Abstract
Purpose
To investigate the feasibility, efficacy and safety of one-lung ventilation for percutaneous thermal ablation of liver tumors in the hepatic dome.
Materials and methods
From 5 January 2017 to 16 April 2019, 64 patients who underwent ultrasound-guided thermal ablation with a total of 75 liver malignant tumors located in the hepatic dome were enrolled in the present study. One-lung ventilation was employed to improve the acoustic window and protect the lung and diaphragm. If the one-lung ventilation was unsuccessful, artificial pleural effusion was added. The technical efficacy was confirmed by contrast-enhanced computed tomography/magnetic resonance imaging (CT/MRI) 1 month later. After that, CT/MRI was performed every 3–6 months.
Results
Among the enrolled patients, the technical success rate of one lung ventilation was 92.2% (59/64). The visibility scores of tumors were improved significantly after one-lung ventilation compared to those before one-lung ventilation (p < .001). Finally, 78.6% (55/70) of the tumors achieved clinical success of one-lung ventilation to become clearly visible and underwent thermal ablation. Fourteen of the remaining 15 tumors achieved a satisfactory acoustic window after combination of artificial pleural effusion. One lesion remained inconspicuous and partly affected by pulmonary gas. The follow-up period was 8 months (3–30 months). The technical efficacy rate was confirmed to be 100% (75/75). During the follow-up period, local tumor progression occurred in 2 patients (2/75, 2.7%). Major complications occurred in two patients (2/64, 3.1%) receiving one-lung ventilation.
Conclusions
One-lung ventilation is a promising noninvasive method for the thermal ablation of hepatic dome tumors due to its efficacy and safety.
Introduction
Locoregional ablation therapy is recommended by the updated guidelines [Citation1,Citation2] as a first-line treatment option for early and very early stage hepatocellular carcinoma (HCC) or patients not eligible for surgical resection. Among the varies imaging modalities, ultrasound (US) is one of the most widely used guiding means because it is convenient, real-time and radiation-free [Citation1]. However, challenges still exist in the clinical practice of US-guided thermal ablation, such as ablation of tumors in the hepatic dome due to the poor acoustic window and vulnerability to thermal damage.
To solve this problem, artificial pleural effusion has been the most frequently reported ancillary procedure to improve the visualization of tumors in the hepatic dome and create a liquid barrier between the lung and the diaphragm [Citation3–5]. This method has made thermal ablation of tumors in the hepatic dome feasible. However, the catheterization process is technically difficult and strongly depends on the operator experience. Besides, it is an invasive procedure that may increase the incidence of post-ablation complications. Some literature has also mentioned that the visualization of tumors in the anterior position of the hepatic dome may not be improved [Citation6] even with a large amount of artificial pleural effusion injection. In addition to artificial pleural effusion, several ancillary approaches have been proposed, including artificial ascites [Citation7], artificial pneumothorax [Citation6,Citation8,Citation9], transthoracic ablation under computer tomography(CT) guidance [Citation6,Citation9–11] or thorascopy [Citation12], usage of epipericardial fat pad [Citation13]. However, all of these approaches were considered as invasive procedures and have not been widely adopted as a routine practice.
Therefore, researchers are seeking a noninvasive procedure to assist thermal ablation of liver tumors in the hepatic dome. D’Amico et al. [Citation14] recently reported that thermal ablation with one-lung ventilation could achieve US-guided percutaneous ablation of hepatic dome tumors. However, this study was only a case report with a limited follow-up period. Further evidence was needed to validate the efficacy and safety of this method.
Therefore, in the present study, we aimed to investigate the feasibility, efficacy and safety of one-lung ventilation as an ancillary approach for thermal ablation of liver tumors in the hepatic dome.
Materials and methods
Inclusions and exclusions
Between January 2017 and April 2019, patients with liver tumors treated by US-guided thermal ablation were enrolled. The inclusion criteria were as follows: (1) patients diagnosed as malignant liver tumor; (2) nodules located below the diaphragm and adjacent to the air-filled lung, causing the acoustic window to be affected by pulmonary gas. Diffuse pulmonary disease and pulmonary tumors were excluded according to the pre-ablation chest CT, X-ray or pulmonary function test.
The diagnosis of hepatocellular carcinoma was based on typical imaging or pathological results [Citation1], while the diagnosis of other liver malignancies was based on pathological results.
