Abstract
Purpose
To identify the beneficial body mass index (BMI) for patients with hepatocellular carcinoma (HCC) to achieve longer survival time following curative microwave ablation (MWA).
Methods
This retrospective study evaluated 474 patients with solitary primary HCC who underwent MWA. BMI at initial admission and other characteristics were collected. The associations of the BMI with the overall survival (OS) and disease-free survival (DFS) were analyzed by Cox proportional hazards regression analysis in multiple models. A two-piecewise linear regression model was applied to examine the threshold effect of the BMI on OS and DFS by maximized log likelihood method. The threshold level was determined by using trial and error.
Results
Patients with a normal BMI range achieved improved survival outcomes but similar DFS in multiple models. In the model with adjustments of the age, size, and Charlson score, patients with BMI ≤ 22.9 and ≤24.9 kg/m2 exhibited a lower death rate than patients with BMI ≤18.5 kg/m2 (p < 0.05). U-shaped relationships between the BMI and OS were illustrated when the BMI was set as a continuous variable. The death prevalence decreased with an increasing BMI up to the first turning point of 21.5 and increased with an increasing BMI up to the second turning point of 23.1 (p = 0.00). The threshold effect analysis indicated that no turning point was selected in the DFS results (p = 0.10).
Conclusions
The beneficial BMI level for HCC patients following MWA, with a more likely favorable survival outcome, is 21.5 to 23.1 kg/m2.
Introduction
Overweight and obesity, which are referred to as 'excess body weight,’ are defined as abnormal or excessive fat accumulation that lead to chronic diseases and reduce life expectancy [Citation1]. Many studies have proven that excess body weight is associated with a highly elevated risk of adverse health outcomes, such as cardiovascular disease, cancers, diabetes, and musculoskeletal disorders [Citation1]. The global prevalence of excess body weight and the associated cancer burden has been increasing over the past decades. Between 1975 and 2016, the prevalence of excess body weight in adults increased from nearly 21% in men and 24% in women to approximately 40% in both sexes. Consequently, excess body weight accounted for approximately 544,300 cases of all cancers in 2012 [Citation2]. Excess body weight is typically reported and classified by the body mass index (BMI), which is the most commonly used anthropometric index to approximate overall body fatness. A BMI ≥25 kg/m2 is defined as excess body weight, while ≤18.5 kg/m2 is defined as underweight. Unfavorable consequences of underweight include the high risk of infectious diseases and adverse pregnancy outcomes [Citation3].
The association between abnormal body weight and hepatocellular carcinoma (HCC) has been illustrated in several studies [Citation4]. HCC is the most common form of liver cancer and is the fourth most frequent cause of cancer deaths globally [Citation5,Citation6]. Hepatic viral infection remains its primary risk factor, while other exposures including diabetes, smoking, and alcohol consumption also play a role in the occurrence of HCC [Citation1,Citation7,Citation8]. Zhao et al. [Citation9] found that patients with HBV-related HCC exhibited significantly high mean BMI levels. Moreover, Yang et al. [Citation10] determined that obesity was associated with approximately 80% higher HCC incidences. A Korean population-based cohort study demonstrated that high BMI was significantly associated with a higher risk of HCC among patients with chronic HBV infections [Citation11]. Furthermore, Hagstrom et al. [Citation12] verified that high BMI was associated with an increased risk of severe liver disease in a national, population-based cohort study of 1.2 million men.
In addition to the negative influence of an abnormal BMI on the occurrence of HCC, it has been found to be associated with an inferior survival outcome following HCC treatment. Calle et al. [Citation13] reported that obesity was associated with a higher risk of HCC-related deaths in a large prospective study. Moreover, a higher BMI was associated with more progressive diseases or poor survival outcomes in HCC patients following liver transplantation, resection, and transcatheter arterial chemoembolization (TACE) treatment [Citation14–16]. Certain studies have also demonstrated that a lower BMI resulted in poor survival outcomes in HCC patients [Citation17,Citation18]. However, the majority of research has only indicated the initial tendency between the BMI and HCC morbidity or survival outcomes. In recent studies, BMI levels have typically been categorized into five groups and studied as a category variable. Weight loss is the ideal method for reducing the risk of HCC morbidity in obese patients. However, the ideal BMI for achieving the longest survival time in HCC patients has not yet been determined. Furthermore, additional factors such as the tumor size, tumor number or pathologic differentiation degree that affect survival are clinical indexes that patients cannot change or choose, while BMI is a definite factor that can be controlled in an environment conducive to a healthy diet and active living. Therefore, it is meaningful to identify the beneficial BMI range for prolonging survival time. To the best of the authors’ knowledge, at present, no studies exist that recommend the beneficial BMI for HCC patients.
