Abstract
Purpose: Adenomyosis is a relatively common disease among women of childbearing age. A minimally invasive alternative technique with low risks, faster recovery and decreased side effects is desired. We hypothesized that percutaneous microwave ablation (PMWA) under laparoscopic guidance would substantially reduce the risk of collateral thermal damage to the intestinal tract and relieve the pelvic adhesions. This study aimed to evaluate the feasibility, safety and efficacy of transvaginal ultrasound- and laparoscopy-guided PMWA for the treatment of adenomyosis.
Materials and methods: From May 2015 to October 2017, a total of 70 patients with symptomatic adenomyosis who underwent transvaginal ultrasound- and laparoscopy-guided PMWA were included in this study. The technical efficacy and complications of PMWA were assessed. Meanwhile, the uterine volume, lesion volume, symptom severity score (SSS) and visual analog scale (VAS) score before PMWA and at 1, 6 and 12 months after PMWA were recorded.
Results: PMWA was successfully performed with transvaginal ultrasound guidance and laparoscope assistance in all patients. No major complication was found after PMWA in any patients. The uterine volume, lesion volume, SSS and VAS were all decreased significantly at follow-up (p < .01).
Conclusion: Transvaginal ultrasound- and laparoscopy-guided PMWA, which significantly decreased the uterine volume, lesion volume, SSS and VAS score, is a feasible minimally invasive technique for the treatment of adenomyosis.
Introduction
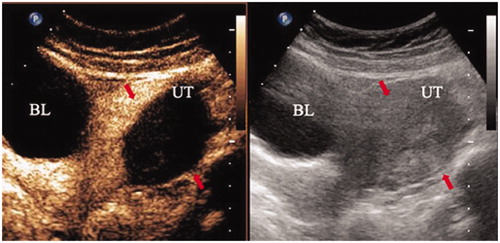
Adenomyosis is a relatively common disease among women of childbearing age with prevalence ranging from 8% to 27% [Citation1–3]. Symptomatic patients often complain of dysmenorrhea, metrorrhagia, chronic pelvic pain, dyspareunia and infertility, which seriously influence the quality of daily life. Adenomyosis is often identified by uterine enlargement, and it is frequently found in the posterior uterine wall [Citation4].
Medical therapy may relieve symptoms of adenomyosis to a certain degree, but medical therapeutic approaches may be ineffective in the long run, and relapse of symptoms frequently occurs.
Hysterectomy remains the most popular treatment for adenomyosis, while it is not suitable for women of reproductive age who have a strong desire to preserve the uterus and fertility [Citation5]. Recently, some less invasive treatments have emerged, such as uterine artery embolization (UAE), image-guided ablation techniques, including high-intensity focused ultrasound ablation (HIFU), radiofrequency ablation (RFA) and microwave ablation (MWA) [Citation6–12].
UAE is considered safe and effective, but its effects on ovary function and fertility is still under evaluation [Citation13–15]. A minimally invasive alternative technique with low risks, faster recovery and decreased side effects is desired.
HIFU with ultrasound or magnetic resonance imaging (MRI) guidance is safe and effective in the treatment of adenomyosis, but the technical eligibility limits its wide range of applications [Citation9]. RFA is also an effective minimally invasive treatment, but it has low thermal efficiency and relatively long treatment time. MWA has gained increasing attention because of its high thermal efficiency. Moreover, ultrasound-guided percutaneous microwave ablation (PMWA) for adenomyosis can relieve symptoms of adenomyosis and reduce uterine volume [Citation12,Citation16,Citation17], and it does not affect ovary function [Citation11,Citation12,Citation18]. However, PMWA has the potential risk of collateral thermal damage to the nearby intestinal tract, and a percutaneous approach is technically challenging because of bowel interference. PMWA performed with artificial ascites may improve access for treatment of adenomyosis [Citation16], while it is still limited for patients with pelvic adhesions.
We hypothesized that PMWA guided with laparoscope would substantially reduce the risk of collateral thermal damage to the intestinal tract and relieve the pelvic adhesions. Thus, this study aimed to assess the feasibility, safety and efficacy of transvaginal ultrasound- and laparoscopy-guided PMWA for adenomyosis.
Materials and methods
Patients
From May 2015 to October 2017, 70 patients with diffuse adenomyosis who underwent transvaginal ultrasound- and laparoscopy-guided PMWA at our center were included in this prospective study.
