Introduction
Radiation recall, which was first described in 1959, remains a rare and erratic inflammatory phenomenon that manifests in previously irradiated areas after the subsequent administration of chemotherapeutic agents [Citation1]. Anthracyclines, taxanes, antimetabolites, and topoisomerase inhibitors have all been associated with radiation recall [Citation2], as have specific agents such as dabrafenib, pazopanib, sorafenib, and trastuzumab [Citation3–5]. The interval between the cessation of radiation and manifestation of the inflammation can range from a few days to 15 years [Citation6].
While dermatitis is the most commonly reported recall reaction, other manifestations include mucositis [Citation7], myositis [Citation8], myocarditis [Citation9], pneumonitis [Citation10], and gastroenteritis [Citation11]. The management of recall reaction differs according to presentation; persistent reactions can require the discontinuation of therapy [Citation12] or the co-administration of corticosteroids and anti-inflammatory agents [Citation13], whereas mild reactions allow for the continuation of the prescribed chemotherapeutic regimens with no anti-inflammatory agents required [Citation14].
Deep regional hyperthermia is a therapeutic approach for pelvic solid tumors by which the temperature of the lesion is elevated to 40–43 °C to damage the cancer cells and potentiate the efficacy of chemotherapy or radiotherapy. Heat can modulate the vascularization, oxygenation, and immune responses by its induction of hypoxia-inducible factor, (VEGF and heat shock protein (HSP) pathways [Citation15]. The ability of deep regional hyperthermia to potentiate radiosensitivity and chemosensitivity has led to combined treatment modalities that incorporate hyperthermia with radiotherapy and chemotherapy. Nishimura et al. revealed that hyperthermia combined with radiotherapy can inhibit angiogenesis through heat-induced vascular damage [Citation16]. The role of hyperthermia combined with molecular inhibitors such as regorafenib, a multi-targeted receptor tyrosine kinase inhibitor that is active against VEGF receptor (VEGFR), platelet-derived growth factor receptor, fibroblast growth factor receptor, and angiopoietin receptor has not yet been investigated.
To the best of our knowledge, the occurrence of radiation recall hematuria (cystitis) after hyperthermia and regorafenib treatment has not been investigated. This is the first patient reported to have radiation recall triggered by hyperthermia two years after palliative radiation therapy which was resolved by sparing the bladder from the field of hyperthermia.
Case presentation
A 66-year-old woman was diagnosed with stage IIIC rectal adenocarcinoma (cT3N2M0) with wild type K-ras in November 2013. Neoadjuvant chemotherapy with induction FOLFOX (5-fluroruracil, leucovorin, and oxaliplatin) and concurrent chemoradiotherapy (CCRT) with FOLFIRI (5-fluroruracil, leucovorin, and irinotecan) and 5040 cGy of radiation in 28 fractions was administered between November 2013 and January 2014, followed by definitive surgery in April 2014 and a complete course of adjuvant chemotherapy using bevacizumab plus FOLFOX. In March 2015, abdominal computed tomography (CT) and positron emission tomography-CT (PET-CT) revealed local recurrence with regional lymphadenopathy; hence, palliative CCRT with bevacizumab plus FOLFOX were administered. In December 2015, a transverse colostomy was created because a rectocutaneous fistula had formed with the tumor invading into the skin over the presacral area, and the patient received capecitabine. After further disease progression, capecitabine was replaced by regorafenib on March 2nd, 2017, and the patient was referred to our oncology clinic after PET-CT revealed that the presacral tumor continued to grow and had invaded the anus and focal right gluteus maximus muscle.
Hyperthermia was induced weekly using a BSD-2000 regional deep hyperthermia device (Pyrexar, Salt Lake City, Utah, USA) with 300 W/77.0 MHz of power starting on March 28th, 2017, approximately 1 month after regorafenib initiation. The patient experienced gross hematuria 3 days after commencing hyperthermia while under regorafenib therapy. Coagulation profiles, urine biochemistry, and cytology studies revealed no evidence of infection. Flexible cystoscopy revealed mild inflammation at the right lateral bladder wall, which was initially suspected to be Foley catheter-related. Her hematuria improved after receiving irrigation of the bladder during the first and second hyperthermia sessions. Nevertheless, she experienced recurrent hematuria following the third hyperthermia session even after removing the Foley catheter; however her hematuria ceased after sparing the bladder from the hyperthermia field during the fourth session. She ultimately underwent a total of 15 hyperthermia sessions within 5 months (March 28th–September 3rd, 2017) using 350–400 W of power with average rectal temperatures ranging between 39.9 °C and 41.1 °C. The temperature of the bladder could not be recorded because of the unavailability of three-way catheters at the time.