For this reason, we studied the association between the BMI and survival outcomes in HCC patients following curative microwave ablation (MWA) treatment. The beneficial BMI range was identified to guide patients in maintaining their body weight at an ideal level, with the aim of achieving a longer survival time.
Materials and methods
Patient selection
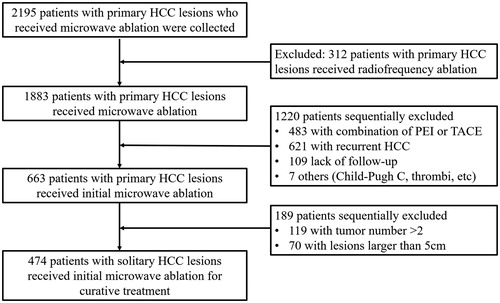
This retrospective study was approved by the institutional review board of the Chinese PLA General Hospital. Written informed consent was obtained from all patients prior to treatment, in accordance with clinical protocols. We reviewed the electronic medical records of 2195 patients who received MWA for HCC in our department from January 2008 to October 2017. Patients exhibiting the following indications were included in this study: (a) a solitary tumor ≤5 cm in diameter; (b) HCC confirmed at histologic or clinical examination; (c) Child–Pugh class A or B classification; (d) no evidence of extrahepatic metastasis, vein or bile duct tumor embolus at the time of diagnosis; (e) an Eastern Cooperative Oncology Group performance status of 0 to 1; (f) no previous anticancer treatment; and the exclusion criteria were as follows: (a) serious comorbidities, including severe heart or kidney dysfunction; (b) severe coagulation disorders (e.g. prothrombin time >30 s, prothrombin activity <40% or platelet count <30 × 109/L); (c) alcohol consumption or cigarette smoking after the HCC diagnosis; and (d) other malignancies in addition to HCC. The flowchart for the patient selection is presented in .
Figure 1. Flow diagram of patient selection in this study. HCC: hepatocellular carcinoma; PEI: percutaneous ethanol injection; TACE: transcatheter arterial chemoembolization.
The HCC diagnostic criteria were based on the guidelines of the European Association for the Study of Liver and the American Association for the Study of Liver Disease [Citation19].
Data collection and variable definitions
We collected the weight and height of the patients at their admission. The BMI was calculated as the weight in kilograms divided by the height in meters squared. The BMIs were further divided into five levels: ≤18.5 kg/m2 (underweight), 18.5 to 21.9 kg/m2 (normal), 22.0 to 24.9 kg/m2 (normal), 25 to 29.9 kg/m2 (overweight), and ≥30 kg/m2 (obese) according to the World Health Organization classification [Citation20]
The overall survival (OS) rate was calculated from the date of the first ablation treatment until either the date of death or the last visit to our outpatient clinic before 30 December 2017. Patients who remained alive at the last follow-up time were termed as ‘censored’ in the statistical analysis. The disease-free survival (DFS) was defined as the time during which the patient did not experience local tumor progression, intrahepatic distant recurrence or extrahepatic recurrence following the initial ablation treatment [Citation21]. The safety of all patients was assessed using the Society of Interventional Radiology classification system [Citation22]. Major complications were defined as an event that led to substantial morbidity and disability, which increased the care level, resulted in hospital admission or substantially lengthened the hospital stay (Society of Interventional Radiology classifications C to E). All other complications, such as fever or pain, were considered as minor.
Study outcomes
The primary outcome was the event of death due to HCC progression, or death due to complications of hepatic cirrhosis. The secondary outcome was tumor progression, including local tumor progression, intrahepatic distant recurrence, and extrahepatic recurrence.
