Abstract
Purpose: To evaluate the clinical value of transarterial chemoembolization (TACE) combined with microwave ablation (MWA) for unresectable hepatocellular carcinoma (HCC).
Patients and methods: Eligible studies were identified using PubMed, MedLine, Embase, the Cochrane Library, and Web of Science, investigating the synergistic effect of TACE + MWA in the treatment of advanced HCC. Endpoints were the 1-, 2- and 3-year survival rates, local control rate (LCR), objective remission rate (ORR), and adverse event (AE). Odds ratio (OR) with 95% confidence interval (CI) was used to determine the effect size.
Results: Nine studies including 351 patients in the TACE + MWA group and 653 patients in the TACE group were enrolled in this meta-analysis. The pooled OR for the 1-, 2-, and 3-year survival rates were in favor of TACE + MWA (OR = 3.29, 95% CI 2.26–4.79; OR = 2.82, 95% CI 2.01–3.95; OR = 4.50, 95% CI 2.96–6.86; respectively). The pooled OR for the ORR and LCR were also in favor of TACE + MWA (OR = 4.64, 95%CI 3.11–6.91; OR = 3.93, 95% CI 2.64–5.87; respectively). No significant difference in the incidence of severe AE was observed between TACE + MWA group and TACE group (p > .05). However, subgroup analysis showed that patients with tumor size >5 cm were more likely to be benefited from TACE + MWA, rather than patients with tumor size ≤5 cm.
Conclusion: With the current data, we concluded that combination TACE and MWA was safe, and should be strongly recommended to unresectable patients with tumor size >5 cm, but TACE alone was enough for unresectable patients with tumor size ≤5 cm. However, the conclusion needs further validation.
Introduction
Hepatocellular carcinoma (HCC) remains one of the most common kinds of tumors worldwide, although the incidence has been decreasing stably [Citation1]. Approximately 782000 patients are diagnosed as HCC every year, and 746000 patients die of HCC [Citation1,Citation2]. Unfortunately, 70% of patients have lost chances of resection at diagnosis [Citation2,Citation3]. For unresectable HCC, transarterial chemoembolization (TACE) is often taken as the first-line treatment [Citation4–6]. However, restrictions exist in the treatment of TACE [Citation7,Citation8], and the prognosis of patients with unresectable stage receiving TACE alone remains poor [Citation6,Citation9].
Microwave ablation therapy (MWA) is an emerging strategy for HCC [Citation10,Citation11]. The efficiency of MWA has been confirmed repeatedly in the treatment of patients with tumor ≤5 cm, and is reported to be superior to radiofrequency ablation (RFA) [Citation10,Citation12]. Combination of TACE and MWA has been tried in clinic in the recent decades [Citation13–16], but the conclusion has yet to be reached. Hence, a comprehensive systematic review and meta-analysis is badly warranted to evaluate the clinical value of combination of TACE and MWA for unresectable HCC.
Material and method
This meta-analysis was conducted according to the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature search
A comprehensive literature search was conducted by two independent researchers to identify studies evaluating the clinical value of combination of TACE and MW for unresectable patients with advanced HCC. PubMed, MedLine, Embase, the Cochrane Library, and Web of Science. Free words including ‘hepatocellular carcinoma’, ‘transarterial chemoembolization’ and ‘microwave ablation’ were searched within the title, abstract and key words using these databases. Variations of ‘hepatocellular carcinoma’ including ‘HCC’, ‘liver tumor(s)’, ‘liver carcinoma’, ‘liver cancer(s)’, ‘hepatic tumor(s)’, ‘hepatic carcinoma’, and ‘hepatic cancer(s)’, variations of ‘transarterial chemoembolization’ including ‘TACE’, and ‘transcatheter arterial chemoembolization’, and variations of ‘microwave ablation’ including ‘microwave coagulation’, ‘MWA’, and ‘MWC’, were also searched. The literature search, filtering and reading began in Jan 2018, and the final systematic search was conducted in Aug 2019. Potential eligible studies were forward-tracked manually through the references of the included studies, reviews, letters and comments. Additional searches were also conducted in Google Scholar, using key words including ‘hepatocellular carcinoma’, ‘transarterial chemoembolization’ and ‘microwave ablation’.
