Abstract
Background: To evaluate the efficacy and safety of radiofrequency ablation (RFA), microwave ablation (MWA) and laser ablation (LA) for treating papillary thyroid microcarcinoma (PTMC).
Materials and methods: PUBMED and EMBASE were searched for studies on the efficacy and safety of RFA, MWA and LA for treating PTMC. The standard mean difference of the tumor volume before and after therapy and the proportion of complete disappearance, local recurrence, distant metastasis and complications were assessed using both fixed or random-effects modeling. Heterogeneity among studies was determined using the Q statistic for the pooled estimates and the inconsistency index I2.
Results: A total of 12 eligible studies, including a sample size of 1187 patients and 1284 PTMCs, were used. RFA, MWA and LA all showed a significant reduction in tumor volume of PTMCs (p < 0.05). Though MWA demonstrated superior efficacy over the other two therapies for volume reduction, the differences were not statistically significant. Additionally, the pooled proportion of complete disappearance after RFA was the highest (76.2%), and the pooled proportion of recurrence for RFA was the lowest (0.01%) among the three therapeutic methods, but no significant difference was detected. There was no event of distant metastasis during the follow-up in all of these studies. Few major complications were encountered; the pooled proportion of complications for RFA (1.73%), MWA (6.0%) and LA (0.92%) was low, revealing no significant differences (p > 0.05).
Conclusion: RFA, MWA and LA are acceptable treatments to manage PTMCs in terms of efficacy and safety for non-surgical candidates.
Introduction
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, and lesions of this type with a diameter less than 10 mm are termed papillary thyroid microcarcinomas (PTMCs) [Citation1]. Due to the advent of high-resolution ultrasound (US) detection and more regular fine-needle aspiration (FNA) biopsies, the reported incidence of PTMCs has increased worldwide [Citation2]. PTMCs are often considered indolent tumors and have a good prognosis [Citation3]. In a previous study, non-surgical observation of 300 PTMCs over 1–17 years revealed that 7% had increased in size, 90% were unchanged and 3% had decreased in size; none of the patients developed extrathyroidal invasion or distant metastasis [Citation4]. Sugitani et al. [Citation5] observed 415 patients with asymptomatic PTMC for a period of 2–22 years, and the results suggested that only 6% had increased in size, 91% showed no change, and 3% had decreased in size. Similarly, Ito et al. [Citation6] reported that none of the 1235 patients with low-risk PTMC, who chose observation without immediate surgery, showed distant metastasis or died of PTMC during an observation period ranging from 1.5 to 19 years.
Although surgery is recommended for PTMC by current guidelines [Citation7–9], there are some circumstances when the patients are ineligible for surgery due to systemic disease. Moreover, thyroidectomy may have complications such as laryngeal nerve paralysis, hypothyroidism, hypoparathyroidism, lifelong medication and scarring [Citation10–13]. Thus, surgery may lead overtreatment for PTMC, which is generally considered a harmless disease. The 2015 American Thyroid Association (ATA) guideline recommended that patients with low-risk PTMCs can take an active surveillance management approach [Citation8]. However, active surveillance management is not acceptable for many anxious patients with PTMC. Therefore, thermal ablation, which considered a moderate approach between surgery and surveillance, may be attempted for patients with PTMCs. According to the 2017 Korean Society of Thyroid Radiology guideline, patients with PTC who refuse or cannot undergo surgery can be treated by thermal ablations [Citation14]. Although indications of thermal ablations for PTMCs have not been clearly established, radiofrequency ablation (RFA) [Citation15–23], microwave ablation (MWA), [Citation24–28] and laser ablation (LA) [Citation29–32] have recently been applied to non-surgical candidates with PTMCs. Accumulating evidence indicates that these alternative approaches can effectively eliminate PTMCs with a low complication rate over a certain follow-up period [Citation33].
