Abstract
Purpose
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel approach for delivering intraperitoneal chemotherapy and offers perspective in the treatment of peritoneal carcinomatosis. Concept is based on a 12 mmHg capnoperitoneum loaded with drug changed in microdoplets. It was postulated to guarantee a more homogeneous drug distribution and tissular uptake than hyperthermic intraperitoneal chemotherapy (HIPEC). The aim of this study was to compare cisplatin peritoneal distribution and pharmacokinetic between HIPEC and PIPAC procedures in a healthy swine model.
Methods
Two groups of eight pigs underwent either HIPEC with cisplatin (70 mg/m2) at 43 °C for 60 min, or PIPAC with cisplatin (7.5 mg/m2) for 30 min. Postoperatively, peritoneal areas were biopsied allowing peritoneal cavity cartography. Tissular and plasmatic cisplatin concentrations were analyzed.
Results
Cisplatin distribution was heterogeneous in both the groups with higher concentrations obtained closed to the delivery sites. Median total platinum peritoneal concentration by pig was higher in the HIPEC group than in the PIPAC group (18.0 μg/g versus 4.3 μg/g, p < .001) but the yield was 2.2 times better with PIPAC. Platinum concentrations were higher in the HIPEC group in all stations. At each time-point, cisplatin plasmatic concentrations were higher in the HIPEC group (p < .001) but beneath the toxicity threshold.
Conclusions
With doses used in clinical practice, HIPEC guaranteed a higher cisplatin peritoneal uptake than PIPAC in this swine model. Spatial drug distribution was heterogeneous with both technics, with hotspots closed to the drug delivery sites. Nevertheless, considering the dose ratio, IP drug uptake yield was better with PIPAC.
Introduction
In digestive, gynecological and primary peritoneal malignancies, peritoneal carcinomatosis (PC) indicates poor prognosis. Different combinations of PC origin and peritoneal spread generate four clinical situations – limited spread, usually peritumoral; extended metastases accessible to complete surgical resection; borderline disease and non-resectable PC – each warranting different therapeutic strategies.
For selected patients with resectable PC, complete cytoreductive surgery (CRS) provides better outcomes and is considered the only potentially curative treatment. Immediate postoperative hyperthermic intraperitoneal chemotherapy (HIPEC) combined with complete CRS can improve local control [Citation1], showing encouraging oncological results [Citation2–4]. Systemic chemotherapy is the current standard for patients with non-resectable disease, but yields insufficient oncological outcomes for PC of most origins [Citation5–7]. Intraperitoneal (IP) chemotherapy could improve local control, increase survival and sometimes convert non-resectable disease to resectable disease.
The rationale behind IP drug administration is to exploit peritoneal–plasma barrier pharmacokinetics-enhancing tumor nodule exposure to chemotherapy drugs, while decreasing systemic passage and associated toxicities [Citation8]. However, IP therapy is limited by two pharmacokinetic issues: poor drug penetration into nodules, and heterogeneous spatial distribution within the peritoneal cavity [Citation9]. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is proposed as a new IP therapy method that could overcome these limitations [Citation10]. The 12-mmHg ‘therapeutic capnoperitoneum’ PIPAC method was developed to ensure better local drug bioavailability with homogenous distribution and tissue penetration [Citation11]. However, recent data from ex vivo and postmortem swine models highlight non-uniform patterns of doxorubicin distribution [Citation12].
In the present study, we aimed to compare distribution and pharmacokinetic profiles of IP drug after HIPEC and PIPAC in an in vivo swine model using protocols similar to those applied in clinical practice [Citation13,Citation14]. Although previous reports investigate doxorubicin as an IP agent, we chose cisplatin due to its wider clinical use and the greater amount of available data [Citation3,Citation15].