The study was conducted according to the principles of the Declaration of Helsinki and approved by the Human Ethics Review Committee of the Central Hospital of Wuhan. Written informed consent for the procedure and study was obtained from all patients.
All patients were diagnosed with adenomyosis using transvaginal ultrasonography and MRI. In this study, the diagnostic criteria of uterine adenomyosis on transvaginal ultrasonography were as follows: (1) no distinction between the endometrium and myometrium, (2) asymmetry of the posterior and anterior myometrium, (3) subendometrial myometrial striations, (4) myometrial cysts and fibrosis and (5) heterogeneous myometrial echotexture [Citation19–21]. Moreover, the diagnostic criteria of uterine adenomyosis on MRI were as follows: (1) a myometrial mass with indistinct margins of primarily low intensity, (2) diffuse or local widening of junctional zones on T2-weighted images, (3) junctional zone thickness >15 mm, (4) uterine enlargement and (5) small hypointense myometrial spots [Citation3].
The inclusion criteria of the study were (a) adenomyosis diagnosed by imaging (transvaginal ultrasonography or MRI) and syndrome (e.g., dysmenorrhea and/or menorrhagia); (b) patients with a strong desire to preserve the uterus and fertility and (c) patients who accepted the PMWA technique voluntarily.
The exclusion criteria were (a) coexistence of severe disease of other important organs such as the brain, heart and liver; (b) patients with menopausal status and (c) patients with a history of other interventional treatment, such as UAE and high-frequency focused ultrasonography.
PMWA procedures
To avoid inter-operator variability, PMWA and ultrasonography were performed by the same operators during the study period. PMWA was performed in an outpatient operating room. The patient was placed on the supine lithotomy position, and laparoscopy (OLYMPUS HD EndoEYE, Japan) was performed under general anesthesia. Artificial pneumoperitoneum widened the distance between the uterus and surrounding organs. The surface color and shape of the uterus could be seen clearly. Pelvic adhesions (if existed) were loosened and treated with laparoscopy first. Under real-time monitoring of laparoscopy and transvaginal ultrasound (4–9 MHz, Philips IU22, Netherlands), a 16-gauge needle (BARD, Tempe, AZ, USA) was inserted into the lesion for biopsies, avoiding important blood vessels. A cooled-shaft microwave tumor coagulator (ECO-100, Yigao Microwave System Corp., Nanjing, China) consisting of a 15-gauge needle antenna with a 1.0 cm exposed tip was used for PMWA, and it can produce 150 W of power at 2450 MHz.
According to the location and size of the lesion, MWA needle insertion was made with the ‘moving shot’ technique, the lesion was divided into multiple, small conceptual ablation units, and WMA was performed unit-by-unit by moving the electrode until the lesion was ablated completely. The antenna was percutaneously inserted across the abdominal wall and was placed close to the serosa of the uterus under laparoscope monitoring; then, avoiding large vessels, it was inserted into the planned site of the lesion under transvaginal ultrasound guidance (). A power output of 60 W was used during PMWA. The lesions were ablated unit-by-unit by moving the antenna tip. The distance between two units was approximately 1.5–2.5 cm, and each unit was ablated for 2.5–3.5 min. On transvaginal ultrasonography, once the hyperechogenic signal covered the entire lesion or reached 3–5 mm near the margin of the serosa or uterine endometrium, the ablation procedure was halted and the needle was withdrawn (). Laparoscopy showed the color of the lesion surface changing from bright red to dark red and finally to pale, which suggested that the ablation effect was satisfactory. Contrast-enhanced ultrasonography (CEUS, SonoVue, Bracco, Italy) was immediately performed trans-abdominally to assess the effectiveness of WMA, and necrotic areas were shown as nonenhanced areas on CEUS ().
Figure 1. Laparoscope and transvaginal ultrasound images of the uterus. Laparoscope image before (A) and after (B) needle antenna puncturing into the uterus. Transvaginal ultrasound image before (C) and after (D) needle antenna (arrow) puncturing into the uterus.