Regarding non-surgical therapeutic options for treating PTMC, several studies have reported the therapeutic efficacy and safety of percutaneous US-guided thermal ablations including RFA, MWA and LA. These studies evaluated the tumor volume reduction ratio, complete disappearance, recurrence and complications of the minimally invasive treatments for treating PTMC and demonstrated that thermal ablation could be an alternative to surgery based on the high rates of disease-free survival and low rates of complications. In addition, some studies revealed that MWA has several advantages over surgery in PTMC treatment, including lower rates of complications, less hospitalization time, lower cost and higher post-operative quality [Citation24,Citation25]. However, majority of these studies were retrospective and descriptive or used a small number of patients with short-term follow-up, lacking large-scale studies that evaluate the procedures. Therefore, to acquire the exact value of these minimally invasive approaches in the treatment of PTMC, it is necessary and timely to collect the published data and perform a meta-analysis. To the best of our knowledge, the present systematic review and meta-analysis is the first to evaluate the efficacy and safety of RFA, MWA and LA as treatments of this disease.
Materials and methods
Literature search strategy
A comprehensive computer literature search of PubMed and Embase.com was conducted to find published articles on the topic of the review. The following search terms were used: in Pubmed, ((((((((radiofrequency[Title/Abstract]) OR radio-frequency[Title/Abstract]) OR radio frequency[Title/Abstract]) OR microwave[Title/Abstract]) OR micro-wave[Title/Abstract]) OR micro wave[Title/Abstract]) OR laser[Title/Abstract])) AND Thyroid Neoplasms [MeSH Terms]; in Embase, (radiofrequency:ti,ab,kw OR ‘radio frequency’:ti,ab,kw OR microwave:ti,ab,kw OR ‘micro wave’:ti,ab,kw OR laser:ti,ab,kw) AND (‘thyroid cancer’/exp). The search was updated until 30 July 2019, without language restrictions. To identify additional suitable articles and expand our search, the references of the retrieved articles were also screened.
Inclusion criteria
Studies (or subsets of studies) that investigated RFA, MWA, or LA for the treatment of PTMCs were eligible for inclusion. Studies (or subsets of studies) that satisfied all of the following criteria were included: (a) Population: patients underwent RFA, MWA, or LA for treating primary PTMCs without clinically apparent lymph node or distant metastasis at diagnosis. Papillary carcinoma was confirmed by US-guided FNA prior to the treatment. Studies that contained more than five consecutive patients were included. (b) Study design: retrospective or prospective studies were both included. (c) Outcomes: results were reported in sufficient detail to assess PTMC volume reduction, complete disappearance, complications, recurrence and distant metastasis.
Exclusion criteria
The exclusion criteria included the following: (a) Case reports and series with a sample size less than five relevant patients, or studies with potential selection bias (e.g., nonconsecutive series). (b) Review articles, editorials, letters and comments. (c) Studies on topics other than therapeutic efficacy and safety. (d) Studies with insufficient data on PTMC volume reduction, complete disappearance, complications, recurrence and distant metastasis. Two reviewers independently selected potentially relevant studies by screening the retrieved literature.
Data extraction
We extracted the following data from the included studies into a standardized data form: (a) study characteristics: first author, year of publication, affiliation, study period, study design, method of thermal ablation and sample size; (b) demographic and clinical characteristics: age, sex and follow-up interval; (c) therapeutic efficacy characteristics: initial and post-operative tumor volume (on the final follow-up), frequencies of complete disappearance (during follow-up after treatment), local recurrence and distant metastasis; (d) safety characteristics: frequencies of major and minor complications.
Complete disappearance was defined as any of the following descriptions in the included studies: ‘completely disappeared’, ‘completely absorbed’, ‘completely resolved’, or ‘only left needle tracks’. On the other hand, descriptions such as ‘calcified residues’, ‘volume reduction and disappearance of color Doppler signal’, ‘volume unchanged and Contrast-Enhanced Ultrasound (CEUS) confirmed no enhancement’, ‘FNA showed necrosis’, or ‘cicatricial hyperplasia’ were not considered as complete disappearance. Local recurrence was defined as newly-presented thyroid tumors, cervical lymph nodes, or non-necrotic ablation areas with pathologic diagnosis of malignancy during the post-operative follow-up period. Complications introduced by US-guided thermal ablation were defined by the Society of Interventional Radiology, including major and minor complications [Citation34,Citation35]. In the present review, both major complications (such as persistent or transient voice hoarseness, post-operative hypothyroidism, hypoparathyroidism or hyperthyroidism, brachial plexus injury and dysphagia) and minor complications (such as bleeding, vomiting, skin burn, fever, local infection and severe pain that need medication) were included. One reviewer extracted the data from the included studies, and the other reviewer verified the accuracy of these data.
