Abstract
Objectives: This randomized controlled trial (RCT) aims to compare the clinical application values of contrast-enhanced ultrasound (CEUS), computed tomography/magnetic resonance-CEUS (CT/MR-CEUS), and three-dimensional ultrasound-CEUS (3DUS-CEUS) Fusion imaging (FI) techniques in the assistance of thermal ablation for hepatocellular carcinoma (HCC).
Methods: A RCT was conducted on 374 patients with 456 HCCs between January 2016 and September 2017. CEUS, CT/MR-CEUS, and 3DUS-CEUS FI techniques were randomly used to assist HCC ablation. All lesions were ablated according to a previously determined plan, and FI groups required a 5-mm ablative margin. The primary endpoints were technical efficacy of thermal ablation and local tumor progression (LTP).
Results: According to randomization, 153 (18.8 ± 8.0 cm), 153 (18.3 ± 6.6 cm) and 150 (19.1 ± 6.9 cm) HCCs were assigned to CT/MR-CEUS, 3DUS-CEUS and CEUS groups respectively. Technical efficacy rates (99.3% vs. 100% vs. 100%) were achieved in the three groups, showing no statistical differences (p = 1.000). The median follow-up time was 24 (1–37) months. LTP rates at 1 and 2 years were 3.4%, 12.2% for CT/MR-CEUS FI, 4.8%, 9.0% for 3DUS-CEUS FI, and 8.6%, 19.9% for CEUS, respectively (p = .105). The results of subgroup analysis for LTP were statistically significant when patients with albumin-bilirubin (ALBI) grade 2 and 3 (p = .000), and tumor located at risky positions (p = .042). In addition, the p value in group of multiple tumors was close to .05 (p = .052).
Conclusions: All the three techniques are feasible for intraoperative HCC thermal ablation. Compared with CEUS, FI techniques are more suitable in patients with ALBI grade 2 and 3, multiple tumors, and in tumors at risky locations.
Introduction
Thermal ablation is a safe and curative treatment for hepatocellular carcinoma (HCC) and has been widely used for patients with early-stage HCC [Citation1–3]. Imaging is used through the whole process of ablation, including planning, targeting, monitoring, intraprocedural modification, and assessing treatment response [Citation4]. Because of its convenience, real-time display, lack of radiation, ultrasound (US) is well established and commonly used for the whole ablation procedure. Moreover, the European Federation of Societies for US in Medicine and Biology (EFSUMB) guidelines have highlighted the role of CEUS as a cost-effective technique with a good safety profile for detecting lesions, monitoring and guiding ablation, and evaluating treatment effect [Citation5]. However, for tumors with poor blood supply, it is hard for CEUS to locate and detect residual tumor, which may result in incomplete ablation and increase the incidence of local tumor progression (LTP) [Citation6]. Additionally, CEUS is unable to visually display the spatial relationship between the original tumor and the ablative zone, and the ablative margin (AM) is difficult to accurately measure [Citation7], since the AM is an independent factor for LTP [Citation8].
The development of fusion imaging (FI) brings hope to the task of overcoming the above problems. FI is the overlay of two or more imaging datasets together as one display [Citation9]. Furthermore, it fuses the pretreated index tumor range and that of ablative zone, to precisely determine whether sufficient AM is achieved [Citation5,Citation9]. US-based FI techniques are more suitable for intraprocedural application due to its simplicity, portability and real-time guidance. Generally this technique includes CT/MR-CEUS FI and 3DUS-CEUS FI. CT/MR-CEUS FI combines the high contrast resolution of CT/MR with the real-time and convenient performance of US, having distinctive advantages in HCC ablation procedures, especially in the identification, localization, and subsequent ablation of lesions that are not visible with B-mode US in the liver [Citation10,Citation11]. Moreover, our group has validated that CT/MR-CEUS FI is feasible for intraoperative use and is an accurate method to evaluate AM [Citation12,Citation13]. However, translocation, rotation, or deformation of the liver may interfere the registration accuracy [Citation6]. In contrast, three-dimensional (3 D) US-CEUS FI can minimize the effect of anatomical change and is more convenient in practice [Citation14,Citation15]. Furthermore, the feasibility and value of 3DUS-CEUS FI in HCC thermal ablation has been confirmed, but its application is restricted for lesions visible in B-mode US [Citation14].
