Abstract
Purpose: To evaluate the complications encountered during microwave ablation (MWA) for primary and secondary hyperparathyroidism (HPT).
Materials and methods: The retrospective study enrolled 213 secondary hyperparathyroidism (SHPT) and 51 primary hyperparathyroidism (PHPT) patients who received MWA between July 2015 and September 2018. The major and minor treatment-related complications were documented. The baseline data, clinical parameters, laboratory indices and characteristics of the parathyroid glands were analyzed to assess the risk factors associated with these complications.
Results: The incidence of post-MWA complications in HPT patients was 12.1% (32/264). In total, five (5/264, 1.9%) patients with SHPT had major complications, including aphonia/hoarseness (n = 4) and Horner syndrome (n = 1). A total of 27 (10.2%, 27/264) HPT patients had minor complications, including neck hematoma (0.8%, 2/264), bucking (4.2%, 11/264) and phonasthenia (5.3%, 14/264). The incidence of severe hypocalcemia (SH) after MWA was 18.2%. Cutaneous necrosis occurred in two SH patients after intravenous calcium supplementation. There were no significant differences in the incidence of overall complications, major complications and minor complications between SHPT and PHPT patients (12.7% vs 9.8%, p = 0.811; 2.3% vs 0, p = 0.587; 10.3% vs 9.8%, p = 1.000). A history of parathyroidectomy (PTX) (p = 0.031) and multiple symptoms (p = 0.000) were risk factors for the occurrence of complications in SHPT patients. One patient sustained a permanent injury to a unilateral recurrent laryngeal nerve (RLN), and the two patients who experienced cutaneous necrosis underwent debridement plus autologous skin transplantation. The remaining patients recovered without sequelae.
Conclusion: The incidence of major complications was low which only occurred in SHPT patients. Most of the patients with complications recovered spontaneously. MWA is safe for the treatment of HPT.
Introduction
Image-guided thermal ablation has been applied to the treatment of hyperparathyroidism (HPT) in recent years [Citation1–4]. As an ablation method, US-guided percutaneous microwave ablation (MWA) has yielded promising clinical results [Citation5–9]. Since this technique causes minimal injuries and effectively reduces intact parathyroid hormone (iPTH) levels, it has been gradually accepted in clinical practice. However, investigators in previous studies have mainly focused on assessing the feasibility and therapeutic effectiveness of this method. The complications of treating HPT with MWA, such as voice changes, hematoma formation, hypocalcemia and pain, have been reported but not systematically evaluated [Citation5–7,Citation10–12]. Therefore, the purpose of this study was to evaluate the clinical aspects of the complications encountered in the treatment of primary and secondary HPT with MWA, analyze the potential risk factors associated with the occurrence of complications, and provide information for preventing and managing these complications.
Materials and methods
Patients
This retrospective study protocol was approved by the Human Ethics Review Committee of the China-Japan Friendship Hospital (2015-GZR-77). Written informed consent was obtained from each patient before MWA.
From July 2015 to September 2018, 264 patients with 457 hyperplastic parathyroid glands treated by MWA were assessed; 213 patients with 402 parathyroid glands had secondary HPT (SHPT), and 51 patients with 55 parathyroid glands had primary HPT (PHPT). The patients consisted of 144 men and 120 women. The mean age was 52.5 ± 13.9 years (range, 18 to 84 years). The mean maximum diameter of the glands was 1.4 ± 0.6 cm (range, 0.6 to 4.8 cm). There were 430 parathyroid glands in their typical locations and 27 ectopic glands (19 in the suprasternal fossae, 3 in the anterior mediastinum, 3 in the submandibular glands, 1 within the thyroid, and 1 inside the carotid sheath).
