic therapy. Other inclusion criteria included: age ≥18 years, ECOG PS ≤1, normal organ function, no previous radiotherapy, and no concurrent chemotherapy. The exclusion criteria were as follows: rash or unhealed wound in the radiation field, pregnancy or lactation, and the presence of connective tissue disorder.
Routine radiotherapy and assessment
Patients lay in a supine treatment position. 3DCRT or IMRT was delivered to the target volume, including the chest wall and ipsilateral lymph nodes (supraclavicular and level III axillary). All patients were treated with 6 MV X-rays. Additional boluses were added according to chest wall thickness variation. The protocol allowed for a dose variation (in the planning target volume) between 95% and 105% of that at the reference point on the central axis [Citation5–6]. A total dose of 50-Gy radiotherapy was delivered in daily fractions of 2 Gy for five consecutive weeks. Radiation-induced dermatitis was assessed according to two scoring systems, the RTOG and the patients-reported symptoms scale. The latter was measured by the Skin Toxicity Assessment Tool (STAT) [Citation12]. All staff in the study group received an education on how to use the two scales. RSI was scored by two senior physicians with more than 5 years of experience. Physicians who graded radiation dermatitis were blinded to the temperature measurements. Radiation-induced skin toxicity was evaluated at baseline (registration) and then weekly from the start of radiotherapy to 2 weeks after the end of treatment.
Skin temperature assessment
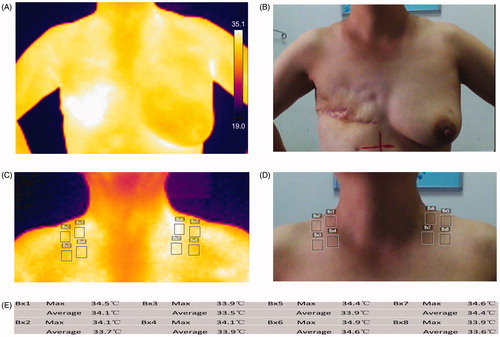
The timing of the baseline measurement was the day of enrollment before radiotherapy. Measurements were also taken weekly during radiation and additionally 2 weeks after radiotherapy (total 7 weeks, 8 times). The testing room temperature was about 24–27 °C. The patients also needed to disrobe from the waist up and stand at rest 5 min before the examination. We defined specific areas of interest according to . A frontal thermal image of torso from neck to upper abdomen was obtained 2 h before routine radiation using a digital Infrared thermal imager (FLIR E5 Serial No.63985976, Wilsonville, OR, USA). The maximum and average skin temperatures of four testing areas were measured on the supraclavicular radiation field, and outlined and automatically calculated by a specific software package (FLIR Systems 6.3.17227.1001). Identical measurements on the contralateral supraclavicular region were used as controls in our assessments. Skin care products were allowed including topical corticosteroids, oil-in-water emulsions, silver sulfadiazine and so on. No topical treatment was allowed for at least 6 h before tests.
Statistical analysis
DST was the value of the treated area minus that of the untreated area (controls): DSTaverage = the average skin temperature in treated areas – the average skin temperature in untreated areas; DSTmax = the maximum skin temperature in treated areas – the maximum skin temperature in untreated areas. To calculate the extent of weekly DST variation (DSTW) during radiotherapy, the following formula was used:
where n = 0–7, indicating the time point observed weekly including the baseline. # DSTaverage/max was sometimes a negative number with the minimum value greater than −2, according to our preliminary experiment and this trial. In order to correctly reflect the extent to which DST changes over time, the data were adjusted by the statistical method.
We used SPSS version 17.0 (SPSS Inc, Chicago, IL) for statistical analysis. Continuous variables were expressed as mean ± standard deviation. Paired samples tests were performed to compare DST and patient-reported symptoms before, during and after treatment. One-Way ANOVA was performed for DST comparisons between different RTOG scores. Two-tailed Spearman’s correlation analysis was used for RTOG scores and changes in DST. The area under receiver operating characteristic (ROC) curve was used to evaluate the predictive ability of risk factors. Multiple logistic regression analysis was performed to determine the simultaneous influence of various factors on RTOG score. Backward elimination was used as the regression variable selection technique in this study. All statistical tests were conducted at a two-sided significance level of 0.05.