Since the accuracy and feasibility in both FI techniques have been respectively validated [Citation12–14], and CEUS also plays a good role in the ablation procedure, it is necessary to conduct a randomized controlled trial (RCT) to evaluate their clinical application values in HCC ablation, and find out the most appropriate method targeting specific circumstances. Thus, this RCT aims to compare the clinical application values of CEUS, CT/MR-CEUS, and 3DUS-CEUS FI techniques in the assistance of thermal ablation for HCC.
Materials and methods
Study design
This was a prospective and parallel-group RCT that was registered as a Chinese domestic clinical trial (ChiCTR-ICQ-15007315) at clinicaltrials.gov in September 2015, China. This study followed the Declaration of Helsinki and was approved by the Ethical Review Board of our hospital. Informed consent was obtained from all patients. Enrolled patients were randomized at a 1:1:1 ratio into the CT/MR-CEUS group, 3DUS-CEUS group, or CEUS group, and the corresponding technique was used to assist the ablation procedure. Because of the nature of the interventions, the physicians and data collectors were not blinded to arm allocation. However, the radiologists who assessed technical efficacy of thermal ablation one month after ablation were blinded to arm allocation. The primary endpoints were technical efficacy of thermal ablation and LTP. The secondary endpoints were the applicable rate of the three techniques, technical success rate of thermal ablation, the rate of supplementary ablation, duration of ablation procedure, major complication and overall survival (OS) rates.
Sample size
A previous study with similar inclusion criteria as ours showed that the cumulative incidence of LTP was 14.5% at 5 years [Citation16]. In this study, reducing the incidence of LTP to 5% was determined to be clinically significant. For the application of a non-inferiority trial, 139 events were required in each group to achieve a power of 0.80 and a significance level of .05. Considering a dropout rate of 10%, a sample size of 150 lesions was, therefore, included for each group.
Patients
All patients recruited in this study received thermal ablation for curative purpose in our hospital between January 2016 and September 2017. The diagnostic imaging criteria of HCC was based on American Association for the Study of Liver Diseases (AASLD) [Citation17] or pathology. The inclusion criteria were as follows: (1) age 18 to 80 years; (2) a solitary tumor measuring < 5 cm, or multiple tumors (≤3 in number), each measuring < 3 cm; (3) Child-Pugh class A or B; (4) prolonged prothrombin time < 6 s or a platelet count of >50,000/μL. The exclusion criteria were: (1) allergy to the ultrasound contrast agents (UCA); (2) pacemaker; (3) no contrast-enhanced CT(CECT) or contrast-enhanced MR (CEMR) within one to three months after ablation.
Randomization
Dividing the patients’ hospitalization number by three, enrolled patients were randomized into three groups at a 1:1:1 ratio by the remainder. Patients with a remainder of one were assigned to CEUS group, those with a remainder of two were assigned to CT/MR-CEUS group, and those with a remainder of zero were assigned to 3DUS-CEUS group. The patients’ hospitalization numbers were generated by a computerized random number generator.
Instruments
The cooled-tip radiofrequency ablation (RFA) system (Covidien, Mansfield, MA, USA) and an internally cooled-tip electrode with a 3 cm tip were used. For microwave ablation (MWA), a microwave generator (Kangyou Cor., Nanjing, China) of 2450 MHz and internally cooled microwave antenna were used. The Mylab Twice (ESOATE, Italy) machine with Virtual Navigator (VN) and three-dimensional software were employed. A convex array probe CA541 was used for US examination and FI. CEUS was performed using the real-time contrast-enhanced imaging technique with a mechanical index less than 0.05.
Thermal ablation procedures
All ablation procedures were performed under general anesthesia by three senior interventional sonographers who had at least six years of experience.
Selection of RFA and MWA
RFA was the priority, especially for lesions near critical organs and structures (major hepatic vessel, diaphragm, gastrointestinal tract, gallbladder, and major intrahepatic bile duct, etc.) <5 mm or in difficult puncture locations. However, in some circumstances, including for tumors >3 cm in maximum diameter, distance to critical organs and structures >5 mm, or patients with poorer coagulation function, MWA was preferred.