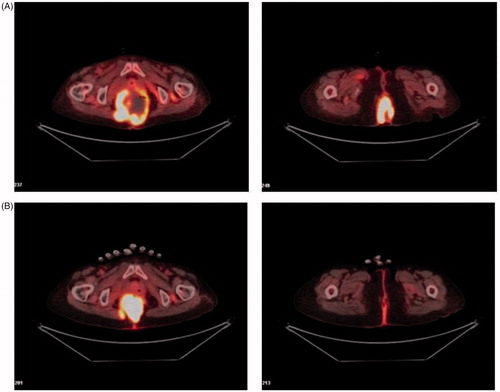
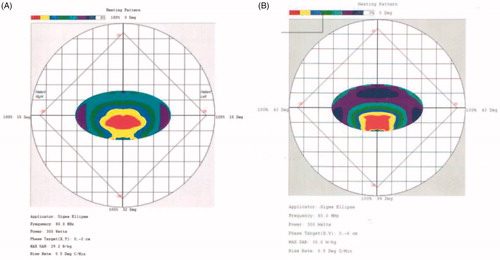
Partial response was achieved, as PET-CT revealed that a proportion of the tumor tissue became necrotic and that the tumor markedly shrank (). Levels of the tumor marker CA-19.9, initially 46.5 ng/dL before hyperthermia, rose to 107 ng/dL after the first hyperthermia session but declined to 67.3 ng/dL after the third session and to 32 ng/dL (which was within normal range) after the seventh session; these values correlated well with the clinical response. The recall hemorrhagic cystitis that occurred in our patient was resolved by sparing the bladder from the hyperthermia field and reducing the power density distribution around the bladder from 50% to 75% () to below 50% (.
Discussion
Excessive temperature elevation in the bladder is a key cause of hematuria. The initial thermal dose (in cumulative equivalent minutes at 43 °C (CEM43 °C)) reached 29.5 (CEM43 °C) in the vagina and was adjusted to 27.8 (CEM43 °C) for the third hyperthermia session. The subsequent thermal doses in the vagina after bladder-sparing were 11.2 (CEM43 °C) and 14.5 (CEM43 °C) during the fourth and fifth hyperthermia sessions, respectively for durations between 60 and 70 min in each session. The thermal dose of the rectum ranged from 2.6 (CEM43 °C) during the first hyperthermia session to 7.2 (CEM43 °C) during the third. The initial rectal thermal dose was lower because the thermometer was placed more distally, near the protruding tumor. The rectal thermal dose rose to 28.9 (CEM43 °C) during the subsequent fifth hyperthermia session owing to more proximal recordings after the tumor’s shrinkage. Data regarding the thermal dose in the bladder was limited because of the lack of intravesical temperature recording (which could be obtained either via MRI-guided temperature recording techniques or a three-way catheter that was not available that time).
A history of previous irradiation provides an important clue when diagnosing radiation recall regardless of how the phenomenon is triggered (e.g., by regorafenib, hyperthermia, or both combined in our patient). That no hematuria was observed during the administration of regorafenib monotherapy minimizes the likelihood of recall being triggered by regorafenib alone when administered for a short duration (1 month). However, as an anti-angiogenic agent that targets VEGFR, we could not exclude the possibility of hyperthermia catalyzing a regorafenib-induced recall reaction that would otherwise have occurred at a higher dose of the inhibitor drug. As such, attention should be paid to the unusual side effects of radiation recall toxicity. Potential synergistic effect of hyperthermia combined with angiogenesis inhibitors and anti-angiogenic tyrosine kinase inhibitors such as pazopanib have previously been demonstrated to improve clinical outcomes significantly [Citation17], as was the case in our patient. The addition of hyperthermia to offset the initial poor response of single-agent regorafenib warrants further clinical trials to further explore this combination.