Informed consent
One-lung ventilation was occasionally employed as a routine clinical practice of general anesthesia to assist thermal ablation of hepatic dome tumors for the first time in 2016 with no intent to carry out a clinical research. The data was retrospectively collected in the present study. The study on thermal ablation was approved by the IRB; Since one-lung ventilation was a routine practice conducted by the anesthesiologist as a portion of general anesthesia, an ethics approval for one-lung ventilation assisted thermal ablation was not applied additionally. Before the ablation procedure, every patient was informed of one-lung ventilation implementation and the potential risks. So informed consent of the ablation procedure as well as the implementation of one-lung ventilation was obtained from every patient as part of the routine management. Inform consent of the study was not obtained in this retrospective study.
Implementation of one-lung ventilation
Patients underwent chest CT, X-ray or pulmonary function test to rule out diffuse pulmonary disease and pulmonary tumors before the ablation procedure. Each patient was placed in a supine position with the right arm extended. All ablation procedures were performed under endotracheal general anesthesia. During the anesthesia process, a double cavity endotracheal tube (Covidien, Mansfield, MA, USA) was intubated under the guidance of a bronchofiberscope. One-lung ventilation (tidal volume, 5–7 mL/kg; respiratory rate, 15–18/min; positive end-expiratory pressure, 5–10 cmH2O; fraction of inspired oxygen, 70–80%) was induced immediately before every puncture of the electrode or antenna and assessment of the treatment effect with moderate pressure to collapse one lung. The visibility of tumors was graded by the operator on a 3-point scale: 1 score- Completely invisible due to the pulmonary gas; 2 score- Partially visible and partially affected by the pulmonary gas; 3 score- Clearly visible of the whole tumor. If the acoustic window was improved and the visibility score was raised to three, one-lung ventilation was considered as clinically successful and the ablation would subsequently be performed. If the acoustic window was not improved to display the whole tumor, then one-lung ventilation was considered clinically unsuccessful, and artificial pleural effusion was added to improve the visibility of the tumor. Artificial pleural effusion enabled the injection of saline solution into the pleural cavity, which acted as the acoustic window. The detailed steps refer to the previous literature [Citation5].
The patient’s oxygen saturation and vital signs were closely monitored during the ablation procedure. If oxygen saturation decreased obviously (≤93%) during one-lung ventilation, the collapsed lung was pumped up again, and bilateral lung ventilation was employed. In this situation, artificial pleural effusion was performed instead.
Thermal ablation
Equipment
MyLab Twice and MyLab Class US machines (Esoate, Genoa, Italy) with convex probe CA541 (frequency: 1–8 MHz) or CA431 (frequency: 4–10 MHz) were employed for image guidance. Radiofrequency ablation (RFA) and microwave ablation (MWA) were used in the present study. RFA was performed with a cooled-tip RFA system (Covidien, Mansfield, MA, USA), and MWA was performed with an internally cooled microwave antenna (Kangyou Cor., Nanjing, China) and an microwave generator (Kangyou Cor., Nanjing, China) of 2450 MHz.
Ablation procedure
The thermal ablation procedure was performed by three senior interventional US physicians (ZRQ, LK, and XEJ) with more than 5 years of experience in ablation procedures from the beginning of the study. The selection of ablation techniques was mainly based on the location of lesions and the condition of patients. For lesions directly adjacent to the diaphragm or in difficult puncture locations, radiofrequency ablation was preferred. For larger lesions (≥2 cm) with a distance of no less than 10 mm to the diaphragm or patients with abnormal coagulation function (prothrombin time between 14 and 20 s or a platelet count of 50–100 *109/L), microwave was preferred. With the utilization of one-lung ventilation, the image of the whole tumor could be achieved, and the electrode or antenna was inserted under US guidance and precisely positioned in the tumor. Single or overlapping multiple punctures were performed to encompass the index tumor and a circumferential 5-mm ablative margin if possible. When the ablation procedure was completed as planned, contrast-enhanced US was employed 5–10 min later to evaluate whether the index tumor was ablated completely. Supplementary ablation would be performed if necessary. If the avascular zone completely covered the whole tumor, it was considered a technical success.
Post-ablation surveillance and follow-up
Symptoms were monitored after the ablation procedure. US examination was performed to exclude early complications on the first day after ablation. Contrast-enhanced CT/magnetic resonance imaging (MRI) one month after ablation was used to confirm the technical efficacy. Then, contrast-enhanced CT/MRI combined with serum testing was used to monitor the incidence of recurrence every three months.