Auxiliary methods for thermal ablation
When lesions adjacent to the diaphragm or appearing to be interfered with by gas in the lungs, artificial pleural fluid was employed. When lesions were adjacent to the gastrointestinal tract and gallbladder, artificial ascites were employed. Warm normal saline (0.9%; 300–2000 ml) was injected into the peritoneal or right pleural cavity via an inserted tube to produce artificial pleural fluid or artificial ascites.
Implementation of thermal ablation
Generally, the RF generator was set in impedance mode with a maximum output. Each insertion of an RF electrode took approximately 12 min. The MW generator was set at 60 watts and maintained for 6 min in each MW antenna insertion. Firstly, the corresponding technique would be used to locate the tumor. Secondly, all lesions were ablated according to a previously determined plan, and multiple insertions were required to obtain a sufficient ablation zone and a 5-mm AM, as much as possible. If patients had poor liver function, lesions were in a difficult puncture location, or were <5mm from critical organs and structures, AM was not always required. Thirdly, the corresponding technique was performed to monitor the ablation. After thermal ablation, the technical success of thermal ablation was immediately assessed with the corresponding technique. Supplementary ablation was applied if residue was found in the CEUS group, and the adequate 5 mm AM was not achieved in the CT/MR-CEUS and 3DUS-CEUS groups, until the previously determined ablation plan was finished.
Implementation of CEUS and FI techniques
CEUS
SonoVue (Bracco, Italy) was used as the UCA, which was infused through a peripheral vein at a dose of 2.4 ml and washed with 5 ml sterile saline. As for treatment response assessment, CEUS was performed 10 min after ablation, in order to decrease the interference of gas in the ablative area. During the CEUS evaluation, the non-perfusion zone covered the whole lesion, which was defined as complete ablation.
CT/MR-CEUS FI
First, one CT/MR portal or delayed phase serials in digital Imaging and Communications in Medicine (DICOM) data, which were acquired within two weeks before the thermal ablation procedure, were transferred into the navigation system, and the margin of the index lesion plus a 5-mm AM were outlined in two different colors in the CT/MR images. Second, preliminary registration was performed based on a fixed reference plane on both CT/MR images and real-time US images. Adjacent structures, such as bifurcations or confluences of the portal, hepatic veins, and organ contour were frequently used as anatomical landmarks. After preliminary registration, the CT/MR was fused and interacted with real-time US. Additional refinement was conducted to precisely match the CT/MR images with the real-time US images. All preliminary registrations and refinements were performed at the end of expiration. As for treatment response assessment, CEUS with a comprehensive sweep scan of the ablation zone was exerted in the overlapped mode of CT/MR with CEUS, in order to determine whether the non-enhanced ablative area encompassed both the tumor and the AM. The detailed steps have been previously described [Citation13,Citation18].
3DUS-CEUS FI
First, 3DUS volume data of the index lesion was acquired in free-hand scanning mode at the end of an expiratory breath before ablation. These 3DUS volume images were taken as reference images and were used to outline the margin of the tumor and a 5-mm AM in different colors. Second, because the 3DUS volume images were acquired immediately before the ablation procedure and the position of the patient was fixed, the 3DUS volume images could be automatically fused with the real-time US images, with the aid of the magnetic positioning system. After ablation, refinement was required to obtain a satisfactory registration. The treatment response assessment was similar with CT/MR-CEUS FI. The detailed steps have been previously described [Citation14].
Follow-up
US examination was performed within 24–72 h to observe possible early complications, and all major complications were recorded. One month after ablation, CECT or CEMR was employed to evaluate the technical efficacy of thermal ablation. If ablation was considered technically effective, a follow-up was repeated every 3 months.
Data analysis
1. The applicable rate: The applicable rate refers to the percentages of lesions applicable to a specific technique. For FI techniques, successful performance with a registration error distance less than 3 mm was considered applicable. For CEUS, the clear visualization of the perfusion for lesion and non-perfusion zone for ablative zone were considered applicable. If FI techniques failed in the ablation procedure, CEUS was the compensation, and US was used to compensate if CEUS failed. The failed case would not be used to analyze other rates.
2. Duration of ablation procedure was defined as the time between the beginning of lesion localization and the end of treatment effect assessment.