Quality assessment
The methodological quality of the included studies was independently evaluated by the two reviewers using the tailored questionnaires and criteria provided by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) [Citation36].
Data synthesis and statistical analysis
For each included study, the standard mean difference (SMD) of the tumor volume before and after therapy, and the proportion of complete disappearance, local recurrence, distant metastasis and complications were used as the main indices for this meta-analysis. First, meta-analysis for all of the included studies was performed. The pooled proportion was estimated using the method of Freeman–Tukey Double arcsine transformation. Both the fixed-effect model and the random-effects model were taken into account. We estimated heterogeneity using the inconsistency index I2 (considering a value of 25% to suggest low heterogeneity; 50%, moderate; and 75%, high [Citation37]) with the Q statistic and its p value. If the test of heterogeneity indicated p ≥ 0.05, the fixed-effect model would be used; otherwise, data were analyzed using the DerSimonian–Laird random-effects model [Citation38,Citation39]. Second, sensitivity analysis was conducted to estimate the effect of the remaining studies without the effect of the larger one. Visual inspection of the funnel plot was utilized to test publication bias, and the statistical significance was further evaluated by Egger’s linear regression asymmetry test [Citation40]. Furthermore, we compared estimates of independent meta-analyses between different therapies by mixed-effects meta-regression model [Citation41]. All statistical analyses were calculated by (The R Foundation for Statistical Computing) R version 3.6.1 with the ‘metafor’ package.
Results
Literature search
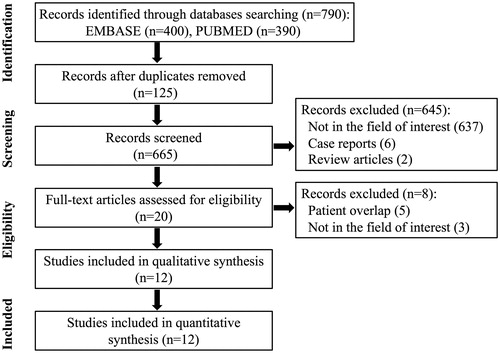
The study selection procedure is displayed in . The literature search of the PubMed and Embase.com generated 790 initial records, of which 125 records were removed based on duplication, leaving 665 records to be screened for further review. Of those records, 645 were excluded after reviewing the titles and abstracts, including 637 records that were not in the field of interest, 6 case reports [Citation18,Citation21,Citation30,Citation42–44] and 2 review articles [Citation33,Citation45]. In the remaining 20 records, 5 were further excluded after reviewing the full text, including 5 studies with a partially overlapping patient cohort [Citation19,Citation24,Citation32,Citation46,Citation47] and 3 studies lacking relevance. In the case of patient overlap, we included the studies with a more significant number of patients. Finally, 12 eligible studies, including a total sample size of 1187 patients and 1284 thyroid tumors, were used in the present meta-analysis. Though the retrieval date for the articles was not limited, studies contained in the final analysis were all published after the year 2014.
Characteristics of the included studies
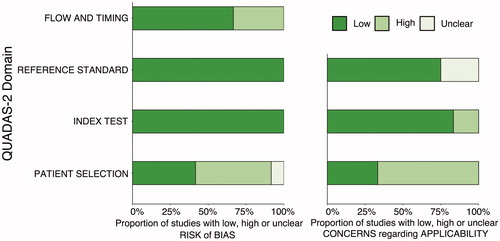
The detailed characteristics of the 12 included studies are shown in , of which 9 studies were retrospective, and 3 were prospective. In terms of the demographic characteristics of the patients in the included studies, the mean ages of these cases ranged between 42.2 and 72 years, and about 74.3% of the patients were female. The mean follow-up time after the treatment was greater than 7.8 months. In terms of study design and therapeutic approach, 11 studies were single-arm studies (6, 3 and 2 studies treated with RFA, MWA and LA, respectively) and 1 study was comparative (MWA vs. surgery). In terms of patient characteristics, 10 studies included consecutive patients with primary PTMC, and 2 studies included primary small PTC, whose mean maximum diameter of tumors were 4 ± 2.1 mm [Citation17] and 9 ± 2.8 mm [Citation22], respectively. The quality of the included studies was moderate overall, and all of the studies satisfied at least 4 of the 7 items assessed by QUADAS-2 ().