Selection criteria
Inclusion criteria: i) patients diagnosed as HCC; ii) patients with unresectable tumors or unwillingness of resection; iii) experiment group was combination of TACE and MWA, and control group was TACE alone; iv) outcomes must include at least one of the followings: ①short-term outcomes, ②long-term outcomes, ③adverse events (AE).
Exclusion criteria: i) patients including intrahepatic cholangiocarcinoma or metastatic liver cancer; ii) patients receiving therapy intended to transplant; iii) combination therapy including RFA or other local therapies; iv) data on the outcomes was not available; v) reviews, comments, letters, case report, and conference abstract.
Intervention
TACE was usually conducted under the local anesthesia and was guide of digital subtraction angiography (DSA) [Citation17]. However, the embolization agent and chemotherapy drug were different from different period.
MWA was performed before or after TACE with an interval of 1–2 weeks under general anesthesia [Citation18]. The procedure was guided by real-time ultrasound or CT, and the number of needles and range of distribution were dependent on the characteristics of tumors (location, size and number). Of note, the power and duration were slightly different from each center.
Tumor response were evaluated according to the standard of the modified RECIST [Citation19], including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Endpoints
Long-term outcome included OS, which was OS was calculated from the treatment to either the data of death or the latest follow-up.
Short-term outcomes included objective remission rate (ORR) and local control rate (LCR). ORR was defined as the rate of CR plus PR, and LCR was the rate of CR, PR plus SD.
AE in this study was referred to the severe AE in particularly, such as upper gastrointestinal bleeding, liver abscess, severe hepatic and renal dysfunction.
Data extraction
Data such as the author’s first name, year of publication, study methods, patient’s characteristic, interventions, and outcomes were extracted and assessed by Qiao Ke and Nanping Lin with predefined forms. The odd ratios (ORs) of 1-, 2-, and 3-year survival rates, ORR, LCR, and AE were extracted directedly from the original data. In case of disagreement, a third investigator was intervened to reach a conclusion.
Quality assessment
Either randomized controlled trials (RCT) or retrospective cohort studies (RCS) were included in this study, which were evaluated by Cochrane risk assessment tool and Newcastle-Ottawa Scale (NOS) [Citation20,Citation21], respectively. For RCTs, any studies were assessed to be high risk of bias should be reevaluated. For NOS, a full score was 9, and studies scoring above 5 were considered high quality. The quality of non-randomized studies was assessed by the modified Newcastle-Ottawa Scale (NOS), and more than 7 stars were defined as high quality, 4–6 star as medium quality, and <4 stars as low quality.
Statistical analysis
The meta-analysis was registered at http://www.crd.york.ac.uk/PROSPERO/ (Review registry 150883) and was performed using RevMan Version 5.3. ORs and 95%CIs was used to evaluate the 1-, 2-, and 3-year survival rates, ORR, LCR, and AE comparing between TACE + WMA group and TACE group. Heterogeneity was determined by the χ2 test and I2 statistics. When p > .10 and I2 <50% was considered as no significant heterogeneity among the included studies, and then the effect size was evaluated using a fixed-effect model; otherwise, the effect size was evaluated using a random-effect model [Citation22], Subgroup analyses were conducted stratified by the tumor size. Publication bias was determined using Begg’s and Egger’s tests by Stata 14.
Results
Base characteristic of the included studies
In a total, nine records with one RCT were enrolled in this meta-analysis [Citation23–31], including 351 patients in the TACE + MWA group, 613 patients in the TACE group. The search strategies and results were shown in .