3. Technical success of thermal ablation refers to the percentage of lesions that technical success was achieved according to FI techniques or CEUS. Technical efficacy of thermal ablation refers to the percentage of lesions that the complete tumor necrosis according to the CECT/CEMR one month after ablation. The applicable rate, the technique success and technical efficacy of thermal ablation and LTP were assessed on a tumor by tumor basis. Duration of ablation procedure, Major complications and OS were evaluated on a per-patient basis.
Statistical analysis
Comparisons among three groups were performed with Pearson’s χ2 text. For the duration of ablation procedure, comparisons were performed with the Mann–Whitney test. OS, LTP rates and the subgroup analyses of LTP rate were analyzed with Kaplan–Meier method. Statistical analyses were conducted using SPSS 25.0 software (SPSS, Chicago, IL, USA). p < .05 was considered statistically significant.
Results
Enrollment
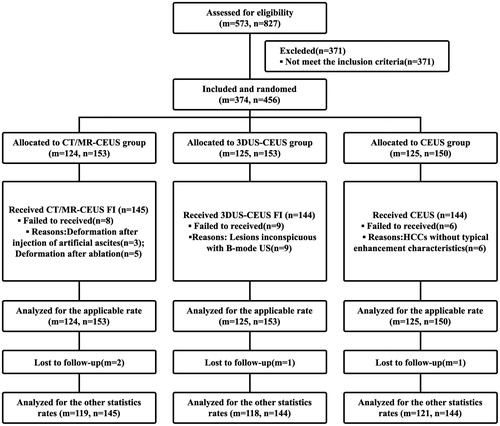
The consort diagram for this study was shown in . Finally, 374 patients with 456 HCCs were included from January 2016 to September 2017. According to randomization, 124 patients with 153 HCCs were assigned to CT/MR-CEUS group, 125 patients with 153 HCCs were assigned to 3DUS-CEUS group, and 125 patients with 150 HCCs were assigned to the CEUS group. summarizes the baseline characteristics of the patients and tumors. There were no significant differences among the three groups for any of the variables. Two patients in the CT/MR-CEUS group, one in the 3DUS-CEUS group, and one in the CEUS group were lost to follow-up.
Table 1. Baseline patient and tumor characteristics.
The applicable rate and duration of ablation procedure
The applicable rates for CT/MR-CEUS, 3DUS-CEUS, and CEUS groups were 94.8% (145/153), 94.1% (144/153), and 96.0% (144/150), respectively, and there was no significant difference among the three groups (p = .749). Besides, there were eight lesions in CT/MR-CEUS group, nine in 3DUS-CEUS group and six in CEUS group failed to apply the certain technique during ablation, and the reasons were showed in .
The duration of the ablation procedures for CT/MR-CEUS, 3DUS-CEUS FI, and CEUS were 56 (20–183) min, 55 (10–179) min, and 53 (10–165) min, respectively, and the difference was not significant either (p = .206).
Technical success rate and the rate of supplementary ablation
There were 13.8% (20/145) lesions in CT/MR-CEUS group, 20.1% (29/144) lesions in 3DUS-CEUS group and 9.0% (13/144) lesions in CEUS group received supplemental ablation to meet the requirement of complete ablation after the immediate assessment, and the differences among the three groups were statistically significant (p = .026). Finally, all lesions were identified as completely ablated (), and the technical success rates of thermal ablation were 100% in all three groups.
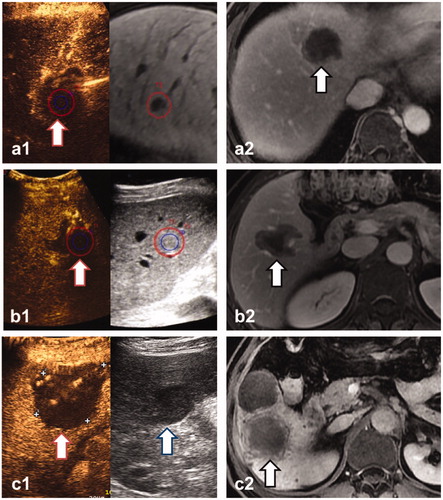
Figure 2. Three lesions were evaluated by CT/MR-CEUS FI (a), 3DUS-CEUS FI (b) and CEUS (c) respectively, and met the requirement of complete ablation. a1, b1: The real-time US is shown at left, the pre-ablation CEMR(a1) and 3DUS(b1) images are shown at right, and the margin of lesion (blue circle) plus a 5-mm AM (red circle) were outlined. The non-perfusion area had entirely covered the lesion and 5-mmAM (red arrow), showing that the lesions were ablated completely and sufficient AM were obtained. c1: The index lesion is shown at right (blue arrow). After ablation, the non-perfusion area covered the whole lesion (red circle), showing complete ablation. a2, b2, c2: CEMR one month after ablation demonstrated the technical efficacy of the ablation (black arrow).