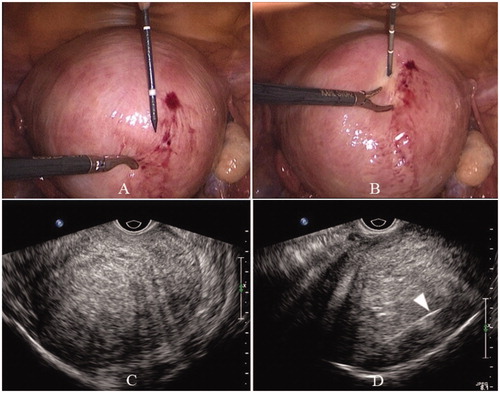
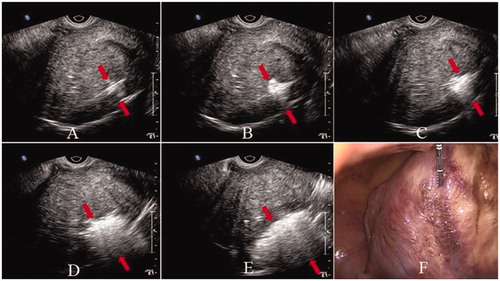
Figure 2. Laparoscope and transvaginal ultrasound images during ablation. Hyperechogenic signal under transvaginal ultrasound guidance indicates ablation area (A–E), and the scope of the hyperechogenic signal becomes larger and larger. Uterus shrinkage and color change from bright red to pale on laparoscope (F). (Red arrows indicate the area of the hyperechogenic signal.)
Complications and follow-up
The complications of all patients during and after the procedure were recorded. The patients were asked to return to the hospital at 1, 6 and 12 months after the ablation procedure for follow-up. Measurements of uterine volume and lesion volume based on MRI were obtained. A symptom severity score (SSS) questionnaire and a visual analog scale (VAS) questionnaire [Citation16] were completed before PMWA by all patients and 1, 6 and 12 months after the PMWA by patients who returned to hospital for follow-up. The SSS questionnaire included the following eight items: amount of menstrual blood loss, menstrual blood clotting, prolonged menstruation, menstrual disorders, pelvic pressure, frequency of urination at day and night and fatigue. The VAS was used to assess the extent of dysmenorrhea.
Statistical analysis
SPSS 19 (SPSS Inc., Chicago, IL, USA) software was used for statistical analysis. Normally distributed data were expressed as mean ± SD. Student’s t test was used to compare data with normal distributions. The Wilcoxon signed rank test was used to compare data with skewed distributions. Fisher’s exact tests were used to compare the proportions. p < .05 was considered statistically significant.
Results
Patient information
A total of 70 patients (age range 32–49 years, 42 ± 6 years) underwent transvaginal ultrasound- and laparoscopy-guided PMWA for adenomyosis were evaluated. All women included in the study were premenopausal patients, and patients’ baseline information is summarized in .
Table 1. Baseline information.
Technical feasibility
The technique of transvaginal ultrasound- and laparoscopy-guided PMWA was feasible in all 70 adenomyosis patients, with a success rate of 100%. On laparoscopy, pelvic adhesions between the uterus and surrounding organs were found in 36 patients (51%) and were detached with laparoscope for safe ablation. If the adhesions are extensive and severe, they were detached at least to the level of the cervix. The mean operation time was 29.4 ± 7.6 min.
Safety
No major complication was found during and after the procedure. Vaginal discharge was found in 11 patients (16%), which spontaneously disappeared in 2 weeks. Nine patients (13%) experienced abdominal pain, which spontaneously disappeared within 3 days.
Technical efficacy
Of the total 70 patients, 51 patients returned to the hospital for reevaluation at 1, 6 and 12 months after ablation. As shown in , the uterine volume and lesion volume measured on MR images decreased statistically from the baseline of 213.5 ± 88.6 cm3 and 73.7 ± 35.4 cm3 to 95.5 ± 44.6 cm3 and 16.5 ± 8.6 cm3 at 12 months after ablation, respectively (p < .01; ). Patient VAS scores for dysmenorrhea showed a significant reduction from the baseline of 7.1 ± 0.8 to 1.3 ± 0.4 at 12 months after the ablation procedure (p < .01). The SSS significantly decreased from 21.6 ± 6.3 to 8.6 ± 3.7 at the 12-month follow-up (p < .01). Forty-three patients (84%) showed improvements of dysmenorrhea, and 40 patients (78%) showed improvement of menorrhagia.
Figure 4. Change in lesion volume before PMWA and at follow-up on MRI. A: Lesion size before PMWA (A), 1 month (B), 6 months (C) and 12 months (D) after PMWA.