The characteristics and baseline demographic data of the patients in each research were listed in . Quality assessment of each included study was exhibited in , and seven of nine studies were evaluated as quality of high [Citation23,Citation25,Citation27–31]. Detail of treatments in the included studies was depicted in .
Table 1. Characteristics of the clinical trials included in the meta-analysis.
Table 2. Interventions of included studies.
Table 3. Subgroup analysis stratified by tumor size. (A) tumor size ≤5 cm. (B) tumor size >5 cm.
Long-term outcome
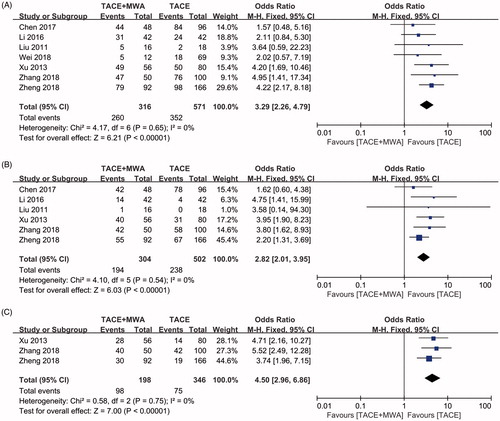
1-, 2-, and 3-year survival rates comparing between TACE + MWA group and TACE group were evaluated in seven [Citation23,Citation24,Citation26–28,Citation30,Citation31], six [Citation23,Citation24,Citation26–28,Citation30] and three included studies [Citation24,Citation28,Citation30], respectively. Significant heterogeneities were not observed in all three subgroups (I2=0, p = .65, ; I2=0, p = .54, ; I2=0, p = .75, ; respectively). Using a fixed-effect model, the pooled OR for the 1-, 2-, and 3-year survival rates were in favor of TACE + MWA group (OR = 3.29, CI 95% 2.26–4.79, ; OR = 2.82, CI 95% 2.01–3.95, ; OR = 4.50, CI 95% 2.96–6.86, ; respectively). Publication bias was not detected in all three subgroups using Egger’s test (p = .452, p = .542, p = .172, respectively) and Begg’s test (p = .548, p = 1.000, p = .296, respectively).
Short-term outcome
ORR comparing between TACE + MWA group and TACE group was evaluated in six included studies without significant heterogeneities (I2=0, p = .41, ) [Citation23,Citation25–27,Citation29,Citation30]. Using a fixed-effect model, the pooled OR for the ORR was in favor of TACE + MWA group (OR = 4.64, CI 95% 3.11–6.91, ). Publication bias was not detected in all three subgroups using Egger’s test (p = .152) and Begg’s test (p = .452).
Figure 3. Forest plot of the tumor response between TACE + MWA and TACE alone. (A) Objective remission rate. (B) Local control rate.
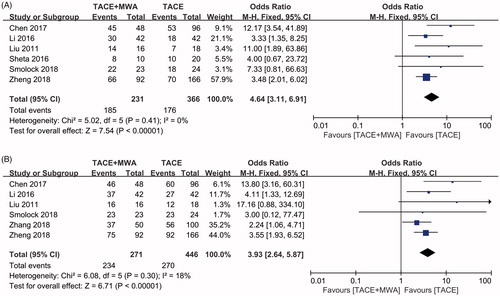
LCR comparing between TACE + MWA group and TACE group was evaluated in six included studies without significant heterogeneities (I2=18%, p = .30, ) [Citation23,Citation26–30]. Using a fixed-effect model, the pooled OR for the ORR was in favor of TACE + MWA group (OR = 3.93, CI 95% 2.64–5.87, ). Publication bias was not detected in all three subgroups using Egger’s test (p = .242) and Begg’s test (p = .707).