Results
A total of 41 patients enrolled in the study and underwent RT without treatment delay or interruption. Their median age was 45 years (mean, 46 years; range, 28–67 years). Patient characteristics were summarized in . The maximum RSI for the whole radiation field during the treatment according to RTOG scoring system as follows: Grade 1 toxicity, 43.9% (18 patients); Grade 2 toxicity 51.2% (21 patients) and Grade 3 toxicity 4.9% (2 patients).
Table 1. Patient demographics and disease characteristics.
For the supraclavicular radiation field we focused on, the maximum dermatitis Grade 0, Grade 1, Grade 2, Grade 3 and Grade 4 toxicity was seen in 0, 31, 10, 0 and 0 patients, respectively. Eleven patients began to develop RSI in the second week after radiotherapy, 22 patients in the third week, 6 patients in the fourth week and 2 patients in the fifth week. Patients developed a typical radiation erythema, which set in after a mean interval of 2.88 weeks (median, 3 weeks; range, 1–5 weeks). Grade 2 erythema occurred in 33 patients with an average time of 4.64 weeks (median, 4 weeks; range, 3–7 weeks). The patient-reported symptom scores were also assessed by the Skin Toxicity Assessment Tool. As the radiation dose exposure increased, the symptom scores (burning-feeling, itching, pulling, pain and tenderness) rose during the second or third week of radiotherapy, peaked at 1 week after the end of radiation and then gradually declined (). When comparing baseline values, patient-reported symptom scores including burning-feeling, itching, and pulling increased significantly at 2 weeks after radiotherapy, but pain score began to differ significantly 3 weeks after radiotherapy. Most symptom scores were significantly higher than those in the previous week. There were no statistically significant differences in tenderness symptom scores during the observation.
Table 2. The patient-reported symptoms and temperature changed along with time.
Alteration in skin temperature
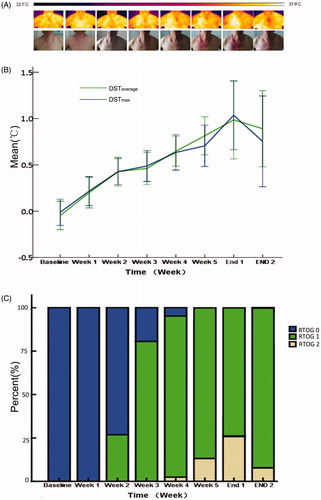
The increases of DSTaverage and DSTmax were also noted during radiotherapy. When making a pairwise comparison, the following data were statistically significant: DSTmax and DSTaverage at week 1 versus baseline; DSTmax and DSTaverage at week 2 versus at week 1 and DSTaverage at week 5 versus at week 4 (). The mean values of DSTWaverage at each observation point were 1.197 ± 0.431, 1.128 ± 0.217, 1.032 ± 0.189, 1.114 ± 0.311, 1.087 ± 0.188, 1.046 ± 1.077 and 0.923 ± 0.964, respectively. The mean values of DSTWmax were 1.177 ± 0.401, 1.123 ± 0.225, 1.039 ± 0.202, 1.082 ± 0.245, 1.090 ± 0.368, 1.207 ± 0.366 and 0.936 ± 0.296. Only the mean difference in the values of DSTWaverage between the second and the third week was statistically significant (T = 2.194, p= .034).