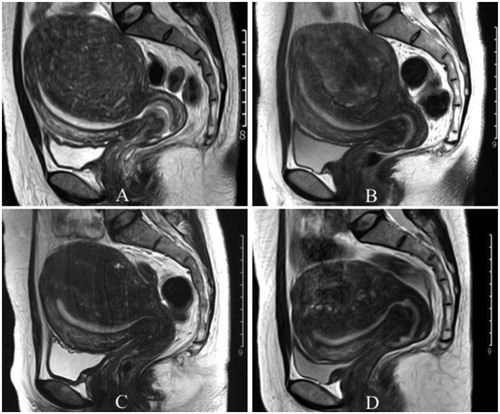
Table 2. Uterine volume, lesion volume, SSS and VAS score before and after microwave ablation of group 1.
Discussion
Adenomyosis is a common uterine pathological change. Hysterectomy is the standard treatment, but it is more invasive. In recent years, patients prefer some less invasive treatment options. As such, PMWA is a minimally invasive treatment for adenomyosis by inducing regional tissue necrosis through heat energy. However, PMWA may damage the nearby intestinal tract, and the percutaneous approach is also technically challenging because of bowel interference. Therefore, finding a feasible and safe method is necessary to avoid potential risk of PMWA. Laparoscopy could observe the surface of the uterus and movement of the bowel and could combine with PMWA to reduce potential risk.
The results of this study showed that transvaginal ultrasound- and laparoscopy-guided PMWA is a feasible technique with a 100% success rate without severe side effects, and it is also an effective technique. The uterine volume, lesion volume, SSS and VAS score of each patient all decreased significantly in the 1-year follow-up period.
In 2011, Zhang et al. reported the transabdominal ultrasound-guided PMWA technique for the treatment of adenomyosis [Citation22]. Transabdominal ultrasound-guided PMWA can effectively relieve symptoms, and it provides high microwave thermal efficiency and short treatment time. In another study, the efficacy of treatment and effect on ovarian function have been evaluated in 142 women with adenomyosis [Citation11]. As an advantage, this technique only needs local anesthesia and the technique is simple and easy. However, it has some drawbacks. The intestine may interfere with antenna insertion, and thermal ablation under transabdominal ultrasound guidance alone may cause severe complications such as penetration or thermal injuries of other pelvic organs and abdomen hemorrhage, especially in patients with adhesion between the uterus and surrounding organs. Several studies have reported bowel perforation after hepatic or renal thermal ablation with image guidance alone [Citation23–25]. In addition, many adenomyosis lesions are located in the posterior wall of the uterus, and the antenna has to pass through the endometrium to enter the posterior wall of the uterus, which may damage the endometrium.
To achieve a clear antenna path in some cases and avoid damaging the surrounding organs during PMWA, Hai et al. [Citation16] introduced artificial ascites during the procedure. However, artificial ascites could not separate the adhesions between the uterus and surrounding organs and could not reduce the heat conduction from the uterus to other organs through adhesions. In our procedure, we introduced laparoscopy to separate pelvic adhesions and provide a visually wide antenna path. In our study, pelvic adhesions between the uterus and surrounding organs were found in 51% of the patients (36/70), which could be detached with laparoscope at least to the level of the cervix.
Laparoscopy also has the following roles in the procedures: (1) it could remove subserosal uterine fibroids before ablation; (2) the blood vessels on the surface of the uterus could be seen during antenna insertion, which is useful to avoid bleeding; (3) laparoscopy could observe the color change of the surface of the uterus to evaluate the ablation effect; (4) the cavitas pelvis and uterine surface could be cleaned with the laparoscope, which may reduce the possibility of pelvic adhesions after the ablation and (5) the operator could find the hemorrhagic spots in the abdomen and stop bleeding with laparoscope immediately.
In this study, the antenna tip was clearly displayed on transvaginal ultrasound screen and was inserted into the optimal site in the lesion of all patients. On transvaginal ultrasonography, once the hyperechogenic signal covered the whole lesion or reached 3–5 mm near the margin of the serosa or uterine endometrium, the MWA procedure was halted. Meanwhile, the operator could observe the color change of the uterine surface on the laparoscope screen during ablation. The procedure was stopped when the color of the uterine surface changed from bright red to dark red or pale to avoid damage to the uterine serosa. No bowel perforation or severe hematuria was found in any patients in our study. CEUS was used immediately post ablation to assess the range of ablation. If the nonenhanced areas were much smaller than the lesion evaluated with transvaginal ultrasonography or MRI before ablation, further ablation was needed. In addition, the nonenhanced areas after ablation corresponded to the hyperechogenic signal areas from transvaginal ultrasonography during ablation.