Follow-up and survival
Major complications
There were no treatment-related deaths. The rates of major complications for the CT/MR-CEUS, 3DUS-CEUS, and CEUS groups were 0.84% (1/119), 0.85% (1/118), and 0.83% (1/121), respectively, and the difference was not statistically significant (p = 1.000). In the CT/MR-CEUS group, there was one case of infection related to a bile leak, which was controlled after percutaneous drainage. One case of needle track bleeding occurred in the 3DUS-CEUS group, which was cured after complementary ablation and blood transfusion. In addition, a case of liver abscess in the CEUS group was controlled after percutaneous drainage.
Technical efficacy rate
Within one to three months after the ablation, all patients received CECT/MR. One residual lesion was detected, which was in CT/MR-CEUS group. Hence, technical efficacy rate for the CT/MR-CEUS, 3DUS-CEUS, and CEUS groups were 99.3% (144/145), 100% (144/144), and 100% (144/144), respectively, and the difference was not statistically significant (p = 1.000). The residual HCC in CT/MR-CEUS group, was located in the subcapsular area, which was in a fairly deep location of liver, and inconspicuous with B-mode US.
LTP
The median follow-up time was 24 (1–37) months. There were six, six and 14 LTPs in the CT/MR-CEUS, 3DUS-CEUS, and CEUS groups, respectively. The 1-, and 2-year LTP rates were 3.4%, 12.2% in the CT/MR-CEUS group, 4.8%, 9.0% in the 3DUS-CEUS group, and 8.6%, 19.9% in the CEUS group, respectively. There were no significant differences in the rate of LTP (p = .105) among the three groups (). Subgroup analysis was carried out for six potential prognostic factors among the three groups, which were showed in . Because the numbers of patients with no liver cirrhosis, Child-Pugh class B, AFP level >200 ng/ml and tumor measuring >3cm were too less to be assessed, subgroup analysis did not include these factors. The results were statistically significant when patients with ALBI grade 2 and 3 (p = .000, ) and tumor located at risky positions (p = .042, ) for LTP. Besides, the p value in group of multiple tumors for LTP was close to .05 (p = .052, ).
Figure 3. LTP curves among CT/MR-CEUS, 3DUS-CEUS and CEUS groups. (A) There was no significant difference among the three groups in the whole lesions (p = .105). (B and C) The results by subgroups of patients with ALBI grade 2 and 3 and tumors located at risky positions were significantly associated with higher LTP rates (p = .000, .042, respectively). (D) The results by subgroups of multiple tumors was close to 0.05 (p = .052).
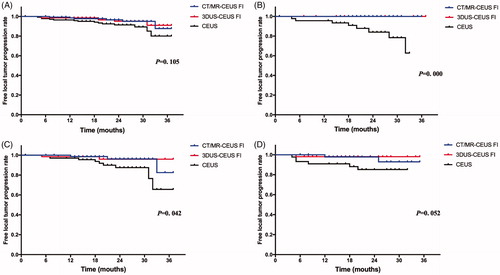
Table 2. Subgroup analysis of LTP among CT/MR-CEUS, 3DUS-CEUS and CEUS groups.