Adverse events
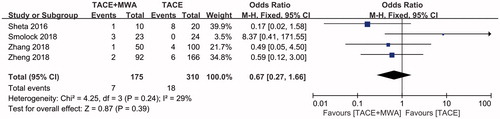
Severe AE comparing between TACE + MWA group and TACE group was evaluated in four included studies without significant heterogeneities (I2=59%, p = .24, ) [Citation25,Citation28–30]. Using a fixed-effect model, there was no significant difference in the incidence of severe AE between TACE + MWA group and TACE group (OR = 0.67, CI 95% 0.27–1.66, ).
Subgroup analysis stratified by tumor size
For patients with tumor size ≤5 cm, 1-, and 2-year survival rates comparing between TACE + MWA group and TACE group were evaluated in two included studies without significant heterogeneities [Citation27,Citation28]. Using a fixed-effect model, there were no significant differences in 1-, and 2-year survival rates between TACE + MWA group and TACE group (OR = 1.83, CI 95% 0.61–5.47; OR = 1.69, CI 95% 0.75–3.81; respectively) ().
For patients with tumor size >5 cm, 1-, 2-, and 3-year survival rates comparing between TACE + MWA group and TACE group were evaluated in four [Citation23,Citation24,Citation30,Citation31], three [Citation23,Citation24,Citation30] and two [Citation24,Citation30] included studies without significant heterogeneities. Using a fixed-effect model, the pooled OR for the 1-, 2-, and 3-year survival rates were in favor of TACE + MWA group (OR = 3.82, CI 95% 2.38–6.13; OR = 3.83, CI 95% 2.37–6.19; OR = 4.12, CI 95% 2.51–6.77; respectively) ().
Discussion
Comprehensive treatment is the optimal choice of patients with unresectable tumors [Citation32], but which is the better remains controversial. To the best of our knowledge, this is the first meta-analysis evaluating the synergistic effect of TACE and MWA for patients with unresectable HCC. Results including nine trials with 351 patients showed that the pooled OR for both the long-term outcomes including the 1-, 2-, and 3-year survival rates, and the short-term outcomes including the ORR and LCR were all in favor of TACE + MWA (all p < .05). In addition, significant difference in the incidence of severe AE was not observed between TACE + MWA group and TACE group (p > .05). Hence, combination of TACE and MWA was efficient and safe, and should be recommended to the patients with unresectable HCC.
Approximately 70% of HCC patients are at unresectable stage with the median OS of 20 months [Citation1]. TACE is the preferred strategy for those with well-tolerated liver function, which is conducted prevalently worldwide. The median OS has been reported to prolonged from 20 to 30 months [Citation1,Citation2]. However, the treatment of TACE has its own inherent restrictions: 1) TACE alone could be insufficient to some tumors, which are also nourished by portal vein [Citation27]; 2) some of the tumors are hypovascular [Citation33], which are resistant to TACE; 3) repeated TACE often lead damage of normal liver, 4) TACE could stimulate the expression of vascular endothelial growth factor receptor and increase the population of circulating tumor cells, both of which would induce the recurrence to some extent [Citation7,Citation8,Citation34]. Hence, comprehensive treatment including TACE and other strategies is badly warranted in clinic.
In the recent decades, various kinds of TACE-based combination therapy have been explored to improve the prognosis of unresectable patients [Citation13,Citation35–38]. Among the strategies, TACE + RFA is used widely, and the synergistic effect is confirmed by recent meta-analysis [Citation39,Citation40]. However, as an emerging strategy, MWA is found to be an alternative to RFA in the treatment both for patients with small tumors and for patients with unresectable tumors [Citation10,Citation12]. MWA has been conducted prevalently in China, but now it has been carried out in the west with similar result [Citation29,Citation41]. The mechanisms underlying MWA and RFA are both to coagulate issue induced by localized high temperature, although their mechanisms of action are different from each other [Citation12,Citation42]. However, MWA is superior to RFA in the following terms: 1) decreased heat-sink effect mainly because it takes less time for MWA to achieve ablation than that for RFA [Citation43]; 2) better conformability both in the shape and size of the ablation area in MWA due to higher temperature induced by MWA than that by RFA [Citation10].