Correlation between DST and dermatitis score
As shown in , DSTaverage and DSTmax increased significantly with the progress of radiotherapy, and radiation dermatitis was also generally aggravated. The proportion of Grade 2 RSI was the highest at 1 week after the end of radiotherapy, and DSTaverage and DSTmax also reached the peak. The correlations were found between DSTaverage, DSTmax and RTOG score. The mean values of DSTaverage in patients with Grade 0, Grade 1 and Grade 2 were 0.198 ± 0.542, 0.674 ± 0.588 and 1.075 ± 0.799, respectively (F = 28.434, p < .001). Similarly, the mean values of DSTmax were 0.211 ± 0.499, 0.626 ± 0.620 and 1.046 ± 0.863 (F = 22.353, p < .001). The variables included in multiple regression analysis were BMI, breast size, endocrine therapy, trastuzumab therapy, DSTaverage, DSTmax, radiation dose, itching, pain, tenderness, burning-feeling and pulling. Radiation dose (B = 0.014, T = 8.879, p < .001), DSTaverage (B = 0.065, T = 2.136, p < .034), burning-feeling (B = 0.146, T = 3.951, p < .001) and pulling (B = 0.241, T = 5.07, p < .001) were significant associated with RTOG scores. The correlations were also found between DSTaverage, DSTmax and patients-reported symptoms scores except tenderness (DSTaverage: itching F = 11.223, p < .001; pain F = 5.654, p = .001; burning-feeling F = 6.860, p = .001; pulling F = 11.002, p < .001; DSTmax: itching F = 8.949, p < .001; pain F = 3.864, p = .010; burning-feeling F = 9.381, p < .001; pulling F = 13.032, p < .001).
Figure 2. The correlation of the difference of skin temperature and dermatitis score. (A) A representative case with thermography and photograph every week. The color was a presentation of the surface temperature in thermography from 22.7 to 37.9 °C. (B) DST gradually increased with the progress of radiotherapy and peaked at the first week after the end of radiotherapy. (C) With the progress of radiotherapy, the RSI became more severe. The proportion of RSI Grade 2 was the highest at 1 week after the end of radiotherapy. Combined with the above three parts, it can be intuitively found that the severity of RSI is positively correlated with skin temperature changes.
The predictive value of DSTW for RSI
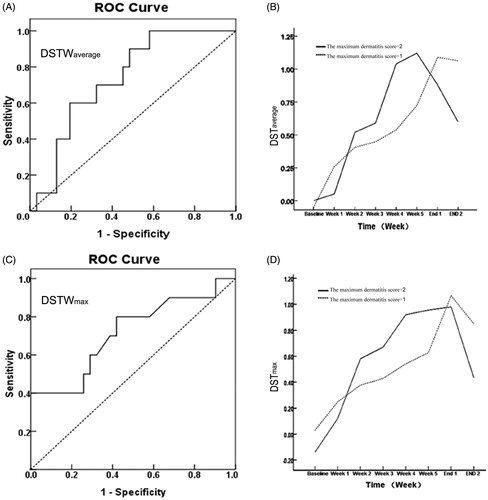
Spearman’s correlation analysis illustrated a positive correlation between the maximum RTOG score and DSTWaverage at week 2 (R = 0.350, p = .025) as well as DSTWmax at week 2 (R = 0.319, p = .042). ROC curve showed that patients with DSTWaverage at week 2 > 1.223 or DSTWmax at week 2 > 1.114 [sensitivity: 60.0% or 80.0%; specificity: 80.6% or 58.0%; area under the curve of ROC: 0.735 or 0.715; p = .027 or 0.044; Youden index = 0.406 or 0.380] could be optimally separated from patients with low grade dermatitis (RTOG score <2; ). No other correlation was significantly found between the maximum RTOG score and DST or symptoms scores within 1–3 weeks after the onset of radiotherapy.
Figure 3. Receiver–operator curve of the difference in temperature as a predictor of future RSI and the tendency chart of DST according RTOG grade. (A and C) The ROC curve of DSTWaverage and DSTWmax, respectively (p = .027 and 0.044). (B and D) The change of difference in average and maximum temperature based on the maximum dermatitis score groupings (Grade 1 or Grade 2), respectively. The slope value of temperature at the second week end compared with the first week was significant increase in a higher toxicity group.
Discussion
The skin is a functional organ acting on temperature control. Increased local skin temperature indicates augment of blood perfusion or vascularization, which can reflect changes in metabolic activity or inflammation [Citation13,Citation14]. An increase in temperature is emitted as infrared radiation, which can be measured by infrared thermal imager, such as FLIR. Since 1982, the US Food and Drug Administration has approved infrared thermography as an auxiliary tool for the diagnosis of breast cancer [Citation15]. With the development of high-resolution digitalized images and image analysis system, it is more sensitive to temperature changes at 0.08 °C and has a high sensitivity of 100% in detecting malignant tumors without direct contact with patient. Recently, more and more studies have focused on another application area, such as the assessment of breast reconstruction, severe hidradenitis suppurativa, burn wound and glucocorticoid-induced obesity [Citation13,Citation16–19]. Positive conclusions are obtained in most studies.