Inclusion criteria
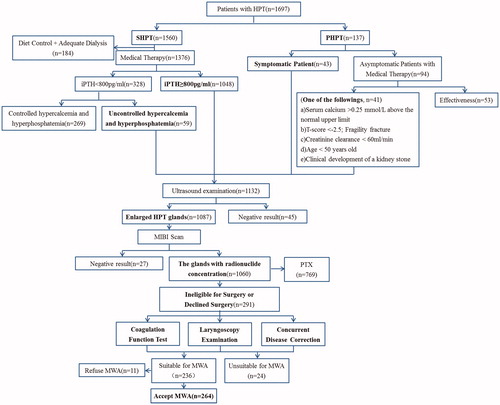
For the PHPT group, the inclusion criteria were as follows: (1) symptomatic patients (etc. urinary calculus, bone pain); or (2) asymptomatic patients who were unable or unwilling to comply with the observation protocols [Citation13]. For the SHPT group, the inclusion criteria were as follows: (1) intolerance to medication; and (2) iPTH greater than 800 pg/mL or less than 800 pg/mL with uncontrolled hypercalcemia and hyperphosphatemia [Citation14]. In addition, the following criteria must be met: (1) unable or refused to undergo surgery; (2) at least one enlarged parathyroid gland with sharp margins clearly shown on US; (3) elevated 99mTc-sestamibi (MIBI) radionuclide concentrations in both the early and delay phases; (4) no recurrent laryngeal nerve (RLN) impairment in patients with a voice change, confirmed by laryngoscopy; (5) prothrombin time less than 18 s, prothrombin activity greater than 60%, and platelet count greater than 60 × 109 cells/L; and (6) no intractable coexisting morbidity, such as cardiac insufficiency or severe hypertension ().
MWA equipment and techniques
MWA was performed by a radiologist with 5 years of experience in MWA for thyroid nodules and hyperplastic parathyroid glands. A microwave generator and a 17-gauge internally cooled applicator antenna with a 0.4-cm tip (Intelligent Basic Microwave Tumor Ablation System; Nanjing ECO Microwave System, Nanjing, China) were applied. An Aplio 500 (Toshiba Medical Systems, Tokyo, Japan) with a 10.0-MHz linear probe was used.
MWA was performed under local anesthesia with 2% lidocaine (Synera, Salt Lake City, Utah) at the puncture site. The hydrodissection technique was performed for nerve protection [Citation5]. Under US guidance, 40–60 ml normal saline (NS) was first injected into the area around the parathyroid nodule to offer heat insulation and nerve isolation. Then, local anesthesia in the form of a lidocaine and NS mixture (1:3) was injected along the peri-parathyroid capsule. The antenna was inserted freehand into the parathyroid gland under US guidance. A fixed-applicator technique was adopted; for each ablation cycle, the power was 30 W, and the radiation time was 15–25 s. MWA was terminated when the hyperechoic zone covered the target nodule. Contrast-enhanced US (Sonovue, Bracco, Milan, Italy) was performed 3–5 min later to assess the efficiency of the ablation procedure. If a non-enhanced zone covered the ablated nodule, complete ablation was achieved; if there was enhancement inside the nodule, further ablation was performed immediately. After complete ablation on one side, the procedure was continued on the contralateral side if there were simultaneously no voice changes and no abnormal vocal cord movements on US.
Clinical data collection and follow-up
The follow-up included US examinations and blood biochemistry panels (e.g., serum iPTH, calcium, phosphate, and alkaline phosphatase [ALP]). The time points were 2 h, 1 day, 7 days, and 1 month postoperatively, and then every 3 months for the first year and every 6 months thereafter.
Follow-up was terminated for the following reasons: (1) death, and (2) secondary PTX, MWA or other ablations for SHPT; as the complications were considered immediate (0–24 h), peri-procedural (1–30 days), or delayed (>30 days), the follow-up time was at least 3 months, and the complications were followed until the final outcome.
Definition of complications
The definitions of complications were consistent with the standardization of terminology and reporting criteria for image-guided tumor ablation [Citation15–16]. A major complication was defined as one that might threaten the patient’s life, lead to substantial morbidity or disability, require an increased level of care, or result in a hospital admission or substantially delayed hospital discharge. All other complications were considered minor. Side effects were defined as unfortunate and undesired consequences that did not require therapy [Citation17].
According to the recovery time, the post-MWA voice changes were classified into major and minor complications. Aphonia and hoarseness, possibly due to thermal injuries, that lasted longer than 7 days were defined as major complications. Phonasthenia, possibly due to compression or edema, that lasted fewer than 7 days was regarded as a minor complication. Pain that persisted for more than 3 days after ablation was regarded as a minor complication [Citation17]. Transient pain during ablation was defined as a side effect. Hypocalcemia was classified as mild-to-moderate serum hypocalcemia when the serum calcium values were 1.875 mmol/L to 2.1 mmol/L and as severe hypocalcemia (SH) when the serum calcium value was less than 1.875 mmol/L [Citation18–20].