Synergistic effect of TACE and MWA is expected, and such combination therapy has been tried [Citation14,Citation27,Citation30]. However, the sample sizes were often small, and the conclusion has yet to be reached. To the best of our knowledge, neither systematic review nor meta-analysis has been published to identify the synergistic effect of TACE and MWA. In this study, nine researches [Citation23–31] including one RCT [Citation25] were identified to evaluate the synergistic effect of TACE and MWA for patients with unresectable stages. Results showed that TACE + MWA was superior to TACE both in the short-term outcomes and long-term outcomes without increasing risk of adverse AE. Mechanisms of synergistic effect of TACE and MWA might be as follows: 1) occlusion of hepatic artery by TACE could reduce the heat sink effect, and in turn enhance the effect of MWA [Citation13]; 2) minor tumors are more likely to be detected by angiography, and tumor sites are easier to be located and evaluated after deposition of iodized oil [Citation44,Citation45]; 3) MWA could wipe out potentially residual tumors following TACE, and TACE could reduce the recurrence of tumor at the margin of MWA [Citation13,Citation30]. Hence, we concluded that combination therapy of TACE + MWA was safe and efficient.
Tumor size is an important independent risk factor of patients with unresectable HCC [Citation35,Citation46]. MWA is found to be not inferior to surgical resection and RFA for patients with tumor size ≤5 cm, but as for patients with tumor size >5 cm, it remains controversial [Citation12,Citation47,Citation48]. On the contrary, in this study we found that the synergistic effect of TACE + MWA was only observed in the subgroup of patients with tumor size >5 cm, rather than in the subgroup of patients with tumor size ≤5 cm. The result indicated that single TACE or MWA was enough for patients with tumor size ≤5 cm, but the finding deserved further validation because only two studies were identified in this subgroup.
There were several restrictions of this meta-analysis. First, eight of nine included studies were retrospective, which indicated an obvious selection and recalling bias. Second, the procedure and course of TACE and MWA might be different from each center, but the detailed data was unavailable. Third, sequence of combination therapy is an important risk factor, but the subgroup of TACE following MWA was only reported in one included study [Citation25]. Fourth, subgroup analysis of age, sex, Child-Pugh and other factors associated with efficacy was not conducted due to data unavailable. The last but not the least seven of the nine included studies came from China [Citation23,Citation24,Citation26–28,Citation30,Citation31], which indicated the conclusion should be restricted in China.
Conclusion
With the current data, we concluded that combination therapy of TACE and MWA was safe, and should be recommended to unresectable patients with tumor size >5 cm, but TACE alone was enough for unresectable patients with tumor size ≤5 cm. However, more RCTs based on multi- centers and large sample size are badly warranted to validate this conclusion.
Disclosure statement
The authors reports no conflicts of interest in this work.
Additional information
Funding
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Benson AB, D’Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;17(4):302–310.
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853.
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589.
- Xiang X, Lau WY, Wu ZY, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombusa multicenter study. Eur J Surg Oncol. 2019;45(8):1460–1467.
- Fang ZT, Wang GZ, Zhang W, et al. Transcatheter arterial embolization promotes liver tumor metastasis by increasing the population of circulating tumor cells. Onco Targets Ther. 2013;6:1563–1572.
- Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529.
- Shimose S, Tanaka M, Iwamoto H, et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 2019;49(8):919–928.
- Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005.
- Kamal A, Elmoety A, Rostom Y, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol. 2019;10(3):562–571.
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):264–272.
- Li W, Ni CF. Current status of the combination therapy of transarterial chemoembolization and local ablation for hepatocellular carcinoma. Abdom Radiol. 2019;44(6):2268–2275.
- Hu H, Chen GF, Yuan W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. 2018;34(8):1351–1358.
- Long J, Zheng JS, Sun B, et al. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int. 2016;10(1):175–184.