RSI was a common side effect involving complex processes of direct radiation injury as well as subsequent inflammatory responses at the functional, cellular and gross levels. Data from the animal models of RSI showed that perivascular inflammatory infiltrated around dilated blood vessels with swollen endothelial cells [Citation20,Citation21]. Our data demonstrated that RSI was associated with changes in skin temperature. The common finding on the treated side compared with the untreated side during radiotherapy was a significant increase in skin temperature difference over time, which showed a statistical difference in most comparisons. The DST values were found to be statistically significantly different among the various RTOG scores and discomfort grades. We found DSTW2, which represented the compared increase amplitude of DST in the second week, could serve as a promising early predictor of more severe dermatitis. Patients with a higher DSTW2 might have a higher RTOG score (≥Grade 2) in the later period of radiotherapy. In a clinical setting, it is important to be able to objectively, accurately and quickly diagnose RSI scores and predict the severity. The findings in this study indicated that this could be achieved by measuring skin temperature during radiotherapy.
The DST has also been observed between the irradiated breast or the chest wall and the contralateral region in a published article [Citation22], and DSTaverage of more than 1.4 °C at 20% of total radiation dose is meaningful for predicting skin reactions. Some results from that study and ours seem to be contradictory, but there are differences in terms of the choice of surgical approach, total radiation dose and the tested radiation field in patients between them. In the study, we choose the supraclavicular radiation field as the assessment area and the contralateral non-radiation area as the control. The selections avoid the influence of confounding factors and explore the variation of skin temperature easily. Because the skin temperatures in symmetrical regions are similar, but the surgical method, breast shape and asymmetry may change this situation. For example, the skin temperature of chest wall after a modified radical surgery (as shown in ) in the following condition is often higher than elsewhere, such as corresponding to heart and great vessels, with thin subcutaneous fat and in the folds. In addition, skin temperature changes in the local supraclavicular field could not be used to assess radiation damage in the distal chest wall field. More research is required to investigate the relationship between the variation of skin temperature and radiation damage in breast area after surgery. One possible direction to overcome this issue may be the development of image analysis software to obtain more parameter information on skin temperature. Another approach is to choose a more reasonable reference region for DST testing. Individual skin temperature fluctuations, different processes of temperature changes in asymmetric skin areas, and environmental factors are considered [Citation23,Citation24].
The main limitation of our study is the relatively low number of patients in our cohort. Skin biopsy specimens are not obtained from the cancer patients enrolled in the study for ethical reasons. Temperature measurements are taken 2 h before radiotherapy and patients need to rest 5 min before examination in the study. This procedure may pose some inconvenience for patients in clinical practice. Detailed information on temperature variation would be detected to select a more appropriate test process. The objective assessment and prediction tool for skin reactions will impact on clinical practice. First, the translation of skin temperature examination to the clinical level will provide a new feasible method for objectively describing RSI. It complements current clinical assessment techniques, which often rely on subjective judgment. Second, the goals of skin protection in radiation are to achieve optimum clinical outcomes, to ensure cost effectiveness and to minimum drug toxicity. A randomized trial has demonstrated mometasone furoate can reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation, but its side effects are also inevitable [Citation25]. Patients can avoid the cost and side effects of drug when early predicting less than Grade 2 RSI. If patients have a trend of severe RSI, radioprotective drugs or other solutions should be applied in advance to reduce medical risks. At last, another application domain of this method is quality assurance during radiotherapy. If the skin temperature rises abnormally in the low dose area of the radiation plan or outside the radiation field, it is necessary to check the accuracy of treatment implementation.