Table 1. Characteristics of the included studies.
Changes in tumor volume after RFA, MWA and LA
The overall pooled estimates for the SMD in tumor volume from baseline to the last follow-up after therapy are summarized in and with corresponding forest plots. Using both the fixed and random effect models, RFA, MWA and LA all induced a statistically significant reduction in nodule volume after ablation (p < 0.0001, p = 0.0189 and p = 0.0002 in the RFA, MWA and LA groups, respectively). Moreover, MWA achieved a higher pooled SMD (−3.82; 95% CI: −7.02; −0.63) than RFA (−1.35; 95% CI: −1.62; −1.09) and LA (−1.80; 95% CI: −2.75; −0.85). However, the differences in the pooled SMD in tumor volume among the three therapies were not statistically significant (p > 0.05).
Figure 3. Results of SMD of tumor volume in the RFA, MWA and LA groups. *Data were analyzed using the random-effects model.
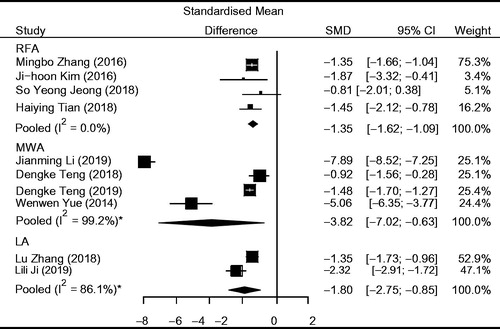
Table 2. Summary of the meta-analytic pooled data for efficacy and safety of RFA, MWA and LA.
Frequencies of complete disappearance after RFA, MWA and LA
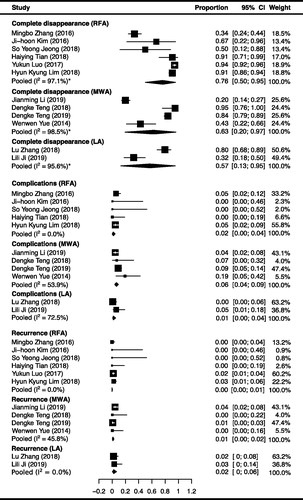
The pooled proportions for complete disappearance of RFA, MWA and LA are summarized in and with corresponding forest plots. The complete disappearance demonstrated a pooled proportion of 76.2% (95% CI: 50.0%; 95.4%; I2=97.1%), 62.9% (95% CI: 19.7%; 96.6%; I2=98.5%) and 57.3% (13.0–95.4%; I2=95.6%) after RFA, MWA and LA therapy, respectively. These results show that the proportion of complete disappearance after RFA was higher than the other two therapies. However, the differences were not statistically significant, revealing p = 0.594 and p = 0.474 compared with MWA and LA respectively. Substantial heterogeneity was observed in the proportions of complete disappearance for RFA, due to the proportion reported from one study [Citation20] (33.7%; 95% CI, 24.4%; 43.9%) as being much lower than the other studies (50%-94%). It probably resulted from the follow-up time of this study (3 ∼ 18 months) was much shorter than the others (12 ∼ 65 months). When sensitivity analysis was performed without this study, the heterogeneity was reduced with a recalculated pooled proportion of 91.6% (95% CI, 83.6%; 97.4%; I2=70.2%).