Survival
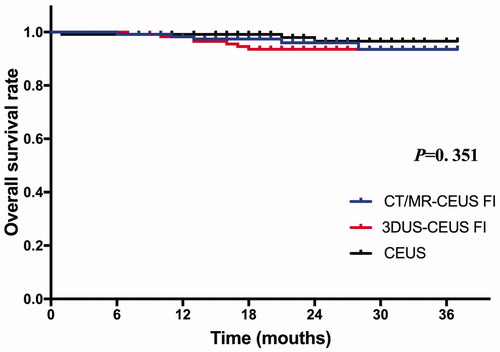
During the follow-up, five patients (4.3%, 5/117) in the CT/MR-CEUS group, seven (6.0%, 7/117) in the 3DUS-CEUS group and three (2.5%, 3/120) in the CEUS group died. The causes of death included HCC progression in four, and variceal bleeding in one (0.7%) in the CT/MR-CEUS group, and HCC progression in four, multiple organ failure in three in the 3DUS-CEUS group, and HCC progression in two and multiple organ failure in one in the CEUS group. The 1-, and 2-year OS rates were 96.0%, 93.5% in the CT/MR-CEUS group, 93.5%, 93.5% in the 3DUS-CEUS group, and 98.0%, 96.6% in the CEUS group, respectively. There was no significant difference in the OS rate (p = .351) among three groups ().
Discussion
In this study, we used the technical efficacy of thermal ablation and LTP as the primary endpoint to evaluate the clinical application values of three kinds of techniques in the assistance of ablation for solitary HCC measuring <5cm or no more than three HCCs, each measuring <3cm in the randomized controlled trial. As for the technical efficacy of thermal ablation, many reports have indicated that CT/MR-CEUS, 3DUS-CEUS FI techniques and CEUS can accurately assess the therapeutic efficacy [Citation13,Citation14,Citation19]. Previous studies have shown that the technical efficacy rates of CT/MR-CEUS, 3DUS-CEUS FI techniques and CEUS range 95.7–100% [Citation10,Citation13,Citation20], 96–100% [Citation14,Citation15,Citation21], 91–100% [Citation19,Citation22,Citation23], respectively. In this study, the technical efficacy rates for CT/MR-CEUS, 3DUS-CEUS FI techniques and CEUS were 99.3%, 100%, and 100%, respectively, which were similar to the results of previous studies. Furthermore, this randomized controlled trial illustrated powerfully that these three kinds of techniques can not only be applied intraoperatively, but also assess the immediate ablation outcomes precisely. In addition, early detection of residual HCC after thermal ablation is critical and can facilitate successful treatment [Citation21]. Once the residue was found, supplementary ablation could be performed immediately to enlarge the ablation zone. Our result showed that the supplementary ablation rates in FI groups were higher than CEUS group. In our opinion, FI techniques can display the spatial relationship between the original tumor and the ablative zone, which can help to find the residue and improve the AM intraoperatively. However, the intraoperative detection of residue by routine CEUS is mainly based on the appearance of characteristic tumor enhancement, which depends more on the experience of an individual surgeon. Besides, Previous study [Citation24,Citation25] showed that sub-capsular tumor location was an important factor affecting mistargeting after FI-guided ablation of HCCs. Because large anatomic landmarks such as the portal vein branch cannot be used to locate this kind of tumor, and the rib shadow could obscure the vision of tumor. It greatly increases the difficulty of ablation. In our study, this technical difficulty of tumor in sub-capsular location was also observed in CT/MR-CEUS group. Thus, we should pay more attention to this kind of tumor.
Secondly, as for LTP, Studies have reported that LTP after thermal ablation for liver malignancies ranged between 1.6 and 26.0% [Citation26]. An AM of 5 mm or more for a typical HCC was reported to be necessary to prevent LTP [Citation27] because of the presence of microsatellite lesions around a main tumor [Citation28]. Therefore, in this study, we not only required complete ablation, but also achieved a 5-mm AM, as much as possible, to decrease the incidence of LTP. Our result showed that the LTP rates for CT/MR-CEUS, 3DUS-CEUS FI techniques and CEUS were 12.2%, 9.0%, and 19.9%, respectively, which were similar to previous studies. Although there was no significant difference among the three techniques in terms of LTP, there was a trend at two years. Then, we continued to conduct a subgroup analysis, and found that FI techniques could reduce the rate of LTP in patients with ALBI grade 2 and 3, and in tumors at risky locations. The ALBI grade is defined using the ALBI score, which is calculated based on levels of bilirubin and albumin in the serum, and could assess the severity of liver dysfunction in patients with HCC [Citation29–31]. To protect liver function for patients with ALBI grade 2 and 3, we had better restrict the ablation margin, which may lead to LTP. FI techniques can precisely evaluate the treatment response, and then reduce the occurrence of LTP. In addition, when tumors are located at risky locations, FI techniques have the unique advantage in better demonstration of the relationship between lesion and the critical organs and structures, including major hepatic vessels, diaphragm, gastrointestinal tract, gallbladder, and major intrahepatic bile duct. Thus, the operators have more confidence in locating lesions and planning for adequate electrode paths. Besides, the precise assessment of AM can prevent damage to surrounding vital tissues, and reduce the occurrence of LTP as well as complications. As for multifocal tumors, the p value among the three groups was close to .05, and we considered that FI techniques were more helpful in multifocal tumors ablation. Multiple tumors need multiple punctures and ablations. The hyperechoic “cloud” of gas caused by heating immediately after ablation, will greatly obscure US images of the lesion, and make the guidance of next puncture difficult [Citation32,Citation33]. FI techniques can locate the lesion and guide subsequent punctures without the interference of vaporization, helping to reduce the difficulty of the procedures. Thus, we think FI techniques are more suitable in patients with ALBI grade 2 and 3, multifocal tumors, and in tumors at a risky location.