In conclusion, infrared thermography could be used to estimate and predict the dermatitis scores as a noninvasive, quantitative and bioengineering method that is not painful, not radiant, and is easily transportable during the course. Special attention should be paid to select comparative reference point and measurement condition. Future prospective studies with more cases and measurement parameters will be helpful to confirm the diagnostic and predictive value of skin temperature in RSI.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Dainichi T, Hanakawa S, Kabashima K. Classification of inflammatory skin diseases: a proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J Dermatol Sci. 2014;76(2):81–89.
- Omidvari S, Saboori H, Mohammadianpanah M, et al. Topical betamethasone for prevention of radiation dermatitis. Indian J Dermatol Venereol Leprol. 2007;73(3):209.
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3):985–993.
- Noble-Adams R. Radiation-induced reactions 1: an examination of the phenomenon. Br J Nurs. 1999;8(17):1134–1140.
- Zhao H, Zhu W, Jia L, et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol. 2016;89(1058):20150665.
- Zhu W, Jia L, Chen G, et al. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget. 2016;7(30):48607–48613.
- McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs. 2006;22(3):163–173.
- Cohn AB, Lang PO, Agarwal JP, et al. Free-flap reconstruction in the doubly irradiated patient population. Plast Surg Nurs. 2008;122:125–132.
- Galloway TJ, Wirth LJ, Colevas AD, et al. A phase I study of CUDC-101, a multitarget inhibitor of HDACs, EGFR, and HER2, in combination with chemoradiation in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21(7):1566–1573.
- López E, Núñez MI, Guerrero MR, et al. Breast cancer acute radiotherapy morbidity evaluated by different scoring systems. Breast Cancer Res Treat. 2002;73(2):127–134.
- Bray FN, Simmons BJ, Wolfson AH, et al. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb). . 2016;6(2):185–206.
- Neben-Wittich MA, Atherton PJ, Schwartz DJ, et al. Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a Phase III trial of mometasone cream versus placebo during breast radiotherapy: the North Central Cancer Treatment Group (N06C4). Int J Radiat Oncol Biol Phys. 2011;81(2):397–402.
- Xue EY, Chandler LK, Viviano SL, Keith JD. Use of FLIR ONE smartphone thermography in burn wound assessment. Ann Plast Surg. 2018;80(4 Suppl 4):S236–S238.
- Wu Y, Nieuwenhoff MD, Huygen FJ, et al. Characterizing human skin blood flow regulation in response to different local skin temperature perturbations. Microvasc Res. 2017;111:96–102.
- Arora N, Martins D, Ruggerio D, et al. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am J Surg. 2008;196(4):523–526.
- Hashimoto Y, Watanabe N, Yuasa T, et al. Breast reconstruction with absorbable mesh sling: dynamic infrared thermography of skin envelope. Gland Surg. 2017;6(1):73–81.
- Derruau S, Renard Y, Pron H, et al. Combining magnetic resonance imaging (MRI) and medical infrared thermography (MIT) in the pre- and per-operating management of severe hidradenitis suppurativa (HS). Photodiagnosis Photodyn Ther. 2018;23:9–11.
- Thuzar M, Law WP, Ratnasingam J, et al. Glucocorticoids suppress brown adipose tissue function in humans: a double-blind placebo-controlled study. Diabetes Obes Metab. 2018;20(4):840–848.
- Boyette-Davis JA, Eng C, Wang XS, et al. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18(11):3180–3187.
- LeBoit PE. Subacute radiation dermatitis: a histologic imitator of acute cutaneous graft-versus-host disease. J Am Acad Dermatol. 1989;20(2):236–241.
- Schmuth M, Sztankay A, Weinlich G, et al. Permeability barrier function of skin exposed to ionizing radiation. Arch Dermatol. 2001;137(8):1019–1023.
- Maillot O, Leduc N, Atallah V, et al. Evaluation of acute skin toxicity of breast radiotherapy using thermography: results of a prospective single-centre trial. Cancer Radiother. 2018;22(3):205–210.
- Te Kulve M, Schlangen LJM, Schellen L, et al. The impact of morning light intensity and environmental temperature on body temperatures and alertness. Physiol Behav. 2017;175:72–81.
- Regulation of body temperature. Br Med J. 1951;1(4696):24–25.
- Ho AY, Olm-Shipman M, Zhang Z, et al. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2018;101(2):325–333.