Outcomes
The primary outcome of the study was the rate of complications. Various secondary outcomes were also assessed, which were as follows: (1) the incidence of overall complications, major complications and minor complications between the PHPT and SHPT groups; and (2) SHPT patients were further grouped as the complication and noncomplication groups. The baseline data, clinical parameters, laboratory indices and parathyroid gland characteristics were analyzed to assess the risk factors for complications associated with thermal injury.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (IBM, Armonk, NY, USA) and Stata version 15.0 (StataCorp LLC, TX, USA). Student’s t-tests and independent Mann–Whitney U-tests were used to compare continuous variables, and the chi-square test was used for categorical variables. Logistic stepwise regression with the forward selection of variables was adopted to explore the risk factors for complications. p < 0.05 was considered statistically significant.
Results
The incidence of complications in the HPT group is shown in . A total of 32 (32/264, 12.1%) complications were encountered. Five (5/264, 1.9%) major complications occurred in the SHPT group, while no major complications occurred in the PHPT group. The incidence of minor complications in the HPT group was 10.2% (27/264). There were no significant differences between the SHPT and PHPT patients in the incidence of overall complications, major complications and minor complications (12.7% vs 9.8%, p = 0.811; 2.3% vs 0, p = 0.587; 10.3% vs 9.8%, p = 1.000).
Table 1. Complications and side effects in 264 patients who underwent MWA of HPT.
Risk factors for complications
In the univariate analysis, SHPT patients with a history of PTX (p = 0.031) and multiple symptoms (p = 0.000) were associated with the occurrence of complications (). In the subsequent analyses, no variables met the criteria to be included in the regression equation of the multivariate analysis.
Table 2. Comparison of relevant clinical parameters between SHPT patients with and without complications.
Major complications
Five (1.9%) major complications occurred, including voice changes in four (1.5%) patients and Horner syndrome (HS) in one (0.4%).
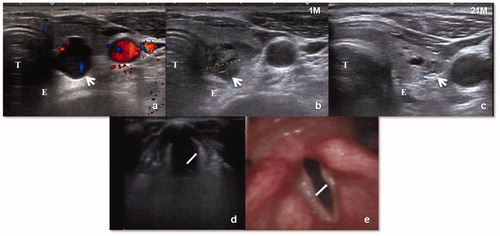
All 4 patients with aphonia/hoarseness had parathyroid glands located close to the tracheoesophageal groove. Aphonia/hoarseness occurred during or just after the procedure. Laryngoscopy showed two cases of bilateral vocal cord paralysis and two cases of unilateral vocal cord paralysis. One case of bilateral RLN injury was comorbid with SH. This patient complained of difficulty breathing 3 days after MWA. After oxygen intake and the SH was corrected, the symptoms disappeared. Three patients’ voices recovered completely 1–3 months after MWA. A permanent injury of a unilateral RLN occurred in one patient who had dysphonia and abnormal tonality during the follow-up period ().
Figure 2. (a)Transverse US scan in a 69-year-old SHPT patient shows a parathyroid nodule (white arrow) behind inferior left lobe of thyroid abutting the trachea (T) and esophagus (E). (b–c) One and 21 months after MWA, US image shows the shrunken ablation zone(white arrow) clings to the area of the recurrent laryngeal nerve. (d–e) The left vocal cord (white arrow) loses tension, atrophies to be arched and becomes thin under eupnea on US. The left vocal cord is located on one side and could not close the glottis. Laryngoscopy revealed atrophic and thin left vocal cord.
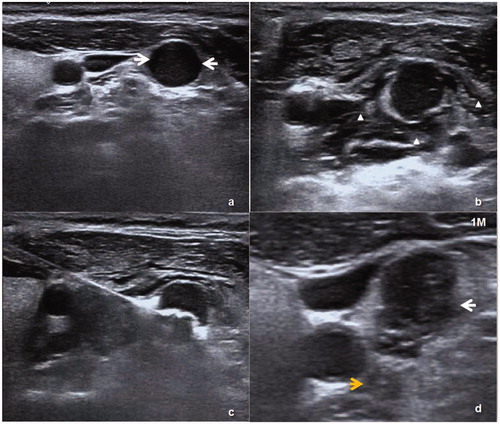
HS was encountered in one patient with an ectopic parathyroid gland located inside the left carotid sheath after PTX (). The patient showed mild miosis and eyelid ptosis on the left side 3 days after ablation. After 6 months of follow-up, the symptoms had been completely alleviated.