The mechanisms underlying radiation recall reactions remain unclear. The tissue reaction that occurs in response to exposing a previously irradiated site to a second precipitating agent resembles a memory effect. Possible hypotheses include the development of an initial localized chronic inflammatory response to radiation in which cytokine upregulation recurs more aggressively with future stimuli [Citation2], or genetic alteration of local stem cells in the irradiated area rendering the remnant surviving cells intolerant to a second insult. Hyperthermia increases vascular permeability and induces HSP expression. Immune activation via HSPs that are released from dying tumor cells might increase the cross-presentation of HSP-bound peptide antigens to major histocompatibility complex class I molecules in dendritic cells, leading to efficient induction of antigen-specific cytotoxic T-lymphocytes and natural killer cell activity [Citation18,Citation19]. The role of the initial inflammatory effect, memory cell formation, and subsequent immune reactivation in eliciting the recall phenomenon requires further investigation.
The clinical courses of patients who experience radiation recall after therapies such as hyperthermia and regorafenib are unpredictable. Outcomes range from ceasing further effective therapy, reducing dosage, or providing pre-medicaton with steroids, depending on the severity. In our patient, bladder-sparing alone was effective, and there was no need to administer steroids or other anti-inflammatory agents to treat her radiation recall hematuria.
Conclusions
Despite its challenging diagnosis, radiation recall should be considered for patients who undergo irradiation of the pelvis plus treatments such as hyperthermia and/or antiangiogenic agents who consequently experience hematuria. Reduction of heating to the bladder might be an effective strategy to limit this effect.
Geolocation information
This case report describes a patient treated in a hospital in Taipei, Taiwan.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- D’Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology. 1959;73:175–177.
- Azria D, Magne N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31(7):555–570.
- Haraldsdottir S, Bertino E, Haglund K, et al. Radiation recall dermatitis with concomitant dabrafenib and pazopanib therapy. JAMA Dermatol. 2016;152(5):587–589.
- Azad A, Maddison C, Stewart J. Radiation recall dermatitis induced by pazopanib. Onkologie. 2013;36(11):5–6.
- Oh D, Park HC, Lim HY, et al. Sorafenib-triggered radiation recall dermatitis with a disseminated exanthematous reaction. Radiat Oncol J. 2013;31(3):171–174.
- Ubukata M, Kamio T, Ohchi T, et al. Radiation recall dermatitis occurring 6 years and 4 months after breast-conserving surgery: a case report. Oncol Lett. 2016;11(5):3071–3074.
- Wallenborn PA, Postma DS. Radiation recall supraglottitis. A hazard in head and neck chemotherapy. Arch Otolaryngol. 1984;110(9):614–617.
- Delavan JA, Chino JP, Vinson EN. Gemcitabine-induced radiation recall myositis. Skelet Radiol. 2015;44(3):451–455.
- Masri SC, Misselt AJ, Dudek A, et al. Radiation recall reaction causing cardiotoxicity. J Cardiovasc Magn Reson. 2014;16(1):25.
- Schweitzer VG, Juillard GJ, Bajada CL, Parker RG. Radiation recall dermatitis and pneumonitis in a patient treated with paclitaxel. Cancer. 1995;76(6):1069–1072.
- Nishimoto K, Akise Y, Miyazawa M, et al. A case of severe rectal hemorrhage possibly caused by radiation recall after administration of gemcitabine. Keio J Med. 2016;65(1):16–20.
- Barlesi F, Tummino C, Tasei AM, et al. Unsuccessful rechallenge with pemetrexed after a previous radiation recall dermatitis. Lung Cancer. 2006;54(3):423–425.
- Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol. 2001;59(3):237–245.
- Lock M, Sinclair K, Welch S, et al. Radiation recall dermatitis due to gemcitabine does not suggest the need to discontinue chemotherapy. Oncol Lett. 2011;2(1):85–90.
- Peeken JC, Vaupel P, Combs SE. Integrating hyperthermia into modern radiation oncology: what evidence is necessary? Front Oncol. 2017;7:132.
- Nishimura Y, Murata R, Hiraoka M. Combined effects of an angiogenesis inhibitor (TNP-470) and hyperthermia. Br J Cancer. 1996;73(3):270–274.
- Lee SY, Lee NR. Positive response of a primary leiomyosarcoma of the breast following salvage hyperthermia and pazopanib. Korean J Intern Med. 2018;33(2):442–445.
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542.
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25(8):610–616.