MWA protocol
A cooled-shaft MW system (KY-2000, Kangyou Medical, China) with a 15-gauge, 18 cm-long cooled-shaft antenna was used in this study. The MWA was performed by doctors with at least 5 years of experience in hepatic MWA. All MWA procedures were performed percutaneously under general anesthesia and real-time ultrasound guidance. The techniques and strategy of the MWA were the same as those described in our previous study [Citation23]. In general, the microwave energy application was set at 50–80 W for 5–10 min in a session. The region of ablation was monitored by real-time US monitoring. MWA emission was stopped when the hyperechoic zone covered the entire tumor including a safety margin. The ablation therapy included an ablative margin of healthy tissue of at least 5 mm surrounding the tumor. For patients with tumors adjacent to the critical organs, an ablation margin of less than 5 mm or conformal ablation was achieved with the assistance of a temperature monitor, artificial pleural effusion or ascites. If a residual tumor was detected within three days following ablation, an additional MWA session was conducted to achieve complete coagulation. If incomplete tumor ablation remained after an additional treatment session, this case was defined as a technical failure, and such cases were excluded from the study.
Follow-up
All patients were required to undergo contrast-enhanced (CE) imaging (CE–MRI, CE–CT or CEUS) three days and one month after the initial MWA to determine its technical effectiveness and success. The patients underwent regular medical imaging and laboratory tests every three months during the first 2 years, and every six months thereafter. The laboratory tests included hematologic and biochemical analyses, such as a complete blood cell count, prothrombin time, a-fetoprotein (AFP), aspartate aminotransferase, alanine aminotransferase, total bilirubin, serum albumin, and creatinine levels. Moreover, a chest CT, pelvic MRI, bone scintigraphy, PET–CT or PET–MRI were also conducted for patients who were suspended with extrahepatic recurrence according to clinical symptoms or unexplained AFP level elevations. Once local tumor progression, intrahepatic distant recurrence or extrahepatic recurrence was detected during the regular follow-up, appropriate treatments such as surgical resection, thermal ablation, TACE, radiation therapy, sorafenib, or liver transplantation were applied according to the liver function, tumor characteristics or patient will.
Statistical analysis
The basic characteristics of the study population were summarized using descriptive statistical methods. The continuous variables were indicated as the mean and standard deviations for the age and size. The categorical variables were indicated as the number and percentage for the following metrics: gender, BMI grade, Charlson score, tumor location, cirrhosis, Child–Pugh, alanine aminotransferase (ALT), platelets, neutrophil-to-lymphocyte ratio (NLR), the Model for End-Stage Liver Disease (MELD), and albumin–bilirubin (ALBI) grade prior to ablation. Possible prognostic factors for OS and DFS were assessed using Cox proportional hazard models in univariate analyses.
The relationship between the BMI and the risk of death and tumor progression was evaluated by using the BMI as both a categorical and a continuous variable. For management of the BMI as a categorical variable, patients were categorized into quintiles according to their BMI at admission. Cox proportional hazards regression analysis was performed to evaluate the risk of outcomes in the quintile of normal BMI variability (reference hazard ratio: 1.0) versus the other four quintile of BMI variabilities. Three models were used: model 1 was adjusted for age and gender; model 2 was adjusted for age, size, and Charlson score; and model 3 was adjusted for age, gender, size, Charlson score, ALT, tumor location, NLR, platelets, MELD, Child–Pugh, ALBI, and cirrhosis. The variables entered into the different models were determined based on clinical knowledge and the results of the hazard ratio (HR) for the OS and DFS in the multivariate analysis.
Further analyses were performed to explore the relation between the BMI as a continuous variable and the survival outcomes. We applied a two-piecewise regression model to examine the threshold effect of the BMI on the OS and DFS. This model uses maximized log likelihood method. It first runs multiple two-piece-wise regression models. Each model uses a percentile (between 10% and 90%) of the BMI as turning point. The output result includes one-line linear regression (model I), two-piece-wise regression (model II) that gives maximum log likelihood, and p-value from log likelihood ratio test comparing model I versus model II. The model with the turning point that provides maximum log likelihood was picked. The variables entered into the adjusted model were determined based on the results of the HR for the OS and DFS in the multivariate analysis.
Two-tailed probability values of <0.05 were considered as statistically significant. All analyses were performed using Empower(R) (www.empowerstats.com X&Y Solutions, Inc., Boston, MA) and R (http://www.R-project.org).
