Abstract
Purpose: To determine the size of the ablation zone after radiofrequency ablation (RFA) of atypical cartilaginous bone tumors (ACT) using temperature-controlled 20 and 30 mm RFA straight non-cooled electrodes.
Materials and methods: Sixteen patients with ACT in their long bones, who had undergone a single-session single-application CT-guided temperature-controlled RFA, were included retrospectively in the study. Tumors with a diameter of 10–25 mm were treated with 20 mm electrodes (n = 10), and tumors of 25–35 mm, with 30 mm electrodes (n = 6). The ablated zone was measured after three months on MRI images.
Results: All the tumors were within the ablated zone on the 3-month follow-up MRI scan. The mean ablation time with the electrode, at a target temperature of 90 °C, was 7.6 minutes (range 6–10). The median of the largest ablation diameters, on applying the 20 and 30 mm electrodes, were 42 mm (IQR 8.5, range 30–51 mm) and 44.5 mm (IQR 4.5, range 42–63 mm), respectively.
Conclusions: All the retrospectively viewed tumors in the long bones of ACT patients treated with RFA were completely ablated. The ablation zone diameters in the bones were larger than expected, when compared to other tissues, such as the liver.
Introduction
Atypical cartilaginous tumors (ACT), previously known as chondrosarcoma grade one [Citation1], are one of the most frequently encountered tumors in orthopedic oncology [Citation2]. These are characterized by the development of cartilaginous neoplastic tissue, mostly in the long bones and pelvis, that permeates marrow spaces and completely replaces the marrow fat and bone trabeculae [Citation3]. Due to its unpredictable nature, and the possibility of local tumor progression, treatment encompasses complete tumor removal with intralesional curettage followed by local adjuvant treatment as the standard technique of choice [Citation4]. However, the latter technique can lead to complications, such as postoperative fractures, infections and local recurrence [Citation5,Citation6].
Radiofrequency Ablation (RFA) is a minimally invasive procedure that has become the treatment of choice for osteoid osteomas (OO), and is also suitable for the treatment of other bone tumors [Citation7]. Contrary to intralesional curettage, RFA is particularly appealing due to its high success and low complication rates [Citation8,Citation9], and because of the need of little to no patient hospitalization, making it a good option to explore as an alternative to the standard surgical care. Although rare, some known complications of RFA on bone tumors include the potential damage to adjacent soft tissue, particularly in the presence of cortical thinning [Citation10], and the risk of fractures [Citation11].
A pilot study by our research group already proved that cartilaginous tumor cells can, potentially, be eliminated with RFA [Citation12]. It also demonstrated that gadolinium-enhanced magnetic resonance (MR) imaging three months post procedure could be a reliable post-operative follow-up monitoring technique for the detection of residual tumors, similar to the findings by Lee et al. for OO [Citation13]. Although the safety of RFA has been attested, accurate and reproducible planning remains challenging because of the lack of available information on the size of RFA ablation zones in bone tumors and on whether it is comparable to RFA zones in soft tissues (e.g., liver).
Consequently, intervention radiologists and oncology surgeons have had to rely on their experience in the treatment of other types of tumors and on procedural recommendations supplied by the RFA manufacturers. The clinical experience is often based on the treatment of small (< 2 cm) tumors such as OO or on the ablation of other tissues such as the liver, whereas the manufacturers’ information is often based on ex-vivo ablations of non-perfused healthy animal livers treated at room temperature. No standard has been set yet, as it is known that differences in tissue composition and in procedure time result in significantly different outcomes [Citation14]. Additionally, some of the few studies of RFA on larger bone tumors, such as osteoblastomas [Citation15–17] and chondroblastomas [Citation18–21], have only focused on clinical outcomes, and not on the measurements of the ablation zones. Thus, the combination of the aforementioned factors makes it difficult to plan and predict the size of a RFA zone for the treatment of bone tumors.
Therefore, the aim of this study was to evaluate and report on the MRI and CT findings after carrying out a RFA on ACT in patients who were evaluated as possible candidates for RFA instead of intralesional curettage, to report the diameters of the tumors and the corresponding resulting diameters of their ablation zones, and to test whether or not RFA can produce large enough ablation zones to completely ablate the target tumor.