Methods
Animals
This study was performed in 16 male Sus scrofa domesticus pigs. All pigs were allowed to acclimatize to the laboratory environment for 7 days, with free access to standard food and water. Then, the operations were performed. On postoperative day 8, the animals were sacrificed with intravenous injection of embutramide–mebezonium–tetracaine (T61; Intervet, France). This project complied with European regulations (Directive EU 86/609), and was approved by the Animal Ethics Committee of the National Veterinary School of Lyon (VetAgro Sup), France (agreement no. 1552).
Surgical procedure
The pigs received intramuscular premedication with 6 mg/kg tiletamine–zolazepam (Zoletil; Virbac, France). Then, a peripheral venous catheter was inserted in the auricular vein, and anesthesia was administered with 4 mg/kg propofol (Diprivan; AstraZeneca, UK), followed by orotracheal intubation. Animals were maintained under anesthesia using isoflurane and intravenous propofol. Heart rate, electrocardiogram, esophageal temperature and oxygen blood saturation were continuously monitored, and recorded every 5 min. Fluid resuscitation was achieved using isotonic saline and ringer lactate, with a mean volume of 2 L/pig. A central venous catheter was positioned in the jugular vein, and a femoral arterial catheter was placed. Hemodynamic parameters were monitored using the PICCO system (Pulsion, Germany).
The pigs were randomly assigned to undergo HIPEC (eight pigs) or PIPAC (eight pigs). Each pig’s abdominal cavity was arbitrarily divided into 10 peritoneal stations, inspired by Sugarbaker’s peritoneal areas [Citation16], as described in Box 1. In the HIPEC group, closed-abdomen HIPEC was performed using the Cavitherm device (Soframedical, France). We placed two inflow tubes under the diaphragm (stations 1 and 3), and one outflow in the pelvis (station 6). One temperature probe was placed close to the mesentery root. For one hour, HIPEC was performed using a 70 mg/m2 cisplatin solution, with continuous abdominal massage, and a target temperature of 43 °C [Citation13]. Upon completion, intra-abdominal fluids were evacuated, and all tubes were removed.
In the PIPAC group, a 12-mmHg capnoperitoneum was insufflated following placement of two balloon trocars (12 mm and 11 mm; Applied Medical, Düsseldorf, Germany). A 10-mm micropump device (Reger Medizintechnik, Rottweil, Germany) was placed in station 8, facing station 4, and connected to a high-pressure injection device (Injektron 82M; MedTron, Saarbruecken, Germany). The micropump was sterilized once and changed in every two pigs. Abdomen tightness was documented using a CO2 zero-flow. Through the nebulizer, we applied pressurized aerosol containing cisplatin (7.5 mg/m2 body surface) in 150 ml NaCl 0.9%. Injection parameters included a flow of 30 ml/min, maw upstream pressure of 200 psi and intra-abdominal pressure of 12 mmHg [Citation11]. Therapeutic capnoperitoneum was maintained for 30 min at body temperature (37 °C), and exhausted using a closed system including a Buffalo particle filter (Medtek Devices, Inc., Lancaster, USA).
Box 1. Peritoneum biopsies stations.
Cisplatin distribution analysis
After IP chemotherapy application, we performed nine parietal peritoneum biopsies (stations 1–9) and a full-wall jejunum biopsy to analyze visceral peritoneum (station 10). Peritoneal samples were divided from sub-serosal fatty tissues, rinsed with an isotonic saline solution and immediately deep-frozen at −20 °C.
Cisplatin pharmacokinetics
Blood samples were collected at the end of chemotherapy administration (T0), and at 30 (T30) and 60 (T60) min after chemotherapy completion. Blood samples were centrifuged to obtain plasma samples, which were deep-frozen at −20 °C.
Platinum assay
Total plasmatic and peritoneal platinum were measured using an inductively coupled plasma mass spectrometer (ICP-MS Nexion 350XX, Perkin Elmer Life and Analytical Sciences, Waltham, MA, USA). Platinum analysis was performed according to a field application report of Perkin Elmer. Total plasmatic platinum concentrations were expressed in mg/L. For peritoneal platinum measurements, peritoneum samples were dried overnight at 40 °C then 3 h at 105 °C, followed by an acid digestion [HNO3 (Plasma Pure, SCP Sciences, Quebec, Canada) at 60 °C overnight]; results were expressed as µg of platinum per gram of dry tissue.