Frequencies of local recurrence and distant metastasis after RFA, MWA and LA
Local recurrence after RFA, MWA and LA demonstrated a pooled proportion of 0.01% (95% CI: 0.0%; 0.57%; I2=0.0%), 0.85% (95% CI: 0.02%; 2.42%; I2=45.8%) and 1.87% (95% CI: 0.0%; 5.96%; I2=0.0%) respectively. These results show that the proportion of recurrence after RFA was lower than that after MWA and LA, but the differences were not statistically significant, showing p = 0.797 and p = 0.563 compared with MWA and LA, respectively. The recurrent patients underwent secondary ablations or open surgeries, revealing complete disappearances in the follow-up periods. Moreover, no adhesion or inflammatory changes induced by initial ablation were observed during the surgery. On the other hand, no event of distant metastasis occurred during the post-operative follow-up period in any of the studies, resulting in a pooled proportion of 0.0% for the three therapeutic methods. The detailed data of recurrence and distant metastasis are summarized in and , and with corresponding forest plots.
Table 3. Frequencies of each recurrence found in RFA, MWA and LA.
Frequencies of complications from RFA, MWA and LA
Two kinds of major complications (voice hoarseness and hypothyroidism) and three kinds of minor complications (pain, bleeding and skin burn) were reported from RFA, voice hoarseness and bleeding were reported from MWA, and hypothyroidism and pain were reported from LA. The complications demonstrated overall pooled proportions of 1.7% (95% CI: 0.1%; 4.5%; I2=0.0%), 6.0% (95% CI: 3.6%; 8.8%; I2=53.9%) and 0.92% (95% CI: 0.0%; 4.4%; I2=72.5%) in RFA, MWA and LA, respectively. These results demonstrated that the complication rate of LA was lowest, but no statistical significance was observed (p > 0.05). The detailed data of complication rates are summarized in and , and with corresponding forest plots. Serious complications, including injury to the esophagus, trachea, spinal accessory, sympathetic ganglion and phrenic nerves, have not been reported.
Table 4. Frequency of each complication found in RFA, MWA and LA.
Discussion
This meta-analysis revealed that US-guided thermal ablation was a valid therapeutic method for PTMCs. RFA, MWA and LA resulted in a significant reduction in the tumor volume of PTMCs (p < 0.05). Though MWA demonstrated superior efficacy over the other two therapies for volume reduction, the differences were not statistically significant. In addition, the pooled proportion of complete disappearance after RFA was the highest (76.2%), and the pooled proportion of recurrence for RFA was the lowest (0.01%) among the three therapeutic methods, but no significant difference was detected. There was no event of distant metastasis during the last follow-up visit in any of these studies. In terms of safety, few major complications were encountered; the pooled proportion of complications for RFA (1.7%), MWA (6.0%) and LA (0.92%) was low, demonstrating the safety of these procedures. Thus, thermal ablations, including RFA, MWA and LA, are effective and safe treatments for patients with PTMC.
Until now, many original studies have described the efficacy and safety of thermal ablation for treating PTMC [Citation24–28]. Li et al. [Citation24,Citation25] retrospectively compared MWA with surgery for PTMC and suggested that MWA could be a minimally invasive alternative to surgery. However, there are currently no definitive guidelines that describe how RFA, MWA, or LA should be used to treat PTMC, and few studies have compared these three therapies. Besides, a direct comparison among them for treating PTMC is difficult because well-designed prospective randomized trials are not available. Therefore, our systematic review and meta-analysis that gathered the currently available evidence was critical and will promote clinical practice.
In our current study, the efficacy of the three methods of thermal ablation was found to be similar. In terms of SMD in tumor volume and the proportion of complete disappearance, significant heterogeneities were detected. This is most likely explained by the following two reasons. First, the follow-up time in some of the studies was not long enough to observe the outcomes, which may have affected the accuracy of these results. For instance, the follow-up time in Zhang’s study [Citation20] was shorter than that reported in other studies, when sensitivity analysis was performed without this study, the heterogeneity for the proportion of complete disappearance after RFA was remarkably reduced (I2: from 97.1% to 70.2%). Second, though some studies reported the proportion of complete disappearance to be low, this was probably because the calcified residues or scar-like lesions remained. These residues were obviously unchanged 1 year later, but CEUS showed no enhancement, and FNA confirmed necrosis.