Materials and methods
Study design
Patients aged ≥18 with indicative signs of ACT, who had opted for surgical treatment (in contrast to watchful waiting), were selected based on a multidisciplinary assessment consisting of location, size, and aspects of the tumor on an MRI. At the time of the evaluation, the options were discussed with the patient: curettage with local adjuvants or RFA. This retrospective study is based on the patients who chose to be treated with RFA.
To study the size of the resulting ablation zones, we examined intra-operative Computed Tomography (CT) as well as pre and post-operative gadolinium-enhanced MR images of patients who underwent RFA to treat ACT from January 2015 to December 2017 at the University Medical Center Groningen (UMCG). Biopsies were taken at the time of the procedure prior to the RF ablation to confirm the characteristic imaging diagnosis of ACT. Only patients whose diagnosis of ACT had been histopathologically confirmed were selected for this retrospective study. Furthermore, only patients treated with a single-session single-application ablation were selected for this retrospective study in order to reduce the variability in ablation zone size produced by the needle placement in the cases requiring multiple overlapping ablations on the same tumor.
The patients were informed about the potential use of their anonymized data for scientific research with a written form at the time of the intervention; any patients who objected to sharing their data were excluded from the study. Since the procedure was part of the usual care, no additional written or verbal consent was necessary, which is in accordance with the regulations of the Medical Ethical Review Board of the University Medical Center Groningen.
The tumors were measured on a pre-operative gadolinium-enhanced MRI scan. The following electrode lengths were used for the ablation: tumor diameters in the 10–25 mm range were treated with a 20 mm exposed tip electrode, while tumors in the 25–35 mm range were treated using a 30 mm exposed tip electrode. Tumors > 35 mm were ablated with multiple overlapping ablations and were not included in this study. This decision was made based on our previous experience with total bone tumor ablations. However, the overall size of the tumor and its location also played an important role when choosing the electrode length and whether overlapping ablations were needed.
RFA procedure
All the RFA procedures were daycare treatments and were performed under CT guidance (Somatom Definition AS, Siemens Medical Systems, Erlangen, Germany) and general or spinal anesthesia by two experienced radiologists.
An 11 G bone needle was used to drill a hole in the bone, both for tissue sampling and to position the RFA electrode (17 G, Covidien CoolTip, Medtronic, USA). The procedure was temperature-controlled, non-cooled, with a target temperature of 90° Celsius at the tip of the electrode, and an ablation time of between 6 and 10 min. The time was estimated based on our prior experience of treating ACT and other bone tumors, particularly OO. Generally, if the temperature rose without a problem to 90° Celsius, the ablation time was 6 min; if there were problems reaching the target temperature (e.g., electrical impedance increasing fast and the system shutting off before reaching it) the electrode was repositioned slightly and the temperature was increased gradually by hand to reach 90° Celsius, which required up to 10 min of ablation. Additionally, in order to estimate the effects of time and temperature on the size of the ablation zones, we measured the duration of the ablation with the electrode at a temperature of ≥ 60° and the duration after it reached the target temperature of 90°.
Given the small size of the lesions, surgical stabilization was not necessary for these cases.
Evaluation RFA
A follow up MRI was performed 3 months after the procedure to assess the ablation zone using a 1.5 T MRI scanner (Siemens, Erlangen, Germany) with a surface coil. Both fat-suppressed Short Tau Inversion Recovery (STIR) T2-weighted sequences (TR/TE/TI: 8270/160/19 ms, slice thickness 4 mm) and T1-weighted images (TR/TE: 500/19 ms, slice thickness 4 mm), before and after the administration of an intravenous gadolinium-based contrast agent (0.1 mmol gadoterate meglumine (Dotarem®; Guerbet) per kg of body weight), were acquired in two planes (transversal and either coronal or sagittal) as part of the routine MRI protocol in the UMCG.
MRI analysis and measurements
The tumors were measured on the pre-operative MR images following the previously described directions. After ablation, the resulting ablation zones were measured on the follow-up gadolinium-enhanced T1-weighted MR images. The assessor was unaware of the size of the RFA needle used. Two diameters were defined and measured, one along the line of the electrode and one perpendicular to it. The diameter perpendicular to the electrode was assessed in both perpendicular planes and the shortest of the two was chosen. Pre- and postoperative MR images were used in order to compare the tumor and ablation diameters.