However, CT/MR-CEUS, 3DUS-CEUS FI techniques and CEUS have their own intrinsic limitations. Our team has compared two FI techniques in a paired study [Citation6]. The result showed that the major reason for the failure of 3DUS-CEUS and CT/MRI-CEUS FI were the inconspicuous lesions on conventional US images and the deformation of liver after pretreatment CT/MR, respectively. Du et al. [Citation22] indicated that applying CEUS to ablation procedure was not always beneficial because some HCCs do not show typical arterial phase hyper-enhancement and portal venous/delayed phase washout on CEUS. The reasons for the failure in this studies were similar to the previous study. When lesions are inconspicuous with B-mode US, clear pre-ablation 3DUS volume images could not be obtained as reference images for fusion with the post-ablation CEUS images, which restricts the appliance of 3DUS-CEUS FI. Ahn et al. [Citation10] demonstrated that US-CT/MR FI can significantly improve the tumor visibility. CT/MR-CEUS FI can be performed without the restriction of vision in B-mode US, since it was equipped with the high contrast resolution of CT/MR, which can display the lesions clearly. However, in CT/MR-CEUS FI, because the CT/MRI images were usually obtained several days before the ablation procedure, the inherent imaging deformations between US and CT/MR images were inevitable, especially when patients received artificial ascites, pleural effusion or other assistant procedures. These kinds of anatomical changes would occur after pretreatment CT/MR, influencing the accuracy for location of lesions. 3DUS-CEUS FI can be the compensation because the pretreatment 3DUS images were obtained immediately during the procedure and would not be influenced by the anatomical changes [Citation14]. The anatomical consistency of US images between pre-ablation and post-ablation was better. Thus, in our opinion, CT/MR-CEUS FI could be the priority in the assessment of treatment response for the lesions inconspicuous with B-mode US, and 3DUS-CEUS FI can be the compensation for the patients receiving assistant procedures.
Furthermore, the duration of the ablation procedures among the three groups has no statistical significance. Compared with CEUS, FI techniques ensure accurate assessment without prolonging the total duration of the ablation procedure. In addition, the incidences of major complications among the three groups were relatively low, indicating that these techniques are safe and will not increase the risk of ablation.
Our study has some limitations. Firstly, because the numbers of death in each group were relatively small, and the median follow-up time was only 24 months, long-term follow-up should be conducted in all the patients to observe the long-term treatment effect. Although there were no significant differences in the rate of LTP (p = .105) among the three groups, there was a trend at two years, which would be likely to show a true difference in the long-term observation. Secondly, the number of HCC measuring ≥3cm was about 10% in this article, which might cause grouping bias and affect the comparison of LTP among the three techniques. Thus, further studies will include more median and big lesions to clarify this problem. Third, 3DUS-CEUS FI had the weakness of lesions inconspicuous with B-mode US. CEUS clearly showed the blood perfusion of tissues and organs, therefore, we attempted to acquire the 3DCEUS as a 3 D volume image before ablation, and compared it with CEUS image after ablation, which showed great improvement of the assessment effect in lesions inconspicuous with B-mode US. Subsequent research will be conducted to evaluate its feasibility and accuracy in HCC thermal ablation.