Figure 3. (a) An ectopic parathyroid nodule (white arrow) was on the lateral side of the left carotid sheath. (b) A liquid-isolating zone around the nodule (white triangle). (c) The procedure of MWA. (d) One month after MWA, the nodule showed a reduction in volume on US and the ablation zone (white arrow) was close to middle cervical sympathetic ganglion (MCSG) (yellow arrow).
Minor complications
Twenty-seven (10.2%) minor complications occurred, including hematoma in 2 (0.8%) patients, bucking in 11 (4.2%) patients and phonasthenia in 14 (5.3%) patients. The hematomas occurred in the anterior superior mediastinum in one patient (intraoperative immediate, range 3 cm × 1.7 cm) and in the sternocleidomastoid in the other patient (intraoperative immediate, range 1.8 cm × 1.0 cm). Both patients were treated successfully with pressure and thrombin injections (Hemocoagulase Bothrops Atrox for Injection, Nuokang Pharmaceutical Company, Penglai, Shandong). All patients with bucking recovered 1–2 days after MWA. All 14 patients with phonasthenia were able to pronounce words normally 1–7 days after MWA.
Side effects
The side effects included pain, numbness and hypoparathyroidism. All patients recovered from pain within 3 days. Hand and foot numbness occurred in 23 patients on the day of MWA or 1 day after MWA and improved after the hypocalcemia was corrected. The differences of serum calcium levels before MWA and 1 day after MWA were greater in patients with numbness than in patients who did not experience numbness (0.59 ± 0.38 mmol/L vs 0.29 ± 0.32 mmol/L, p < 0.001). In addition, numbness was more common in PHPT patients than in SHPT patients (15.7% vs 7.0%, p = 0.002). The incidence of transient hypoparathyroidism was higher in PHPT patients than that in SHPT patients (17.6% vs 2.3%, p < 0.001), and all patients recovered without management within 2 weeks.
Occurrence of hypocalcemia episodes
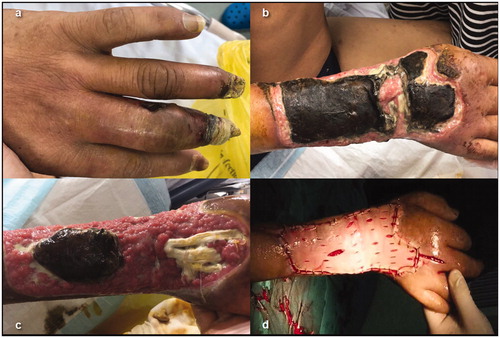
Among 264 patients, 84 (31.8%) developed hypocalcemia episodes 1–3 days after MWA, and 48 (18.2%) in the SHPT group were categorized as SH. No SH was observed in the PHPT group. After receiving oral calcium supplementation, intravenous calcium gluconate and high calcium dialysis fluids, all patients recovered within 3 months. Cutaneous necrosis occurred in two patients with SH after intravenous calcium supplementation. Both patients had preoperative cardiac insufficiency, and one patient suffered from dry gangrene in the fingertips of three digits on his right side. One day after MWA, the iPTH levels were significantly reduced (from 2927 pg/mL to 69 pg/mL and 1703 pg/mL to 95 pg/mL), and SH occurred after 1–2 days. No obvious leakages were found during the calcium gluconate pumping process. However, skin necrosis was observed 3 and 7 days after calcium gluconate pumping, starting from the puncture site and then gradually expanding to the entire forearm. These two patients recovered through debridement plus autologous skin transplantation ().
Discussion
MWA can completely inactivate hyperfunctional parathyroid glands and has several benefits, such as reduced risks, minimal invasiveness, fast recovery and easy repeatability [Citation5–7,Citation10–12]. Although US-guided MWA has many advantages, it also has some associated risks. Compared with the thyroid glands, the parathyroid glands locate on the dorsal side of the thyroid gland and are much closer to the tracheoesophageal groove-the region where the RLN distributes. Additionally, patients with HPT are in a weak condition because of long-term calcium and phosphorus metabolism disorders. Therefore, the incidence of complications is higher after treating parathyroid glands than that after treating thyroid nodules (12.1%, 32/264 vs 3.3%, 48/1459) [Citation17].