Results
Patient characteristics
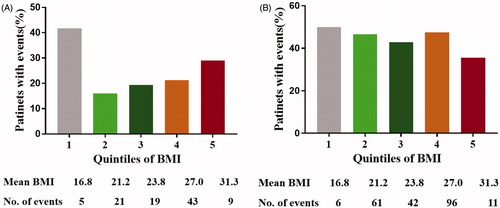
Among the 808 patients enrolled in the trial, 474 patients fulfilled the inclusion criteria for the study. Possible factors that could affect the survival outcomes were collected and are summarized in . The median BMI of patients at their admission was 24.8 ± 3.4, with a range of 14.38 to 36.68 kg/m2. Patients with a BMI lower than 18.5 kg/m2 or higher than 30 kg/m2 accounted for 9% of the total. Patients with a BMI between 24.9 and 29.9 kg/m2 accounted for the largest proportion of the total.
Table 1. Characteristics of included patients and univariate analysis of survival outcomes.
The univariate analysis of the BMI with the OS demonstrated that the BMI quintile was associated with OS results in HCC patients following MWA. Moreover, the NLR and ALBI grade were found to be associated with the OS, while the platelets and ALBI grade were found to be associated with the DFS, in the univariate analysis.
HCC was pathologically proven in 301 patients (63.5%) by percutaneous biopsy during or before ablation, and based on noninvasive criteria for the remaining 173 patients (36.5%).
Ninety-seven patients died in this study. Fifty-three patients died for HCC progression, 44 patients died for the complications due to hepatic cirrhosis. In the 44 patients, six patients died for hepatic encephalopathy, 12 patients die of upper gastrointestinal bleeding, nine patients died of infection, 17 patients died of multiple organ failure.
BMI quintile and survival outcomes
In the unadjusted data, the death rate was the highest in patients with a BMI lower than 18.5 kg/m2 and then increased with each higher quintile of body weight variability (). In the event of tumor progression, the quintile of body weight variability exhibited similar rates at different levels ().
The adjusted model results of the BMI on the survival results are presented in . In all models, a BMI between 18.5 kg/m2 and 22.9 kg/m2 was set as the reference HR (1.0). In model 1, the age and gender were adjusted, as these were common variables to be balanced in the different BMI groups. A decrease in the BMI level increased the risk of death in patients with BMI ≤18.5 kg/m2 (HR: 4.9; 95% confidence interval (CI); 1.8 to 13.2; p = 0.0). An increase in the BMI level decreased the risk of death in patients with BMI ≤ 29.9 kg/m2 (HR: 1.4; 95% CI; 0.8 to 2.7; p = 0.3), and BMI ≥30kg/m2 (HR: 3.4; 95% CI; 1.5 to 7.5; p = 0.0). No significant difference were found between BMI ≤22.9 kg/m2 and BMI ≤ 24,9 kg/m2. Similar results were found for model 2 with the adjustments of age, size, and Charlson score. The age, size, and Charlson score were significant prognostic factors for the OS in the multivariate analysis. In model 3, the age, gender, size, Charlson score, ALT, tumor location, NLR, platelets, MELD, Child–Pugh, ALBI, and cirrhosis were adjusted, as these were the potential factors for DFS. Patients with BMI ≤ 18.5 kg/m2 (HR: 5.2; 95% CI; 1.8 to 15.2; p = 0.0), BMI ≤ 29.9 kg/m2 (HR: 1.9; 95% CI; 1.1 to 3.4; p = 0.0)and BMI ≥30 kg/m2 (HR: 2.7; 95% CI; 1.1 to 6.6; p = 0.0) exhibited a lower death rate than patients with BMI ≤ 22.9 kg/m2. In all three adjusted models, the increase in the BMI had no influence on the tumor progression (p > 0.05).
Table 2. Adjusted effects of BMI on survival results in multiple models.