In conclusion, all the three techniques are feasible for intraoperative HCC thermal ablation. Compared with CEUS, FI techniques are more suitable in patients with ALBI grade 2 and 3, multiple tumors, and in tumor at a risky location. Besides, CT/MR-CEUS FI could be the priority in the assessment of treatment response for the lesions inconspicuous with B-mode US, and 3DUS-CEUS FI can be the compensation for the patients receiving assistant procedures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39(2):187–210.
- Xu E, Long Y, Li K, et al. Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int J Hyperthermia. 2018;35(1):159–167.
- Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI. World J Gastroenterol. 2014;20(15):4160–4166.
- Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23(1):190–197.
- Abi-Jaoudeh N, Kruecker J, Kadoury S, et al. Multimodality image fusion-guided procedures: technique, accuracy, and applications. Cardiovasc Intervent Radiol. 2012;35(5):986–998.
- Ahn S, Lee J, Lee D, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66(2):347–354.
- Bo X, Xu H, Wang D, et al. Fusion imaging of contrast-enhanced ultrasound and contrast-enhanced CT or MRI before radiofrequency ablation for liver cancers. Br J Radiol. 2016;89(1067):20160379.
- Li K, Su Z, Xu E, et al. Evaluation of the ablation margin of hepatocellular carcinoma using CEUS-CT/MR image fusion in a phantom model and in patients. BMC Cancer. 2017;17(1):61.
- Li K, Su Z-Z, Xu E-J, et al. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016;16(1):277.
- Xu EJ, Lv SM, Li K, et al. Immediate evaluation and guidance of liver cancer thermal ablation by three-dimensional ultrasound/contrast-enhanced ultrasound fusion imaging. Int J Hyperthermia. 2018;34(6):870–876.
- Ye J, Huang G, Zhang X, et al. Three-dimensional contrast-enhanced ultrasound fusion imaging predicts local tumor progression by evaluating ablative margin of radiofrequency ablation for hepatocellular carcinoma: a preliminary report. Int J Hyperthermia. 2019;36(1):55–64.
- Lee D, Lee J, Lee J, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909.
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Zhong-Zhen S, Kai L, Rong-Qin Z, et al. A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall Med. 2012; 33:E250–E255.
- Xuan M, Zhou F, Ding Y, et al. Diagnostic accuracy of contrast-enhanced ultrasound in assessing the therapeutic response to radio frequency ablation for liver tumors: systematic review and meta-analysis. Surg Endosc. 2018;32(4):2067–2075.
- Bo XW, Xu HX, Guo LH, et al. Ablative safety margin depicted by fusion imaging with post-treatment contrast-enhanced ultrasound and pre-treatment CECT/CEMRI after radiofrequency ablation for liver cancers. Br J Radiol. 2017;90(1078):20170063.
- Minami Y, Minami T, Hagiwara S, et al. Ultrasound-ultrasound image overlay fusion improves real-time control of radiofrequency ablation margin in the treatment of hepatocellular carcinoma. Eur Radiol. 2018;28(5):1986–1993.
- Du J, Li HL, Zhai B, et al. Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol. 2015;41(9):2400–2411.
- Nishigaki Y, Hayashi H, Tomita E, et al. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2015;45(4):432–440.
- Lee MW, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation of small (1–2 cm) hepatocellular carcinomas inconspicuous on B-mode ultrasonographic imaging: usefulness of combined fusion imaging with MRI and contrast-enhanced ultrasonography. Can J Gastroenterol Hepatol. 2018;2018:1.
- Lim S, Lee MW, Rhim H, et al. Mistargeting after fusion imaging-guided percutaneous radiofrequency ablation of hepatocellular carcinomas. J Vasc Interv Radiol. 2014;25(2):307–314.
- Yu J, Liang P, Yu XL, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25(4):1119–1126.
- Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol. 2007;188(2):480–488.
- Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95(9):1931–1937.
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558.
- Wang J, Zhang Z, Yan X, et al. Albumin-bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51(8):1172–1178.
- Rhim H, Lee M, Kim Y, et al. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. Am J Roentgenol. 2008;190(5):1324–1330.
- Thamtorawat S, Hicks R, Yu J, et al. Preliminary Outcome of microwave ablation of hepatocellular carcinoma: breaking the 3-cm barrier? J Vasc Interv Radiol. 2016;27(5):623–630.