Statistical analysis
All variables are presented as mean (standard deviation) or median (min–max). Due to the small sample size, groups were compared using non-parametric tests, with the exact Mann–Whitney test used for quantitative variables. We compared the topographic distribution of cisplatin tissue concentrations between the HIPEC and PIPAC groups, globally and according to station. Tissue concentrations were also analyzed within groups using non-parametric tests for matched pairs with correction for multiple testing (Friedman). For all tests, a p value of <.05 was the statistical significance threshold.
Results
All experiments were performed as scheduled. Mean weight on the day of surgery was 38 kg (35–43 kg).
Peritoneal platinum concentrations
Peritoneal platinum concentrations are presented in and . Median total platinum peritoneal concentration was significantly higher in the HIPEC group (18.0 μg/g) than the PIPAC group (4.3 μg/g) (p < .001). The ratio between drug posology and median total platinum concentration was 2.23 times higher with PIPAC than with HIPEC. Considering only the parietal peritoneum biopsies (stations 1–9), median platinum concentrations were 18.8 μg/g in the HIPEC group, and 4.8 μg/g in the PIPAC group (p < .0001). In the visceral peritoneum (station 10), mean platinum concentrations were 3.17 μg/g in the HIPEC group, and 0.75 μg/g in the PIPAC group (p = .005). Drug uptake distribution analysis revealed that the mean and median platinum concentrations were higher in the HIPEC group at all stations, with a significant difference at 7 of 10 stations ().
Figure 1. Boxplot showing topographic distribution of cisplatin tissular concentrations in the HIPEC and PIPAC groups. For each station, comparison has been performed between HIPEC and PIPAC groups using non-parametric tests (exact Mann–Whitney test). IQR: interquartile range.
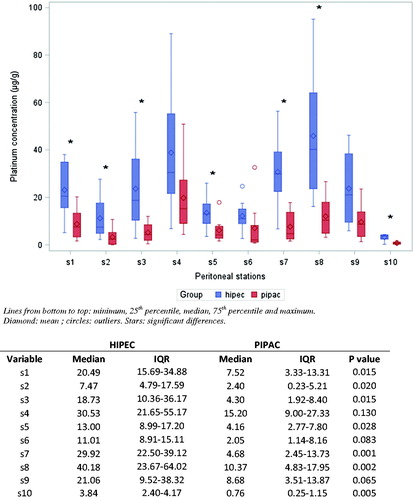
Table 1. Tissular platinum concentrations.
Intra-group analysis of platinum uptake spatial distribution revealed wide heterogeneity among stations in each group (p < .001 for each group) (), with greater platinum concentration variability in the HIPEC group (). The HIPEC group exhibited higher concentrations in samples from the right and left flanks (stations 4 and 8), next to the inflow tubes. The PIPAC group showed higher platinum uptake at station 4, facing the extremity of the micropump ().
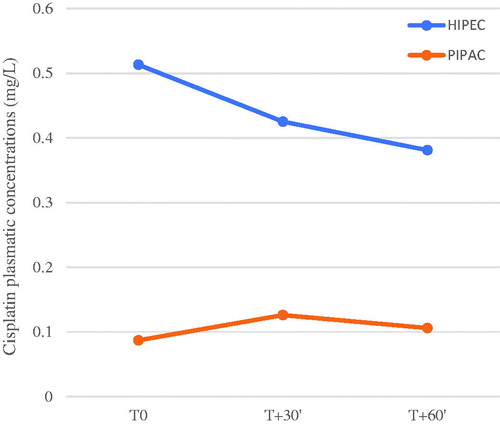
Total platinum plasma concentration
Mean total platinum plasma concentrations are presented in and . At each time-point, concentrations were significantly higher in the HIPEC group than the PIPAC group (p < .001). Peak mean plasma concentrations were 0.513 mg/L (0.174) at T0 in the HIPEC group, and 0.121 (0.021) mg/L at T30 in the PIPAC group.