Threshold effect analysis of BMI on survival outcomes
The U-shaped relationships between the BMI and OS are illustrated in , in which the admission BMI was set as a continuous variable. The variables of age, size, and Charlson score were adjusted, as these were prognostic factors for the OS in the multivariable analyses. A BMI between 20 and 25 kg/m2 exhibited the lowest death rate. The nonlinear relationships between the admission BMI and OS were investigated further by threshold effect analysis to determine the turning points (). The OS rate was the highest when the BMI was between 21.5 and 23.1 kg/m2 (HR: 0.71; 95% CI: 0.26, 1.98; p = 0.52). The prevalence of death decreased with an increase in the BMI up to the first turning point of 21.5 kg/m2 (HR: 0.76; 95% CI: 0.61, 0.94; p = 0.01), and increased with an increase in the BMI up to the second turning point of 23.1 kg/m2 (HR: 1.14; 95% CI: 1.03, 1.25; p = 0.01) in the crude model. The LLR test demonstrated that the tendency was significant (p = 0.01). Similar results were found in the adjusted model and LLR test for the turning points, which were also significant (p = 0.00). The prevalence of death decreased with the increase in the BMI up to the first turning point of 21.5 kg/m2 (HR: 0.72; 95% CI: 0.57, 0.90; p = 0.00), and increased with an increase in the BMI up to the second turning point of 23.1 kg/m2 (HR: 1.13; 95% CI: 1.02, 1.24; p = 0.01) with the adjustments of age, size, and Charlson score.
Figure 3. The relationship between BMI levels and survival outcomes. (A) BMI and overall survival*; (B) BMI and disease-free survival#. *: adjusted for age, size, Charlson Score. #: adjusted for age, gender, size, Charlson Score, ALT, tumor location, NLR, platelet, MELD, Child-pugh, ALBI, cirrhosis.
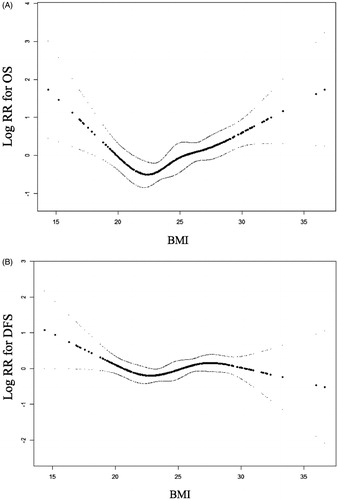
Table 3. Threshold effect analysis of BMI on overall survival by piece-wise linear regression.
Likewise, threshold effect analyses of the BMI on the DFS were conducted. The smoothing curve exhibited mild fluctuations with an increase in the BMI variability (). The threshold effect analysis () demonstrated that no turning point was selected to yield the maximum model likelihood for the DFS results in the crude model (p = 0.28) or adjusted model (p = 0.10).
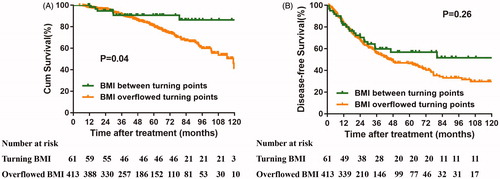
Thereafter, the patients were categorized into two groups according to the BMI level. The Kaplan–Meier curve validated the finding that patients with a BMI between the turning points exhibited longer OS than patients with a BMI beyond the turning points. Although the curve of the BMI between the turning points exhibited a higher tendency than that of the BMI beyond the turning points, the difference was not significant ().
Major complications
The rate of major complications according to the society of interventional radiology classification was 4.2% (20/474). No treatment related deaths were detected in all patients. Eleven patients were detected with pleural effusion and all recovered after aspiration (n = 4) or drainage (n = 7). Tumor seeding was diagnosed in two patients and both received MWA treatments after diagnosis. Biloma occurred in two patients and was cured after 2–3 months of drainage. Hepatic abscess was detected in two patients and was cured after drainage and use of antibiotics. Bleeding occurred in two patients and one was cured after injection of thrombin to the bleeding site, the other was cured after TACE treatment. Ascites occurred in one patient and recovered after drainage and albumin infusion.
Discussion
Excess body weight is pandemic in high-income countries, and its prevalence is increasing in low- and middle-income countries. Consequently, the global cancer burden owing to this condition is estimated to increase continually in the future [Citation2]. To date, the association between body weight loss and cancer risk reduction has not been demonstrated. Related studies are often hampered by the small proportion of individuals successfully maintaining their weight loss and the lack of information on intentional weight loss [Citation24].
The increasing prevalence of obesity has been considered as a contributory factor to the increasing incidence of HCC [Citation25] and poor survival outcomes following treatment [Citation15,Citation26]. Similarly, thus far, no studies have indicated the beneficial weight range for patients to guide weight control. All that can be done is telling patients to lose weight, but this is not a viable solution because underweight may also induce unfavorable survival outcomes. In this study, we firstly provided the recommended body weight level for HCC patients following curative ablation treatment. We found that patients maintaining a BMI between 21.5 and 23.1 kg/m2 could achieve a longer survival time, although this had little effect on the DFS. Both a lower and a higher BMI indicated inferior OS outcomes to that indicated by the BMI range recommended in this study.