Statistics and analysis
SPSS 22.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Continuous measurement data are presented as the mean ± standard deviation if they were normally distributed or as the median (range) if the data were not normally distributed. Enumeration data are presented as percentages.
Technical success rate of one-lung ventilation was defined as the percentage of patients among whom one-lung ventilation was feasible and no oxygen saturation decrease was observed during the ablation procedure. Clinical success rate of one-lung ventilation was defined as the percentage of lesions that was improved to become clearly visible after the application of one-lung ventilation. The hepatic dome was divided into the anterior part and posterior part by the top of the dome. The difference in the clinical successful rate of application between the anterior and posterior dome was compared with the Chi square test. The difference of visibility scores between before and after the implementation of one-lung ventilation was compared with the paired t-test.
Results
Patients and lesions
From 5 January 2017 to 16 April 2019, 64 patients with a total of 75 liver malignant tumors were enrolled in the present study. The baseline characteristics of the enrolled patients and lesions are presented in .
Table 1. Baseline characteristics of patients and lesions.
One-lung ventilation
Among the 75 liver tumors in 64 patients, one-lung ventilation was successfully induced in 59 patients with 70 liver tumors, yielding a technical success rate of one-lung ventilation application of 92.2% (59/64). The reasons for technical failure of one-lung ventilation included unsatisfactory position of the double lumen tube (three tumors in three patients) and obvious decrease in oxygen saturation (two tumors in two patients). For this five patients, bilateral lung ventilation was employed for the rest of the ablation procedure and artificial pleural effusion was injected instead to assist thermal ablation.
After the application of one-lung ventilation, the visibility score of tumors was improved to 2.79 ± 0.41, higher than that before one-lung ventilation (1.53 ± 0.50) (p < .001). The visibility scores of tumors before and after one-lung ventilation were presented in . Finally, 55 tumors in 44 patients achieved a satisfactory acoustic window (graded as 3 scores level) after one-lung ventilation and underwent US-guided thermal ablation, with a clinical success rate of 78.6% (55/70) ( and ). Right side one-lung ventilation (left lung collapsed) was induced in three liver tumors in one patient, while left side one-lung ventilation (right lung collapsed) was induced in 52 tumors in 43 patients. For tumors located in the anterior part of the hepatic dome, the clinical success rate of application was 89.2% (33/37), while that for tumors located in the posterior part was 66.7% (22/33) (p = .039).
Figure 1. A 66-year-old male. (A) Contrast-enhanced MRI indicated a recurrent hepatocellular carcinoma located in segment 4/8 with a maximum diameter of 17 mm. (B) The nodule was partially visible and partially affected by the pulmonary gas. (C and D) The nodule became completely visible after one-lung ventilation and contrast-enhanced ultrasound confirmed the target location. (E) Thermal ablation was performed and immediate contrast-enhanced ultrasound demonstrated that the avascular zone covered the index tumor completely. (F) Contrast-enhanced MRI one month after the ablation procedure confirmed the technical efficacy.
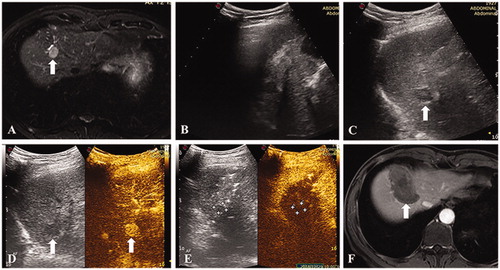
Figure 2. A 48-year-old male. (A) Contrast enhanced MRI indicated a recurrent hepatocellular carcinoma of 12 mm in segment 7. (B) The nodule was completely invisible because of the pulmonary gas. (C) The nodule became completely visible after one-lung ventilation. (D) Thermal ablation was performed and immediate contrast-enhanced ultrasound demonstrated no hyper-enhancement of the tumor. (E) Contrast-enhanced MRI one month after the ablation procedure confirmed the technical efficacy.
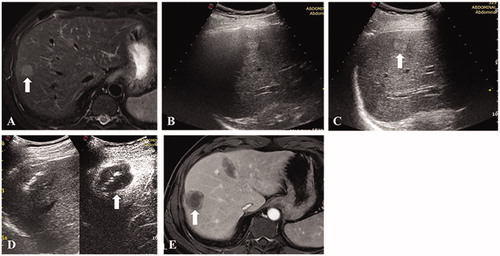
Table 2. Visibility scores before and after one-lung ventilation.