Table 2. Cisplatin plasmatic concentrations according to time of sampling.
Discussion
We compared HIPEC and PIPAC in a swine model, using daily clinical practice treatment settings. Analysis of cisplatin peritoneal uptake revealed that both techniques resulted in heterogeneous spatial distribution, with significantly higher drug concentrations near the administration sites. HIPEC enabled higher peritoneal platinum concentrations than PIPAC, but considering the 10-fold difference in concentrations applied, the PIPAC’s yield was 2.2 times better.
When considering intraperitoneal administration, spatial drug distribution is important. PIPAC is reportedly better than HIPEC in this context, providing a homogeneous, ‘gas-like’ drug distribution throughout the abdominal cavity, via formation of microdroplets carried by the 12-mmHg capnoperitoneum [Citation11]. Initially, a heterogeneous distribution was reported with nebulization of microdroplets smaller than 10 μm and an enhanced staining near the micropump, without staining of the bursa omentalis and inferior liver [Citation17]. In 2012, Solass et al. [Citation11] described a second-generation nebulizer. They compared staining with PIPAC versus conventional lavage, and found that PIPAC yielded a 150-fold higher dye concentration, with more intense staining and a larger stained peritoneal surface [Citation11]. The same team also compared lavage with nebulization in an ex vivo model, assessing agent penetration into a peritoneal fragment inserted into a plastic box mimicking a peritoneal cavity [Citation18]. Lavage was performed with 10 mL, without reference to box volume. The PIPAC group included an electrical gradient to enhance tissue penetration. Compared to lavage, nebulization allowed deeper (up to 1 mm) and more homogeneous agent uptake [Citation18]. Thus, initial comparisons suggested that PIPAC provides more homogeneous distribution than liquid intraperitoneal treatment (considered equivalent to HIPEC).
In contrast, our present experiments demonstrated heterogeneous cisplatin distribution in both the groups, with higher concentrations near the delivery sites. This phenomenon is magnified by the higher concentration used with HIPEC, as attested by the more important variability among stations in that group and by the wide heterogeneity of concentrations in the PIPAC group station 6, the farthest from the MIP. Median total platinum peritoneal concentration was significantly higher following HIPEC than PIPAC (18.0 μg/g versus 4.3 μg/g, p < .001), and platinum concentrations were higher in the HIPEC group at all stations. A research team from Herne studied doxorubicin distribution via PIPAC, and also showed heterogeneous distribution. They reported that doxorubicin reached all the peritoneum parts, showing varying penetration depths among areas [Citation12], with the greatest depth (around 350 μm) near the micropump in the small intestine [Citation12,Citation19].
Khosrawipour et al. [Citation19] evaluated the effects of changing various parameters in an ex vivo model, similar to that described above. They found that micropump position influenced drug distribution and penetration, with better results on the surface directly opposite the micropump. With the micropump closer to tissue, the penetration depth was higher in front of the micropump, but lower elsewhere. Higher doxorubicin concentration promoted significantly increased drug penetration, predominantly localized near the micropump. Enhanced internal pressure did not significantly increase the doxorubicin penetration depth [Citation19].
Another study compared distribution between PIPAC and IP chemotherapy in postmortem swine, applying planar scintigraphy and single-photon emission computed tomography after 99mTc-Pertechnetat administration [Citation20]. IP liquid administration was performed using 150 mL of marked solution. They found deviations from uniform drug distribution of 40% and 74% in the PIPAC group, and of 23% and 34% in the liquid group. All animals exhibited ‘hot spots’ – areas with higher deposition, which uptook 20–30% of the total delivered 99mTc. In addition to regions near the injection sites, the Douglas pouch was a hot spot in both the groups, suggesting a role of gravity in drug repartition. In our present study, we found hot spots of cisplatin uptake in stations near the inlet ports, but not in the pelvic area.