In this study, MRI was used to measure the uterine volume and lesion volume before ablation and at 1, 6 and 12 months after ablation. The volume decreases of the uterus and lesions after ablation and in follow-up were both significant. The SSS and VAS scores were also significantly reduced, indicating the effectiveness of this method.
This study has several limitations. First, this was a prospective study, not a randomized controlled study. Second, the short-term efficacy (1 year) after ablation of adenomyosis was assessed, but long-term efficacy (3 years and longer) needs further observation. Third, the border of the lesion was unclear, and subjective factors influenced the judgment of the extent of ablation.
In conclusion, transvaginal ultrasound- and laparoscopy-guided PMWA is a feasible minimally invasive technique for the treatment of adenomyosis. It has some obvious advantages and no severe side effects. The uterine volume, lesion volume, SSS and VAS score decreased significantly in the follow-up period. Therefore, transvaginal ultrasound- and laparoscopy-guided PMWA is an effective technique and could be an option for the treatment of adenomyosis.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185.
- Graziano A, Lo MG, Piva I, et al. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci. 2015;19(7):1146–1154.
- Maheshwari A, Gurunath S, Fatima F, et al. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–392.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18(4):428–437.
- Wood C. Adenomyosis: difficult to diagnose, and difficult to treat. Diagn Ther Endosc. 2001;7(2):89–95.
- Yoon SW, Kim KA, Cha SH, et al. Successful use of magnetic resonance-guided focused ultrasound surgery to relieve symptoms in a patient with symptomatic focal adenomyosis. Fertil Steril. 2008;90(5):2018.e13–2018.e15.
- Long L, Chen J, Xiong Y, et al. Efficacy of high-intensity focused ultrasound ablation for adenomyosis therapy and sexual life quality. Int J Clin Exp Med. 2015;8(7):11701–11707.
- Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia. 2015;31(7):777–783.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Yang Y, Zhang J, Han ZY, et al. Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep. 2015;5:10034.
- Xu RF, Zhang J, Han ZY, et al. Variables associated with vaginal discharge after ultrasound-guided percutaneous microwave ablation for adenomyosis. Int J Hyperthermia. 2016;32(5):504–510.
- McLucas B, Voorhees WD, Elliott S. Fertility after uterine artery embolization: a review. Minim Invasive Ther Allied Technol. 2016;25(1):1–7.
- Karlsen K, Hrobjartsson A, Korsholm M, et al. Fertility after uterine artery embolization of fibroids: a systematic review. Arch Gynecol Obstet. 2018;297(1):13–25.
- Keung JJ, Spies JB, Caridi TM. Uterine artery embolization: a review of current concepts. Best Pract Res Clin Obstet Gynaecol. 2018;46:66–73.
- Hai N, Zhang J, Xu R, et al. Percutaneous microwave ablation with artificial ascites for symptomatic uterine adenomyosis: initial experience. Int J Hyperthermia. 2017;33(6):646–652.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Xia M, Jing Z, Zhi-Yu H, et al. Research of dose-effect relationship parameters of percutaneous microwave ablation for uterine leiomyomas–a quantitative study. Sci Rep. 2015;4(1):6469.
- Bazot M, Daraï E, Rouger J, et al. Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obstet Gynecol. 2002;20(6):605–611.
- Reinhold C, McCarthy S, Bret PM, et al. Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology. 1996;199(1):151–158.
- Hanafi M. Ultrasound diagnosis of adenomyosis, leiomyoma, or combined with histopathological correlation. J Hum Reprod Sci. 2013;6(3):189–193.
- Zhang J, Han ZY, Feng L, et al. Ultrasound-guided percutaneous microwave ablation in the treatment of diffuse adenomyosis. Zhonghua Yi Xue Za Zhi. 2011;91(39):2749–2752.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.
- Iannuccilli JD, Dupuy DE, Beland MD, et al. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol. 2016;26(6):1656–1664.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.