Based on the manufacturer’s information, the length of the electrode is one of the main factors defining the extent of the ablations. Longer electrodes produce a larger ablation zone, with a longer ablation diameter along the electrode and a shorter ablation diameter perpendicular to it. Therefore, the resulting ablation diameters were classified and analyzed according to the electrode length used.
Statistics
A Pearson’s correlation coefficient was calculated to test for the effect of the duration of treatment and the differences in ablation range. The means and standard deviations (SD) of the normally distributed data, as well as the medians and interquartile ranges (IQR) of the non-normally distributed data, are also presented. p values <0.05 were considered significant. All statistical analyses were performed using Python with the 2.7 version of SciPy [Citation22].
Results
A total of N = 16 patients matched the inclusion criteria namely, those treated with a single electrode position ablation (). The mean age of the patients was 48.8 years (±15.3, range 24–72). The treated anatomical locations were: femur (n = 12), tibia (n = 2), and humerus (n = 2) whereby two tumors resided in the diaphysis (one in the femur, one in the humerus) and 14 in the metaphysis. presents the entire patients’ parameters and outcomes, while gives a summary.
Figure 1. CONSORT diagram showing the inclusion and exclusion criteria and the resulting eligible patients, who were separated into two groups based on the length of the electrode used to treat them.
Table 1. Patients, parameters, and outcomes.
Table 2. Summary of the patients and ablations parameters.
The median ablation zone diameters of the 20 mm electrode were 42 (IQR 8.5) and 24.5 (IQR 19.5) mm for the longitudinal and perpendicular axes, respectively. Regarding the 30 mm electrode, the median ablation zone diameters were 44.5 (IQR 4.5) and 32.5 (IQR 7) mm for the longitudinal and perpendicular axes, respectively. The mean and median tumor and ablation zone diameters of all the data grouped according to electrode length, with their respective SD or IQR, for the perpendicular and longitudinal diameters, are summarized in . gives two examples of the ablation zone measurements.
Figure 2. (A, B) intraoperative CT and postoperative gadolinium-enhanced MR of a tumor ablated close to the cortical wall in a femur metaphysis. (C, D) intraoperative CT and postoperative gadolinium-enhanced MR images of a centrally located tumor ablated in a femur metaphysis.
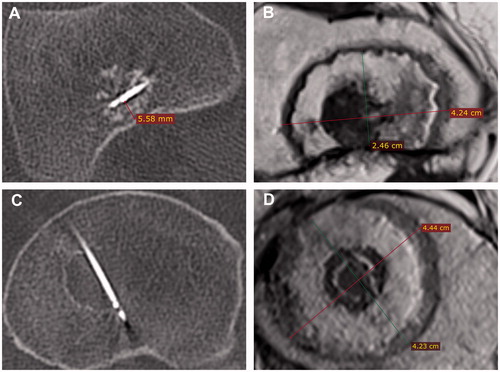
Table 3. Longitudinal and perpendicular diameters of the tumors and ablations. All measurements are in millimeters. Values with a * are normally distributed and represent thus the mean and standard deviation (SD), whereas the rest are non-normally distributed and represent the median and interquartile ranges (IQR), as indicated.
The mean ablation time with the tip of the electrode at 90 °C was 7.6 ± 1.5 min, (range 6–10 min) whereas with a temperature of ≥ 60 °C it was 9.25 ± 1.3 min (range 8–12 min). No significant correlations were found between the differences in the ablation diameters and their duration, neither for the time at 90 °C nor for the time with a temperature of ≥ 60 °C. The P-values for the longitudinal and perpendicular ablation diameters against time at 90 °C were p = 0.94 and p = 0.96 for the 20 mm electrode, and p = 0.76 and p = 1 for the 30 mm electrode. The p values for the perpendicular and longitudinal ablation diameters against time at ≥ 60 °C were: p = 0.94 and p = 0.93 for the 20 mm electrode, and p = 0.82 and p = 0.63 for the 30 mm electrode.
The median, IQR, and the range of the difference in length between the resulting ablation and tumor diameters were as follows: the 20 mm electrode (n = 10) had a perpendicular diameter of 9.5 mm (IQR 15.2, range 1–26), and a longitudinal diameter of 20.5 mm (IQR 9.5, range 4–26); the 30 mm electrode (n = 6) had a perpendicular diameter of 14 mm (IQR 5, range 8–18), and a longitudinal diameter of 20 mm (IQR 8.5, range 13–31). These results are summarized in and illustrated in .