- Si ZM, Wang GZ, Qian S, et al. Combination therapies in the management of large (≥ 5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization. J Vasc Interv Radiol. 2016;27(10):1577–1583.
- Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903.
- Galanakis N, Kehagias E, Matthaiou N, et al. Transcatheter arterial chemoembolization combined with radiofrequency or microwave ablation for hepatocellular carcinoma: a review. Hepat Oncol. 2018;5:P7.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):052–060.
- Julian PTH, Douglas GA. Cochrane Handbook for Systematic Reviews of Interventions. 2008. Chapter 8, Assessing risk of bias in included studies; p. 6–9.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Liu C, Liang P, Liu F, et al. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27(7):654–662.
- Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28(3):456–463.
- Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28(10):1198–1203.
- Li W, Man W, Guo H, et al. Clinical study of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of advanced hepatocellular carcinoma. J Can Res Ther. 2016;12:C217–20.
- Chen QF, Jia ZY, Yang ZQ, et al. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-microwave ablation therapy for hepatocellular carcinoma tumors ≤ 5 cm: a propensity analysis at a single center. Cardiovasc Intervent Radiol. 2017;40(11):1748–1755.
- Zhang R, Shen L, Zhao L, et al. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:219–224.
- Smolock AR, Cristescu MM, Hinshaw A, et al. Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3- to 5-cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abdom Radiol. 2018;43(9):2497–2504.
- Zheng L, Li HL, Guo CY, et al. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19(2):237–246.
- Wei Y, Dai F, Zhao T, et al. Transcatheter arterial chemoembolization monotherapy vs combined transcatheter arterial chemoembolization-percutaneous microwave coagulation therapy for massive hepatocellular carcinoma (≥10 cm). CMAR. 2018;10:5273–5282.
- Daher S, Massarwa M, Benson AA, et al. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol. 2018;6:69–78.
- Lee DH, Lee JM, Kang TW, et al. Clinical outcomes of radiofrequency ablation for early hypovascular HCC: a multicenter retrospective study. Radiology. 2018;286(1):338–349.
- Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. WJG. 2004;10(19):2878–2882.
- Cui W, Fan W, Huang K, et al. Large hepatocellular carcinomas: treatment with transarterial chemoembolization alone or in combination with percutaneous cryoablation. Int J Hyperthermia. 2018;35(1):239–245.
- Veltri A, Gazzera C, Calandri M, et al. Percutaneous treatment of Hepatocellular carcinoma exceeding 3 cm: combined therapy or microwave ablation? Preliminary results. Radiol Med. 2015;120(12):1177–1183.
- Yan JY, Zhang JL, Wang MQ, et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation in single-session for solitary hepatocellular carcinoma larger than 7 cm. Asia-Pac J Clin Oncol. 2018;14(4):300–309.
- Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol. 2019;44(6):2283–2292. NY)
- Chen QW, Ying HF, Gao S, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2016;40(3):309–314.
- Yang DJ, Luo KL, Liu H, et al. Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma. Oncotarget. 2017;8:2960–2970.
- Vogl TJ, Qian J, Tran A, et al. Study on the effect of chemoembolization combined with microwave ablation for the treatment of hepatocellular carcinoma in rats. Diagn Interv Radiol. 2017;23(2):150–155.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009;24(2):223–227.
- Lencioni R, de Baere T, Martin RC, et al. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies - a western perspective. Liver Cancer. 2015;4(4):208–214.
- Stefano GD, Iodice V, Signoriello G, et al. Efficacy and safety of combined sequential treatment with RFA and sorafenib in patients with HCC in intermediate stage ineligible for tace: a prospective randomized open study. J Hepatol. 2015;62:S852.
- Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16(3):661.
- Zhu K, Huang J, Lai L, et al. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288(1):300–307.
- Li X, Zhang L, Fan W, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia. 2011;27(3):240–248.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.