In the remaining 15 patients with 15 tumors, 14 tumors achieved a satisfactory acoustic window after the combination of artificial pleural effusion (medium volume 500 mL, 400–1000 mL). One lesion in one patient was still inconspicuous and partly affected by the pulmonary gas. The operator placed the electrode mainly on the basis of personal experience and estimation of the lesion’s location.
Thermal ablation
RFA was performed in 43 patients (53 liver tumors), while MWA was performed in 21 patients (22 liver tumors). All patients enrolled in the present study achieved technical success in one ablation session. One to six ablations were performed to cover the whole tumor. The mean operation time was 75 min (45–130 min).
Complications related to thoracic injury
After the ablation procedure, the double cavity endotracheal tubes were removed from all patients without complications after re-expansion of their lung.
No dyspnea was observed among the 64 patients with 75 liver tumors. The major complication rate of all enrolled patients was 2.2% (2/64). Among the 44 patients with only one-lung ventilation, one patient (1/44, 2.3%) exhibited a large amount of hydrothorax. The hydrothorax resolved after tube drainage. In contrast, of the 15 patients with one-lung ventilation and artificial pleural effusion application, one patient (1/15, 6.7%) exhibited thoracic bleeding, and thoracoscopy was employed for emergent hemostasis.
Treatment effect
The follow-up period was 8 months (3–30 months). According to the contrast-enhanced CT/MRI, the technical efficacy rate was 100% (75/75). During the follow-up period, local tumor progression (LTP) occurred in two patients (2/75, 2.7%).
Discussion
One-lung ventilation, is a routine technique to control the ventilation of each lung separately during general anesthesia; this application is widely used in thoracic surgery because of its safety and less complications [Citation15,Citation16]. Recently, some researchers began to employ this technique as an ancillary method to asist the thermal ablation of tumors in the hepatic dome. F D’Amico et al. [Citation14] presented a case of subdiaphragmatic and recurrent hepatocellular carcinoma in which the tumor was only reachable after one-lung ventilation and was successfully ablated. This case report indicated that one-lung ventilation may be a safe and efficient option for tumors in the hepatic dome. However, further validation involving larger populations was still needed to clarify the usefulness and clinical value of this technique.
Therefore, to explore the value of one-lung ventilation in the thermal ablation of tumors in the hepatic dome, the present study enrolled 56 patients and lengthened the follow-up period. The results showed a complete ablation rate of 100%, higher than previously reported [Citation4,Citation5,Citation17,Citation18], and an LTP rate of 2.7%, which is lower than that in the previous literature [Citation5,Citation8,Citation18–20]. This finding could be explained by the fact that the application of one-lung ventilation causes the lung to collapse [Citation15], which in turn can reduce the influence of pulmonary gas and improve the acoustic window. According to the visibility scores, the acoustic window after one-lung ventilation was significantly improved compared to that before one-lune ventilation. And the acoustic window could be improved to a satisfactory effect for nearly 80% of tumors. This was important because one-lung ventilation could be a noninvasive alternative for nearly 80% of tumors in the hepatic dome and the implementation of invasive ancillary procedures could be reduced to a considerable extent. In particular, our results indicated that one-lung ventilation may have a good effect on improving the acoustic window of tumors in the anterior part of the hepatic dome. Difference was significant between anterior and posterior hepatic dome in clinical success rate of one-lung ventilation. This specific phenomenon was possibly related to the relatively deep location of posterior hepatic dome, which may cause acoustic attenuation. However, this explanation still needs clarified and further evidence would be collected in the future. The patient’s supine position could possibly make the anterior location in the hepatic dome highly difficult to reach for the artificial pleural effusion. Therefore, one-lung ventilation may be an ideal solution for improving the acoustic window of tumors in the anterior part of the hepatic dome. Besides, the application of right lung collapse was more common than left lung collapse in our study. It could be explained by that the majority of liver tumors in the hepatic dome were influenced by the right lung while for a small portion of tumors located in left lateral lobe, the acoustic window was affected by the left lung.
In addition, only one patient out of the 44 patients (1/44, 2.3%) receiving only one-lung ventilation suffered from major complication, the incidence of which was lower than those previously reported [Citation20]. The possible reason was that the lung and the heat field were separated after one-lung ventilation, thus reducing the risk of lung injury. In this situation, the tumor could be clearly visualized, and the ablation zone could be expanded to ensure a sufficient ablation margin. As a result, complete ablation could be achieved, and lung injury could be avoided.