The results obtained with doxorubicin in ex vivo models are concordant with our present results using cisplatin in an in vivo model. The findings seem to be at least partly explainable by microdroplet size [Citation20,Citation21]. Ex vivo measurements indicate that the micropump delivers droplets with a mean size of 11 μm [Citation3–15,Citation22]. Göhler et al. [Citation21] describes various aerosols generated with a micropump, showing a two-phase gas with 98% of the droplets being >3 μm and a median diameter of 25 μm. From 3 μm upwards, droplets submit to gravitational settling and inertial impaction. The authors calculated that target droplet size should be <1.2 μm for homogenous drug distribution during PIPAC [Citation21].
Although nebulization is a promising approach, neither PIPAC nor HIPEC could guarantee homogenous intraperitoneal drug distribution. The proposed theory that large droplets are affected by gravity seems intuitive, and is a basis for technological improvements. Proposed innovations to homogenize drug uptake include using radiation to decrease doxorubicin penetration [Citation23,Citation24], electrostatic precipitation to accelerate drug uptake [Citation25] and an endoscopic microcatheter to perform microinvasive PIPAC [Citation26]. Acting on droplet behavior seems to be the most promising idea. Adding an electrostatic field may enhance charged droplet precipitation and tissue penetration. Willaert et al. [Citation27] reported in 48 patients that electrostatic PIPAC (EPIPAC) was safe, feasible and efficient [Citation27]. Göhler et al. [Citation28] proposed to reduce aerosol droplet size through a hyperthermic intracavitary nanoaerosol therapy (HINAT), based on extracavitary generation of a heated aerosol. They described an aerosol comprising droplets with a 1.3 μm median diameter, which provides a quasi-uniform distribution over the peritoneum in a swine model. This distribution yields better drug uptake, with a mean penetration depth of 226 μm (88) for HINAT, compared to 102 μm (104) for PIPAC-MIP [Citation28].
The IP drug dosage determines both the efficacy (drug uptake in peritoneum) and toxicity (systemic passage). We used cisplatin concentrations equivalent to those clinically prescribed [Citation13,Citation14]. Schematically, dose ratio between HIPEC and PIPAC was of 9.3, whereas median tissular concentration ratio was of 4.2, suggesting a 2.2 times better yield for peritoneal uptake of IP cisplatin with PIPAC. So intrinsically PIPAC provides a better cisplatin uptake than HIPEC. Clinical used concentrations are constantly evolving. A dose escalation phase I study with a standard 3 + 3 dose escalation design confirmed that cisplatin concentration could be increased up to 10.5 mg/m2 (third step) with no dose limiting toxicity [Citation29]. Nevertheless, a colon perforation occurred during surgical access and other reports suggest an over-risk of anastomotic leakage with PIPAC, suggesting that a clinically usable dose-limit exist for this technic [Citation10,Citation14,Citation30]. While HIPEC was considered to induce 18% of renal insufficiency at 80 mg/m2 [Citation31], nephroprotection with intravenous sodium thiosulfate seems to prevent renal failure, enabling the use of 100 mg/m2 cisplatin in a recent prospective trial [Citation3]. In our study, total cisplatin plasma levels were significantly higher after HIPEC than after PIPAC. Cisplatin’s nephrotoxicity is the main dose-limiting factor in HIPEC, while perturbed renal function is not described after PIPAC [Citation32]. This renal insufficiency risk remains concerning for patients with a poor prognosis [Citation3,Citation33]. Cisplatin pharmacokinetics studies are complex. After intravenous administration, many platinum fractions circulate, including aquated and unchanged cisplatin, and ‘mobile’ and ‘fixed’ metabolites blinded to low or high molecular mass substances, respectively [Citation34]. While the level of unchanged platinum seems to be the most relevant parameter for studying nephrotoxicity, total serum platinum could be used as a risk marker [Citation35]. Data from the literature show that cisplatin-linked renal failure can occur at ≥1 mg/L [Citation34,Citation36–38]. The peak plasma concentration in our experiments was below this threshold, but we did not check serum creatinine, which could have been silently affected.