The potential biological mechanism underlying the association between obesity and poor HCC survival may be related to a number of physiological changes. Obese individuals often have hepatic steatosis, which can potentially progress to steatohepatitis and cirrhosis [Citation27]. The dual role of key transcription factors in the fatty acid oxidation in hepatocellular proliferation and cyclooxygenase-2 expression may explain the final disease progression from steatohepatitis to HCC [Citation28]. Moreover, the development of insulin resistance may exhibit tumorigenic activity by promoting adipose tissue-derived inflammation, oxidative stress, hormonal changes, and lipotoxicity [Citation29–32]. The above two changes could be reasons for HCC development, and may also prompt new HCC lesions following curative therapy. Moreover, obesity has been found to be associated with microvascular invasion [Citation33], thereby resulting in poor HCC survival outcomes. Therefore, poor survival outcomes in obese patients have been attributed to angiogenesis dysregulation caused by adipose tissue. However, this hypothesis has been disputed, as Arsenii et al. [Citation34] reported that visceral obesity did not promote microvascular invasion in patients with HCC. Other factors such as diet, gut microbiomes, and genetic factors [Citation35–39] have been recognized as clinically relevant mechanisms. HCC mechanisms from cirrhosis have been described extensively; however, the mechanisms relating obesity and poor HCC survival remain unclear and require further study [Citation25].
Certain studies have also presented opposing results [Citation40–42]. Hassan et al. [Citation40] found that early adulthood obesity was associated with a higher incidence of HCC at a young age, but did not affect the HCC survival outcomes. In their study, 96.2% of patients were free of viral infections in the control group, while only half were in the study group. Furthermore, other HCC risk factors, such as alcohol consumption, cigarette smoking, and diabetes mellitus, were all distributed significantly unevenly in the two groups. Although statistical methods have been applied to adjust the major risk factors, the selection bias could not be eliminated, and the results may differ in a larger cohort. Early adulthood obesity may have little effect on the HCC survival results, as the development of HCC requires a long time. The body weight in the pretreatment of HCC is more likely to influence the survival outcomes, as it reflects the real-time metabolomics. Concerns have been raised that relying on the BMI at the time of HCC diagnosis is subject to overestimation owing to the presence of ascites among many HCC patients. In this study, patients with moderate and severe ascites were excluded, as this could influence the BMI measurement. We also applied adjustments for the major risk factors of HCC, which were highlighted as potential limitations by authors of other studies. We excluded patients who still consumed alcohol and smoked cigarettes after the HCC diagnosis. In this study, only patients with one lesion were included, as the tumor number itself has been identified as a prognostic factor related to OS [Citation43]. Other factors that may influence OS were calculated by the Charlson Comorbidity Index. We constructed four models to test the relationship between the BMI and survival outcomes, and the results were stable. It was found that a BMI between 21.5 and 23.1 kg/m2 was a favorable condition for patients following curative ablation.
Certain studies have demonstrated similar results, whereby underweight adversely affects long-term outcomes in HCC [Citation17,Citation18]. Cirrhosis could lead to asthenia, anorexia, underweight, and even malnourishment [Citation5,Citation44]. Several studies have reported that a low BMI is correlated with low albumin levels, lymphocyte count, and sarcopenia, which are clinical manifestations of malnutrition and poor immune function [Citation45–47]. All of these findings could explain why underweight patients may have a poor postoperative prognosis.
This study exhibits several limitations. Firstly, this was a retrospective study in one center, involving only patients from China, and few Asians have a BMI above 30 kg/m2. Therefore, our results may underestimate the role of obesity in HCC survival. Secondly, we only analyzed the BMI recorded at patients’ admission and did not analyze weight changes during the follow-up following ablation. Continuous BMI monitoring may provide more valuable information. Thirdly, we only included HCC patients with a solitary tumor and effective liver functioning, so the conclusions could be incorrect for patients with multiple HCC lesions. Fourthly, the influences of patient histories of alcohol consumption and cigarette smoking were not considered. We included patients without alcohol consumption and cigarette smoking following the HCC diagnosis only.