Figure 3. Tumor diameters versus resulting ablation diameters of all 16 patients. Left: longitudinal ablation and tumor diameters. Right: Perpendicular ablation and tumor diameters. The solid diagonal lines in the graphs represent the point where the size of the ablation and the tumor could be the same, indicating the potential risk of recurrence.
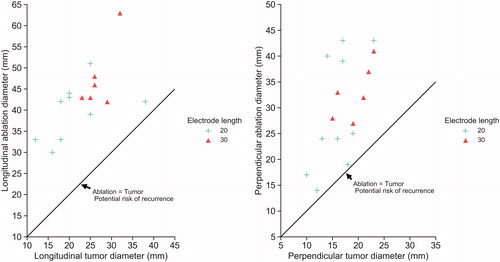
Table 4. Median, IQR, and range of the difference between the resulting ablation diameters and tumor diameters. All values are in millimeters.
All the ablations were technically successful (i.e., the ablated zone was ≥2 mm clear of the tumor on the 3-month follow up MRI, as planned).
Discussion
RFA is an attractive minimally invasive treatment alternative to surgery. The present study addressed an important knowledge gap on the extent of thermal damage by RFA on bone tumors. Applying RFA on ACT in the long bones, using a temperature-controlled algorithm with 20 and 30 mm straight non-cooled electrodes, resulted in ablations with median longitudinal diameters of 42 and 44.5 mm, respectively, which were large enough to completely cover the tumors being treated and more, as planned.
Although literature exists on the resulting ablation zones following RFA in tissues like the liver [Citation23,Citation24], RFA studies of bone tumors other than OO have mostly focused on the clinical outcomes while giving little insight into the extent of the thermal damage. This information is critical for reliable and accurate planning to ensure safe and effective RF ablation of bone tumors. The tumor should be completely ablated while damaging as little healthy surrounding tissue as possible. Therefore, further studies of the extent of RFA ablation zones in bones should be done to explore the possibilities of using this, apparently less risky, technique for the treatment of bone tumors.
Interestingly, since bone has lower thermal and electrical conductivities than soft tissues, such as the liver [Citation25], one could intuitively expect the resulting ablation zones to be smaller than those in soft tissue. However, this was not found in our study. The high water content of cartilaginous tumors together with the lack of heat sinks could be a plausible explanation for the large ablation zones. We have to consider that the main ablated tissue, the tumor, was cartilage instead of bone, and its high water content might result in more favorable conduction of current and heat. Furthermore, the electrical conductive property of cartilage seems to indicate that it is slightly higher than liver tissue (and much higher than bone), but it has similar thermal properties as the liver [Citation25]. Moreover, bone ablations do not suffer from heat sinks caused by nearby large blood vessels, which hinder the ablations. Some studies suggest that tumor tissue has a higher degree of electrical conductivity than healthy tissue [Citation26–28], which could further explain the large ablation zones obtained, since tissues with higher conductivities demonstrate more energy deposition [Citation29].
Another important remark about our study is that applying RFA to an ACT produced ablations with a much shorter perpendicular axis than longitudinal axis (). Although it is true that the longitudinal axis is supposed to be longer, the difference is probably less than it seems according to our numerical results. This discrepancy may be explained by the anatomical characteristics of the tumors and their surroundings, the planning of the procedures, and our aim to report the smallest ablation diameter in the perpendicular axes. Clear examples of this are patients numbers one and three (), whose ablation diameters were particularly shorter in the perpendicularly measured axis. This may have been because the ablations were confined to the small bone cavities (the diaphysis), where the cortical bone could have prevented the ablations from developing further, and therefore resulted in ablations that were as large as possibly allowed by the bone surrounding them. This effect, in turn, resulted in the reported perpendicular ablation diameters, which were as large as the bone in which they were confined i.e., short, yet effective, ablation diameters.