However, for some patients, one-lung ventilation could not be applied successfully or only partly improved the acoustic window due to the technical failure of tube insertion or the specific location of the tumors. This problem can be solved by combining artificial pleural effusion with one-lung ventilation. In the present study, after the combination with the injection of artificial pleural effusion, the acoustic window of over 95% of tumors could be improved to display the whole tumor with a medium volume of 500 mL, which is lower than the injected volume previously reported [Citation5,Citation17]. This result implied that the combination of artificial pleural effusion and one-lung ventilation could have a synergistic role in improving the acoustic window and reducing the volume of injected effusion, in turn reducing the risk of major complications related to artificial pleural effusion.
According to our results, one-lung ventilation may be employed to improve the acoustic window not only of tumors located in the right hepatic dome but also of tumors in segment 2 or 4 by collapsing the left lung.
There are several limitations about the present study. First, the current investigation was a single-arm study without a control group for comparison. Second, the sample size was not sufficiently large. Third, the follow-up period for evaluating the long-term therapeutic effect was relatively short. Consequently, further research is needed to validate the clinical value of one-lung ventilation.
In conclusion, one-lung ventilation is a promising noninvasive method for thermal ablation of hepatic dome tumors with an exact treatment effect and safety. This modality could improve the acoustic window for over 70% of hepatic dome tumors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- American Association for the Study of Liver Diseases. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95(8):996–1004.
- Minami Y, Kudo M, Kawasaki T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38(11):1066–1070.
- Dezhi Z, Ping L, Xiaoling Y, et al. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperth. 2013;29(7):663–670.
- De BT, Dromain CM, Briggs P, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology. 2005;236(2):666–670.
- Hyunchul R, Lim Hyo K, Young-Sun K, et al. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR: Am J Roentgenol. 2008;32(5):109–109.
- Hermida M, Cassinotto C, Piron L, et al. Percutaneous thermal ablation of hepatocellular carcinomas located in the hepatic dome using artificial carbon dioxide pneumothorax: retrospective evaluation of safety and efficacy. Int J Hyperth. 2018;35(1):90–96.
- Fujiwara H, Arai Y, Ishii H, et al. Computed tomography-guided radiofrequency ablation for sub-diaphragm hepatocellular carcinoma: safety and efficacy of inducing an artificial pneumothorax. Acta Med Okayama. 2016;70(3):189–195.
- Shibata T, Shibata T, Maetani Y, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15(11):1323–1327.
- Kim YK, Kim CS, Lee JM, et al. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur J Radiol. 2006;60(1):100–107.
- Park SW, Kim Y, Kang HY, et al. Transthoracic radiofrequency ablation for hepatic tumor located beneath the diaphragm under one-lung ventilation: a case report. Medicine (Baltimore). 2018;97(51):e13863.
- Brennan DD, Ganguli S, Brecher CW, et al. Thinking outside the abdominal box: safe use of the epipericardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol. 2008;19(1):133–136.
- D’Amico F, Serafini S, Finotti M, et al. One-lung ventilation to treat hepatic dome lesion – a further step towards minimally invasive surgery: a case report. J Med Case Rep. 2019;13(1):83–86.
- Bernasconi F, Piccioni F. One-lung ventilation for thoracic surgery: current perspectives. Tumori. 2017;103(6):495–503.
- Mcgrath B, Tennuci C, Lee G. The history of one-lung anesthesia and the double-lumen tube. J Anesth Hist. 2017;3(3):76–86.
- Wang G, Sun Y, Cong L, et al. Artificial pleural effusion in percutaneous microwave ablation of hepatic tumors near the diaphragm under the guidance of ultrasound. Int J Clin Exp Med. 2015;8(9):16765–16771.
- Koda M, Ueki M, Maeda Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR: Am J Roentgenol. 2004;183(3):583–588.
- Ishikawa T, Imai M, Ko M, et al. Effect of treatment support on preventing local recurrence of hepatocellular carcinoma directly adjacent to the diaphragm. Mol Clin Oncol. 2017;7(1):61–66.
- Hsieh YC, Limquiaco JL, Lin CC, et al. Radiofrequency ablation following artificial ascites and pleural effusion creation may improve outcomes for hepatocellular carcinoma in high-risk locations. Abdom Radiol. 2019;44(3):1141–1151.