Our study had several limitations, the main one being that results were obtained in healthy tissues, without peritonectomies. Consequently, the plasma platinum concentrations were probably lower than those observed after CRS-HIPEC, which could influence drug distribution. Additionally, we studied cisplatin penetration into normal peritoneum, which differs from tumor nodules. However, Los et al. [Citation39] described higher cisplatin intratumoral concentration with a single IP administration compared to IV administration, with no increased IP pressure. This concentration was increased by repeated injections, with the advantage extending up to 1.5 mm inward from the tumor periphery [Citation39]. Moreover, hyperthermia enhances platinum penetration, with concomitant survival decreases both in vitro and in vivo [Citation15]. The cisplatin-induced DNA-adducts formation was measured 3–5 mm into the tumor tissue following IP administration and hyperthermia [Citation40].
HIPEC and PIPAC approaches are not dedicated to the same clinical uses, but are often compared [Citation10,Citation11,Citation18]. Our study was a picture of peritoneal cisplatin uptake using clinically established protocols. It showed a heterogeneous distribution in both the groups and a higher tissular drug uptake with HIPEC. PIPAC provided a better yield with lower systemic passage and thus toxicity, confirming this technic as promising. Improvements are needed, possibly including HINAT, increased drug concentration, and repeated administration during the same session with MIP position modified by microcatheter.
Author contributions
Davigo: conception of the experimental study, execution of the experiments (surgical part), manuscript writing and editing. Passot: conception of the experimental study, results analysis, manuscript editing. Vassal: conception of the experimental study, execution of the experiments (anaesthetic part), manuscript writing and editing. Bost: conception of the experimental study, execution of the experiments (veterinary part), manuscript editing. Tavernier: conception of the experimental study, execution of the experiments (surgical part), manuscript editing. Decullier: biostatistics analysis, manuscript editing. Bakrin: conception of the experimental study, manuscript editing. Alyami: conception of the experimental study, execution of the experiments (surgical part), manuscript editing. Bonnet: conception of the experimental study, execution of the experiments (veterinary part), manuscript editing. Louzier: conception of the experimental study, execution of the experiments (veterinary part), manuscript editing. Paquet: conception of the experimental study, execution of the experiments (veterinary part), manuscript editing. Allaouchiche: conception of the experimental study, execution of the experiments (anaesthetic part), manuscript writing and editing. Glehen: conception of the experimental study, manuscript editing. Kepenekian: conception of the experimental study, manuscript writing and editing.
Acknowledgements
The authors thank Dr. Annie-Claude Beaujard and Dhoul-Anrif Alimoundhir for their help conducting experiments. We also thank the Cavitherm, Gamida and Idimed laboratories for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer. 2014;50(2):332–340.
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Rihuete-Caro C, Manzanedo I, Pereira F, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44(11):1805–1810.
- Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719.
- Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
- Miura JT, Johnston FM, Gamblin TC, et al. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol. 2014;21(12):3947–3953.
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62(1):1–11.
- Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89(7):480–487.
- Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21(2):553–559.
- Solaß W, Hetzel A, Nadiradze G, et al. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26(7):1849–1855.
- Khosrawipour V, Khosrawipour T, Kern AJP, et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol. 2016;142(11):2275–2280.
- Di Giorgio A, De Iaco P, De Simone M, et al. Cytoreduction (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: retrospective italian multicenter observational study of 511 cases. Ann Surg Oncol. 2017;24(4):914–922.
- Tempfer CB, Celik I, Solass W, et al. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol. 2014;132(2):307–311.
- Los G, Sminia P, Wondergem J, et al. Optimisation of intraperitoneal cisplatin therapy with regional hyperthermia in rats. Eur J Cancer. 1991;27(4):472–477.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
- Reymond MA, Hu B, Garcia A, et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc. 2000;14(1):51–55.
- Solass W, Herbette A, Schwarz T, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc . 2012;26(3):847–852.