In conclusion, this study provides robust evidence to support the association between the BMI at the initial HCC diagnosis and the survival outcomes. Furthermore, the beneficial BMI level for solitary HCC patients following MWA to achieve favorable survival outcomes has been proposed for the first time. Patients could be guided to achieve the target BMI before treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642.
- Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112.
- Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451.
- Chen Y, Wang X, Wang J, et al. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–2145.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Assi N, Gunter MJ, Thomas DC, et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am J Clin Nutr. 2018;108:117–126.
- Zhu GQ, Sun M, Liao WT, et al. Comparative efficacy and safety between ablative therapies or surgery for small hepatocellular carcinoma: a network meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:935–945.
- Zhao J, Zhao Y, Wang H, et al. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr J. 2011;10:49.
- Yang B, Petrick JL, Kelly SP, et al. Adiposity across the adult life course and incidence of primary liver cancer: the NIH-AARP cohort. Int J Cancer. 2017;141:271–278.
- Kim K, Choi S, Park SM. Association of high body mass index and hepatocellular carcinoma in patients with chronic hepatitis B virus infection: a Korean population-based cohort study. JAMA Oncol. 2018;4:737–739.
- Hagstrom H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut. 2018;67:1536–1542.
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638.
- Wu SE, Charles HW, Park JS, et al. Obesity conveys poor outcome in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Diagn Interv Imaging. 2017;98:37–42.
- Liu X, Xu J. Body mass index and waistline are predictors of survival for hepatocellular carcinoma after hepatectomy. Med Sci Monit. 2015;21:2203–2209.
- Mathur A, Franco ES, Leone JP, et al. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB (Oxford. 2013;15:504–510.
- Li Q, Xing H, Liu D, et al. Negative impact of low body mass index on liver cirrhosis patients with hepatocellular carcinoma. World J Surg Onc. 2015;13:294.
- Okamura Y, Maeda A, Matsunaga K, et al. Negative impact of low body mass index on surgical outcomes after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:449–457.
- Bruix J, Sherman M; Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- World Health Organization. Obesity and overweight fact sheet. Geneva (Switzerland): World Health Organization; 2018 [accessed 2019 Mar 1]. Available from: who.int/mediacentre/factsheets/fs311/en/
- Ahmed M, Solbiati L, Brace CL, et al.; For the International Working Group on Image-guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, and the Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–S390.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19:5430–5438.
- Birks S, Peeters A, Backholer K, et al. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13:868–891.
- Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–117.
- Yu JJ, Shen F, Chen TH, et al. Multicentre study of the prognostic impact of preoperative bodyweight on long-term prognosis of hepatocellular carcinoma. Br J Surg. 2019;106:276–285.
- Wood PA. Connecting the dots: obesity, fatty acids and cancer. Lab Invest. 2009;89:1192–1194.
- Xu L, Han C, Lim K, et al. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006;66:11859–11868.
- Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208.
- Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969.
- Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014;2:3.
- Wu J, Zhu AX. Targeting insulin-like growth factor axis in hepatocellular carcinoma. J Hematol Oncol. 2011;4:30.
- Siegel AB, Wang S, Jacobson JS, et al. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2010;28:1063–1069.
- Arsenii N, Piardi T, Diebold MD, et al. Impact of visceral obesity on microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2016;34:271–278.
- Yki-Jarvinen H. Nutritional modulation of nonalcoholic fatty liver disease and insulin resistance: human data. Curr Opin Clin Nutr Metab Care. 2010;13:709–714.
- Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185.
- Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
- Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20:12945–12955.
- Valenti LV, Baselli GA. Genetics of nonalcoholic fatty liver disease: a 2018 update. Curr Pharm Des. 2018;24:4566–4573.
- Hassan MM, Abdel-Wahab R, Kaseb A, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149:119–129.
- Nishikawa H, Osaki Y, Takeda H, et al. Effect of body mass index on survival after curative therapy for non-B non-C hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:173–181.
- Itoh S, Ikeda Y, Kawanaka H, et al. The effect of overweight status on the short-term and 20-y outcomes after hepatic resection in patients with hepatocellular carcinoma. J Surg Res. 2012;178:640–645.
- Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61:1100–1101.
- Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340–357.
- Ri M, Aikou S, Seto Y. Obesity as a surgical risk factor. Ann Gastroenterol Surg. 2018;2:13–21.
- Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530.
- Ji F, Liang Y, Fu S, et al. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford). 2017;19:695–705.