There was no significant difference in the incidence of complications between SHPT and PHPT patients. Major complications only occurred in the SHPT group, which may be due to the following reasons: (1) SHPT was prevalent in dialysis patients who were frequently frail and chronically ill [Citation21]; (2) SHPT patients generally have severe mineral metabolism disorders because SHPT often involves hyperplasia of multiple parathyroid glands, which causes a high preoperative iPTH value. After ablation, a sharp drop in the iPTH level results in SH; (3) some patients had a history of PTX, and the postoperative adhesions could not be fully isolated during ablation, which increases the risk of thermal injuries to important structures, such as the RLN; and (4) some SHPT patients had comorbidities (e.g., heart failure, vascular calcification), which increases the incidence of complications. SHPT patients, those with a history of PTX or multiple clinical symptoms, are much more likely to suffer from complications.
As a serious complication of MWA, aphonia/hoarseness was observed in four patients, which was a lower incidence than those reported in the literature for PTX (1.5% vs 2.4%) [Citation22–23]. For thyroid nodules treated with radiofrequency (1.0%) and lasers (0.5%), aphonia/hoarseness was also the most common major complication [Citation24–26]. Aphonia/hoarseness mainly occurs in HPT patients because of the specificity of the anatomical site, as targeted HPT lesions lie in close proximity to the RLNs. Thermal injuries, compression and secondary inflammatory responses to treatment are all potential risks. According to our experiences, thermal stimulation of the nerve is similar to a spinal cord shock and results in temporary incapacitation and a lack of coordination that could eventually be recovered. However, complete thermal injury, or necrosis, cannot self-repair or recover with contralateral compensation. Therefore, minimizing heat exposure to the tracheoesophageal groove where the RLNs are positioned is crucial to prevent nerve injuries. There are several strategies to protect the nerve. First, the establishment of a liquid isolating zone that insulates the parathyroid gland can effectively prevent thermal injuries to the RLN. Second, a fixed-applicator technique is more suitable for HPT ablation than moving-shot ablation because the HPT glands are usually small. Third, repeated radiation cycles with a low power of 30 W is helpful in preventing thermal injuries to the surrounding critical structures.
In the present study, there were two cases of bilateral RLN injuries, but the patients’ pronunciation was almost normal before MWA of the contralateral parathyroid gland. Therefore, injuries to the RLN cannot be completely judged by the patients’ pronunciation. More attention should be paid to both the pronunciation (including pitch, tone, and vocalization) and vocal cord movements on US (including the impairment or disappearance of movements) [Citation27]. After the nodules on one side are ablated, the contralateral nodules should be ablated if the RLN has normal function, as confirmed by no voice changes and no abnormal vocal cord movements on US; otherwise, the ablation procedure should be suspended until the RLN function recovers.
In this study, one case of HS occurred. The incidence of HS after MWA was equivalent to that after PTX (0.4% vs 0.27%) [Citation28–29]. This patient had a postoperative ectopic hyperplasic parathyroid gland inside the carotid sheath, which was adjacent to the sympathetic nerve. Despite isolating the gland, HS still occurred, which indicated that this was a high-risk location. A possible reason might be compression or thermal injure to the cervical sympathetic trunk or middle cervical sympathetic ganglion (MCSG) [Citation30–31]. Postoperative adhesions might be another contributing factor. To prevent complications, careful examinations of the parathyroid glands and adjacent structures, sufficient hydrodissection (at least 0.5–1 cm between parathyroid glands and surroundings), proper adjustment of the needle direction, low-power ablation, and US-guided tracing of the antenna are all highly recommended.
In our study, the incidence of hypocalcemia and SH after MWA were 31.8% and 18.2%, respectively, which were lower than those after PTX (hypocalcemia, 27-72%; SH, 37-57%) [Citation19,Citation32], which may be because PTX removes as much of the parathyroid glands as possible, while ablations are only performed for hyperplastic parathyroid glands, which can be clearly visualized on US. The incidence of hypocalcemia can be partially explained by hungry bone syndrome (HBS). HBS could be induced by a high serum iPTH levels before ablation and a precipitous drop in serum iPTH levels after ablation. The revised 2017 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines recommend avoiding hypocalcemia and consider mild hypocalcemia acceptable [Citation33]. SH may cause a series of severe symptoms (e.g., tetany, seizure, cardiac arrhythmia and sudden death) [Citation18]. Other complications combined with SH could also cause serious consequences. Therefore, fractionated ablation should be planned to moderately control iPTH levels and to reduce the incidence of post-MWA SH and prevent SH.