Similarly, this effect was observed when the tumors were located next to, but not completely surrounded by, cortical bone, as shown in , where an electrode was placed immediately next to the cortical bone. The resulting ablation zone seemed to be limited in the direction towards the cortical wall but not in the other directions. However, since we aimed to report the shortest diameter, we chose the diameter that was limited by the cortex. The apparent insulating properties of cortical bone are in accordance with other studies, such as the one by Pinto et al. [Citation30]. In contrast, the ablations seemed to extend more when they were not limited by the cortex, as in the case of the centrically located tumors in the femur metaphysis, as exemplified in . These two phenomena can be seen in the wide variations in the extent of the perpendicular ablations, as shown in , depending on whether they were limited by bone or not.
A limitation of this study is that we could not show the effects of the differences in ablation time on the resulting ablation diameters, as shown by the weak correlation coefficients. This could be because all the ablations were performed in a similar time frame of 6–10 min, after having reached the target temperature of 90 °C. Since ablations tend to grow rapidly in the first few minutes of the procedure and then reach a plateau, whereupon an increase in time results in small differences, we think that any additional changes after 6 min, due to thermal damage, are negligible. This does not mean, however, that this phenomenon occurs after exactly 6 min. More data with shorter and longer ablation times are needed to test this, but such experiments also come with the risk of unsuccessful ablations. Additionally, other factors may be affecting the size of the ablations, such as the histological characteristics of each individual tumor, the position of the electrode, etc.
Another limitation was the impossibility of finding a clear relationship between the results of the ablations and the location of the tumors, as there were many possible confounders and few data points per category (e.g., electrode used, bone type, bone location, etc.). This stresses the main limitation of the study, in that it was a single center study with only 16 patients. In addition, the ranges of the selected electrode lengths were not clear cut because we observed some overlap around the 25 mm zone. This could have been the result of differences in opinion in the assessment of the tumor, other than diameter, and thus as to which electrode to use for the intervention. We also observed one case of a considerably large tumor that was ablated with a 20 mm electrode (patient 1); the reasoning behind the decision to use this electrode is unknown to us. Any repositioning of the antenna was not reported, but it could explain why that electrode was chosen for a tumor of that size.
Likewise, patient 7’s ablation diameter was particularly large along the electrode, but we are not sure what caused this. Furthermore, although an MRI made 3 months after the procedure can be used reliably to show the results of a RFA applied to an OO, perhaps this is not the case for an ACT. The resulting ablated zones might have been underestimated because healing may have already occurred and the ablated zones had shrunk.
Finally, even though the results show that applying RFA to an ACT can produce large ablation zones, it is important not to overestimate the effects of a RFA and the procedures should be planned with care, particularly in complicated cases where overlapping ablations may be necessary. Complete tumor removal is, of course, preferable to having residual tumor tissue, and bone seems to be able to protect the surrounding structures from thermal damage, even if the ablations are larger than expected. However, other studies of RFA on chondroblastomas have shown the risk of damaging articular cartilage [Citation8] and the growth plate [Citation18]. Thus, it seems that bone may not be protective in all cases, which could be related to the thickness of the cortical bone or the perfusion of the surrounding tissues. These factors may also be the result of an underestimation of the extent of the ablations, as highlighted in this study. Therefore, extra caution should be taken when performing ablations close to structures at risk such as the cartilage surface of joints or nerve roots. Additionally, it is important to remember that our results were obtained without using the cool-tip mode, which, if used, could potentially result in even larger ablation zones.
The results of our study should help to understand the effects of RFA on bone tumors and highlight the importance of presenting not only the clinical outcomes of these procedures, but also the resulting ablation zones. A better understanding of RFA effects on bone is still needed for accurate and safe planning of the procedures.
Conclusion
Radiofrequency ablation of atypical cartilaginous tumors in bone with a temperature-controlled mode and straight non-cooled electrodes resulted in large enough ablations to treat the target tumors. The ablations here were larger than those seen in soft tissue.
Acknowledgement
We thank Stella Noach, Silvia Raquel Barrientos-Lopez, and Jadzia Siemienski for reviewing the paper.
Disclosure statement
The authors report no conflicts of interest.
References
- Doyle L. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120(12):1763–1774.
- Damron T, Ward W, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: national cancer data base report. Clin Orthop Relat Res. 2007;459:40–47.
- Marco R, Gitelis S, Brebach G, et al. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(5):292–304.
- Gelderblom H, Hogendoorn P, Dijkstra S, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13(3):320–329.