According to the present meta-analysis, there were no life-threatening complications resulting from these thermal ablations. The pooled proportion of complications of RFA (1.7%), MWA (6.0%) and LA (0.92%) were low, and there was no significant difference among them (p > 0.05). Considering the currently available evidence, these three therapies can be regarded as safe procedures to treat PTMCs. Regarding voice hoarseness, 12 transient and 5 persistent voice changes were reported among 389 patients after MWA, while 5 transient voice hoarseness changes were reported among 276 patients for RFA. This difference is most likely explained by the fact that the heat production rates of the two methods are different. The temperature in RFA increases slowly, and heat is easily taken away. Thus, the coagulation zone size of RFA is limited [Citation48]. Compared with RFA, MWA depends less on tissue impedance and makes the microwave antenna locally obtain high temperatures in seconds, resulting in coagulation necrosis which can inactivate the tumor quickly [Citation49,Citation50]. However, for small-sized tumors such as PTMCs, MWA is not easy to manipulate for operators and may produce a larger zone of active heating than necessary, making it more likely to cause injury to the adjacent nerves.
Although PTMC has a high incidence and excellent clinical outcome, the ideal treatment for PTMC is still controversial. The present systematic review demonstrated the effectiveness and safety of thermal ablations for the treatment of PTMC. Specifically, the included studies in our meta-analysis referred to low-risk PTMCs without lymph node or distant metastasis. For PTMCs which already harbor metastases, surgery is commonly recommended [Citation51]. However, there still exists a small proportion of patients with PTMCs whose micrometastases were difficult to detect by US. These PTMCs may behave aggressively [Citation2]; thus, the accurate risk stratification in PTMC patients has critical clinical implications for the selection of therapeutic strategies. To explicitly distinguish aggressive from indolent PTMCs at an early phase, routine high-resolution US detection and more regular FNA biopsies are currently used; on the other hand, exploitation of radiomics and uncovering prognostic biomarkers would be helpful. Moreover, accumulating evidences indicated the efficacy of secondary ablation [Citation16,Citation17,Citation27] or open surgery [Citation29,Citation31] in the treatment of recurrent PTMCs, and no adhesion or inflammatory changes induced by initial ablation were observed during the surgery [Citation31]. Therefore, even if the thermal ablations were performed on PTMC patients who already have micrometastases and lymph node metastases were later found in the post-operative follow-up period, the majority of these patients may have the opportunity to undergo surgery or secondary ablation.
The present meta-analysis also has some limitations. First, the sample size of the studies was limited, of which most were retrospective, which may have affected the accuracy of the results. Specifically, the MWA and LA articles meeting the inclusion criteria were more concentrated in one geographic area and some overlapping of authorship. These overlapping studies were removed to ensure the objectivity of this analysis. Thus, the number of MWA and LA studies that met our inclusion criteria was smaller than initially anticipated, which could introduce substantial biases and limit the applicability of our findings. Second, our findings were regarded as average effects because detailed individual data were not available. However, we used validated systematic review methods and reported our data using standard guidelines. Despite the above limitations, we applied a robust methodology using the currently available evidence, and demonstrated that RFA, MWA and LA are effective therapies to manage PTMCs in terms of efficacy and safety.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zafon C, et al. Differences in the form of presentation between papillary microcarcinomas and papillary carcinomas of larger size. J Thyroid Res. 2011;2011:639156.
- Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013;63:374–394.
- Mazeh H, Chen H. Advances in surgical therapy for thyroid cancer. Nat Rev Endocrinol. 2011;7:581–588.
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–1231.
- Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014;38:673–678.
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules–2016 Update. Endocr Pract. 2016;22:1–639.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133.
- Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803.
- Gharib H. Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract. 2004;10:31–39.
- Lang BH, Lo CY. Total thyroidectomy for multinodular goiter in the elderly. Am J Surg. 2005;190:418–423.
- Linos D, Economopoulos KP, Kiriakopoulos A, et al. Scar perceptions after thyroid and parathyroid surgery: comparison of minimal and conventional approaches. Surgery. 2013;153:400–407.
- Rafferty MA, Goldstein DP, Rotstein L, et al. Completion thyroidectomy versus total thyroidectomy: is there a difference in complication rates? An analysis of 350 patients. J Am Coll Surg. 2007;205:602–607.