- Khosrawipour V, Khosrawipour T, Falkenstein TA, et al. Evaluating the effect of Micropump© position, internal pressure and doxorubicin dosage on efficacy of pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. Anticancer Res. 2016;36(9):4595–4600.
- Bellendorf A, Khosrawipour V, Khosrawipour T, et al. Scintigraphic peritoneography reveals a non-uniform 99mTc-pertechnetat aerosol distribution pattern for pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a swine model. Surg Endosc. 2017; 26(4):1849–1849.
- Göhler D, Khosrawipour V, Khosrawipour T, et al. Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg Endosc. 2017;31(4):1778–1784.
- Reymond MA, Solass W. PIPAC: pressurized intraperitoneal aerosol chemotherapy–cancer under pressure. 2014.
- Khosrawipour V, Giger-Pabst U, Khosrawipour T, et al. Effect of irradiation on tissue penetration depth of doxorubicin after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo model. J Cancer. 2016;7(8):910–914.
- Khosrawipour V, Khosrawipour T, Hedayat-Pour Y, et al. Effect of whole-abdominal irradiation on penetration depth of doxorubicin in normal tissue after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a post-mortem swine model. Anticancer Res Int Inst Anticancer Res. 2017;37(4):1677–1680.
- Kakchekeeva T, Demtröder C, Herath NI, et al. In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol. 2016;23(S5):592–598.
- Khosrawipour V, Mikolajczyk A, Schubert J, et al. Pressurized intra-peritoneal aerosol chemotherapy (PIPAC) via endoscopical microcatheter system. Anticancer Res. 2018;38(6):3447–3452.
- Willaert W, Van de Sande L, Van Daele E, et al. Safety and preliminary efficacy of electrostatic precipitation during pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable carcinomatosis. Eur J Surg Oncol. 2019;45(12):2302–2309.
- Göhler D, Große S, Bellendorf A, et al. Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurised intraperitoneal aerosol chemotherapy (PIPAC): technical description, experimental validation and first proof of concept. Beilstein J Nanotechnol. 2017;8:2729–2740.
- Tempfer CB, Giger-Pabst U, Seebacher V, et al. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol. 2018;50(1):23–30.
- Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Onc. 2016;14(1):128.
- Gouy S, Ferron G, Glehen O, et al. Results of a multicenter phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer. Gynecol Oncol. 2016;142(2):237–242.
- Blanco A, Giger-Pabst U, Solass W, et al. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol. 2013;20(7):2311–2316.
- Howell SB, Pfeifle CE, Wung WE, et al. Intraperitoneal cis-diamminedichloroplatinum with systemic thiosulfate protection. Cancer Res. 1983;43(3):1426–1431.
- Hanada K, Nishijima K, Ogata H, et al. Population pharmacokinetic analysis of cisplatin and its metabolites in cancer patients: possible misinterpretation of covariates for pharmacokinetic parameters calculated from the concentrations of unchanged cisplatin, ultrafiltered platinum and total platinum. Jpn J Clin Oncol. 2001;31(5):179–184.
- Reece PA, Stafford I, Russell J, et al. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J Clin Oncol. 1987;5(2):304–309.
- Nagai N, Kinoshita M, Ogata H, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39(1–2):131–137.
- Nagai N, Ogata H, Wada Y, et al. Population pharmacokinetics and pharmacodynamics of cisplatin in patients with cancer: analysis with the NONMEM program. J Clin Pharmacol. 1998;38(11):1025–1034.
- Urien S, Brain E, Bugat R, et al. Pharmacokinetics of platinum after oral or intravenous cisplatin: a phase 1 study in 32 adult patients. Cancer Chemother Pharmacol. 2005;55(1):55–60.
- Los G, Mutsaers PHA, van der Vijgh WJF, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitonal chemotherapy: a comparison with systemic chemotherapy. Cancer Res Am Assoc Cancer Res. 1989;49(12):3380–3384.
- van de Vaart PJM, van der Vange N, Zoetmulder FAN, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin–DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34(1):148–154.