Two cases had cutaneous necrosis which were not reported previously. Cutaneous necrosis was related to the intravenous calcium gluconate administration. Both patients presented with cardiac dysfunction, and the dry gangrene in their fingertips reflected a poor arterial blood supply. Therefore, for patients with poor cardiovascular conditions and weak arterial blood supplies, fractionated ablation should be considered to prevent SH, and calcium supplementation should be implemented via the deep vein.
There are a few limitations in the present study. First, this is a single-center study, and further multicenter studies might provide more representative results. Second, future studies are necessary to analyze the relationship between possible related parameters and the occurrence of SH.
Conclusion
The incidence of major complications was low which only occurred in SHPT patients. Most of the complications recovered spontaneously. MWA can be safely used to treat HPT.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol. 2012;199(5):1164–1168.
- Xu SY, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. SMEDJ. 2013;54(07):e137–40.
- Kovatcheva RD, Vlahov JD, Shinkov AD, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism:a feasibility study in four patients. AJR Am J Roentgenol. 2010;195(4):830–835.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282(2):576–584.
- Liu C, Wu B, Huang P, et al. US-guided percutaneous microwave ablation for primary hyperparathyroidism with parathyroid nodules: feasibility and safety study. J Vasc Interv Radiol. 2016;27(6):867–875.
- Zhuo L, Zhang L, Peng LL, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperthermia. 2019; 36(1):29–35.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016; 32(2):180–186.
- Wang G, Liu S, Liu X, et al. Microwave ablation: an effective treatment for mild-to-moderate secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2017;33(8):946–952.
- Diao Z, Liu X, Qian L, et al. Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2016;32(6):614–622.
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968.
- Isakova T, Nickolas TL, Denburg M, et al. KDOQI US commentary on the 2017 KDIGO Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235(3):728–739.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Mittendorf EA, Merlino JI, McHenry CR. Post-parathyroidectomy hypocalcemia: incidence, risk factors, and management. Am Surg. 2004;70(2):114–119.
- Sun X, Zhang X, Lu Y, et al. Risk factors for severe hypocalcemia after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Sci Rep. 2018;8(1):7743.
- Floege J, Tsirtsonis K, Iles J, et al. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with cinacalcet in the EVOLVE trial. Kidney Int. 2018;93(6):1475–1482.
- Oliveira RB, Silva EN, Charpinel DM, et al. Secondary hyperparathyroidism status in Brazil: Brazilian census of parathyroidectomy. J Bras Nefrol. 2011;33(4):457–462.
- Schlosser K, Bartsch DK, Diener MK, et al. Total parathyroidectomy with routine thymectomy and autotransplantation versus total parathyroidectomy alone for secondary hyperparathyroidism: results of a nonconfirmatory multicenter prospective randomized controlled pilot trial. Ann Surg. 2016;264(5):745–753.
- Abdulla AG, Ituarte PH, Harari A, et al. Trends in the frequency and quality of parathyroid surgery: analysis of 17,082 cases over 10 years. Ann Surg. 2015;261(4):746–750.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100(10):3903–3910.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2019.
- Huo SN, Peng LL, Wei Y, et al. Ultrasonic diagnosis of abnormal movement of vocal cord. Chinese J Ultrasound Med. 2018;34(10):878–880.
- Harding JL, Sywak MS, Sidhu S, et al. Horner’s syndromein association with thyroid and parathyroid disease. ANZ J Surg. 2004;74(6):442–445.
- Allen AY, Meyer DR. Neck procedures resulting in Horner syndrome. Ophthalmic Plast Reconstr Surg. 2009;25(1):16–18.
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16(4):749–766.
- Shin JE, Baek JH, Ha EJ, et al. Ultrasound features of middle cervical sympathetic ganglion. Clin J Pain. 2015;31(10):909–913.
- Tsa WC, Peng YS, Chiu YL, et al. Risk factors for severe hypocalcemia after parathyroidectomy in prevalent dialysis patients with secondary hyperparathyroidism. Int Urol Nephrol. 2015;47:1203–1207.
- Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017;92(1):26–36.