- Kim JH, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Tian H, Ma N, Xu D, et al. Analysis of therapeutic effect of ultrasound-guided radiofrequency ablation for thyroid microcarcinoma. Chin J Med Imaging Technol. 2018;34:514–517.
- Luo Y, Zhang M. Ultrasound-guided radiofrequency ablation of low-risk papillary thyroid microcarcinoma: a prospective study. Ultrasound Med Biol. 2017;43:S240–S241.
- Lim HK, Baek SM, Baek JH. US-guided radiofrequency ablation for primary thyroid cancer: efficacy and safety of long-termfollow-up in a large population. Neuroradiology. 2018;60:271.
- Jung ED, Shon HS, Jeon EJ. Radiofrequency ablation for the papillary thyroid cancer in high surgical risk patient. Endocrine Rev. 2014;35:S3.
- Zhang Y, Zhang MB, Luo YK, et al. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8:5450.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26:1581–1587.
- Sun J, Liu X, Zhang Q, et al. Papillary thyroid carcinoma treated with radiofrequency ablation in a patient with hypertrophic cardiomyopathy: a case report. Korean J Radiol. 2016;17:558–561.
- Kim J-h, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33:212–219.
- Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34:611–616.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34:653–659.
- Li J, Liu Y, Liu J, et al. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia. 2019;36:640–646.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144:771–779.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64:109–117.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30:150–157.
- Ji L, Wu Q, Gu J, et al. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19:16.
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917–920.
- Zhang L, Zhou W, Zhan W, et al. Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg. 2018;42:2476–2484.
- Zhou W, Jiang S, Zhan W, et al. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: preliminary results. Eur Radiol. 2017;27:2934–2940.
- Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. Int J Hyperthermia. 2017;33:938–945.
- Lewis CA, et al. Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1997;8:475–479.
- Burke DR, Lewis CA, Cardella JF, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243–S246.
- Whiting PF. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560.
- Kim KW, Lee J, Choi SH, et al. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol. 2015;16:1175–1187.
- Lee J, Kim KW, Choi SH, et al. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. 2015;16:1188–1196.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Berkey CS, Hoaglin DC, Mosteller F, et al. A random-effects regression model for meta-analysis. Statist Med. 1995;14:395–411.
- Lee CU, Kim SJ, Sung JY, et al. Needle track tumor seeding after radiofrequency ablation of a thyroid tumor. Jpn J Radiol. 2014;32:661–663.
- Kim HY, et al. Primary papillary thyroid carcinoma previously treated incompletely with radiofrequency ablation. J Can Res Ther. 2010;6:310–312.
- Valcavi R, Piana S, Bortolan GS, et al. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23:1578–1582.
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: A systematic review and meta-analysis. European Journal of Radiology. 2019;118:58–64.
- Luo Y, Zhang M. Ultrasound-guided radiofrequency ablation of low-risk papillary thyroid microcarcinoma: A prospective study on 421 patients. Thyroid. 2017;27:A105.
- Lim HK, Baek SM, Baek JH. US-guided radiofrequency ablation for primary thyroid cancer: Efficacy and safety of long-term follow-up in a large population. CardioVascular and Interventional Radiology. 2017;40:S187.
- Yekuo L, Shasha W, Feng H. Multipolar radiofrequency ablation in controlling hemorrhage from blunt liver trauma. Am J Emerg Med. 2009;27:197–201.
- Kontos M, Felekouras E, Drakos E, et al. Radiofrequency tissue ablation in an experimental model of grade IV renal trauma: a preliminary report. Surg Endosc. 2005;19:249–253.
- Zhou L, Kuang M, Xu Z, et al. Contrast-enhanced sonographically guided thermal ablation for treatment of solid-organ hemorrhage: preliminary clinical results. J Ultrasound Med. 2015;34:907–915.
- Dobrinja C, Pastoricchio M, Troian M, et al. Partial thyroidectomy for papillary thyroid microcarcinoma: Is completion total thyroidectomy indicated?. Int J Surg. 2017;41:S34–S39.