- Meftah M, Schult P, Henshaw R. Long-term results of intralesional curettage and cryosurgery for treatment of low-grade chondrosarcoma. J Bone Joint Surg Am. 2013;95(15):1358–1364.
- van der Geest I, de Valk M, de Rooy J, et al. Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol. 2008;98(6):421–426.
- Ruiz Santiago F, Castellano García M, Martínez Montes J, et al. Treatment of bone tumours by radiofrequency thermal ablation. Curr Rev Musculoskelet Med. 2009;2(1):43–50.
- Cantwell C, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607–617.
- Rimondi E, Mavrogenis A, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181–188.
- Tins B, Cassar-Pullicino V, McCall I, et al. Radiofrequency ablation of chondroblastoma using a multi-tined expandable electrode system: initial results. Eur Radiol. 2006;16(4):804–810.
- Dierselhuis E, van den Eerden P, Hoekstra H, et al. Radiofrequency ablation in the treatment of cartilaginous lesions in the long bones: results of a pilot study. Bone Joint J. 2014;96-B(11):1540–1545.
- Dierselhuis E, Jutte P, van den Eerden P, et al. Hip fracture after radiofrequency ablation therapy for bone tumors: two case reports. Skeletal Radiol. 2010;39(11):1139–1143.
- Lee M, Ahn J, Chung H, et al. Osteoid osteoma treated with percutaneous radiofrequency ablation: MR imaging follow-up. Eur J Radiol. 2007;64(2):309–314.
- Mertyna P, Hines-Peralta A, Liu Z, et al. Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol. 2007;18(5):647–654.
- Christoph R, Sprengel SD, Lehner B, et al. CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol. 2012;81(11):3426–3434.
- Wang B, Han S, Jiang L, et al. Percutaneous radiofrequency ablation for spinal osteoid osteoma and osteoblastoma. Eur Spine J. 2017;26(7):1884–1892.
- Arrigoni F, Barile A, Zugaro L, et al. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow- up. Int J Hyperthermia. 2018;34(3):1–7.
- Xie C, Jeys L, James S. Radiofrequency ablation of chondroblastoma: long-term clinical and imaging outcomes. Eur Radiol. 2015;25(4):1127–1134.
- Christie-Large M, Evans N, Davies A, et al. Radiofrequency ablation of chondroblastoma: procedure technique, clinical and MR imaging follow up of four cases. Skeletal Radiol. 2008;37(11):1011–1017.
- Petsas T, Megas P, Papathanassiou Z. Radiofrequency ablation of two femoral head chondroblastomas. Eur J Radiol. 2007;1(63–67):63.
- Lalam R, Cribb G, Tins B, et al. Image guided radiofrequency thermo-ablation therapy of chondroblastomas: should it replace surgery? Skeletal Radiol. 2014;43(4):513–522.
- Jones E, Oliphant T, Peterson P, et al. SciPy: Open Source Scientific Tools for Python. 2001. Available from: http://www.scipy.org/
- Heerink W, Solouki A, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol. 2018;28(8):3228–3236.
- Cassinotto C, Denys A, Gay F, et al. Radiofrequency ablation of liver tumors: no difference in the ablation zone volume between cirrhotic and healthy liver. Cardiovasc Intervent Radiol. 2018;41(6):905–911.
- Hasgall P, Di Gennaro F, Baumgartne R, et al. IT’IS Database for thermal and electromagnetic parameters of biological tissues, Version 4.02018. www.itis.ethz.ch/database
- Cheng Y, Fu M. Dielectric properties for non‐invasive detection of normal, benign, and malignant breast tissues using microwave theories. Thorac Cancer.. 2018;9(4):459–465.
- Sha L, Ward E, Stroy B. A review of dielectric properties of normal and malignant breast tissue. Proceedings IEEE SoutheastCon 2002; 2002. p. 457–462.
- Laufer S, Ivorra A, Reuter V, et al. Electrical impedance characterization of normal and cancerous human hepatic tissue. Physiol Meas. 2010;31(7):995–1009.
- Brace C. Radiofrequency and microwave ablation of the liver, lung, kidney and bone: what are the differences. Curr Probl Diagn Radiol. 2009;38(3):135–143.
- Pinto C, Taminiau A, Vanderschueren G, et al. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: tricks of the trade. AJR Am J Roentgenol. 2002;179(6):1633–1642.
