Abstract
Objective
Colorectal liver metastasis is a critical cause of mortality. However, the safety and long-term prognosis of simultaneous colorectal tumor resection along with hepatic lesion ablation are debated. The current analysis was conducted to further clarify the controversy.
Methods
In this retrospective study, we collected data of 68 patients who underwent ablation or resection for liver lesions combined with simultaneous laparoscopic primary colorectal tumor resection between September 2011 and October 2016 at the Third Affiliated Hospital of Sun Yat-sen University. Perioperative outcomes and long-term follow-up data were compared between patients in the resection and ablation groups.
Results
Both groups had similar surgical duration (286.70 ± 78.33 vs. 313.67 ± 80.90 min), conversion rate (2 vs. 0), total expenses (81.51 ± 20.20 vs. 82.21 ± 27.81 kRMB, p = .914) and morbidities (11 vs. 24, p = .667). However, the postoperative hospital stays (12.82 ± 9.25 vs. 8.40 ± 2.38 d) and transfusion rates (56.52% vs. 8.89%) were significantly lower in the ablation group. The long-term overall survival (p = .714), disease-free survival (p = .680) and intra-hepatic recurrent-free survival (p = .496) were comparable between both groups.
Conclusion
With respect to simultaneous treatment for both primary colorectal cancer and liver metastasis, hepatic lesion ablation was associated with lower blood loss and hospital stay duration than liver resection, without compromising the surgical safety and long-term prognosis.
Introduction
With over 1.8 million new cases and 881,000 deaths in 2018, colorectal cancer (CRC) ranks fourth in incidence and second in mortality of all types of malignancies globally [Citation1]. Colorectal liver metastases (CRLM) is a critical cause of mortality [Citation2], with an incidence of 15–25% at the initial diagnosis [Citation3].
Surgical resection remains the gold standard for treating CRLM and can substantially prolong patient survival or even cure the disease in some cases [Citation4]. To minimize the surgical risk, traditional guidelines recommended delayed CRLM resection over simultaneous surgery [Citation5]. However, recent studies have proved similar safety and comparable long-term prognosis of simultaneous resection [Citation6,Citation7]. With the development of modern facilities and minimally invasive principles, thermal ablation has become an alternative for CRLM [Citation8–11].
However, the safety and feasibility of synchronous CRLM ablation and primary tumor resection is still unclear. Hence, we conducted a retrospective analysis of the perioperative safety and long-term prognosis of patients who underwent simultaneous treatment of primary CRC and CRLM, focusing on the role of ablation compared with surgical resection for CRLM.
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Third Affiliated Hospital of Sun Yat-sen University. A total of 68 patients (23 in the resection group and 45 in the ablation group) who underwent ablation or surgical resection of CRLM combined with primary CRC resection between September 2011 and October 2016 were enrolled in the current study and the database of The Third Affiliated Hospital of Sun Yat-Sen University was used.
Criteria
Whether patients underwent ablation or resection was decided by the surgeons. Distribution of both approaches was stable during the entire study period. The inclusion criteria of the study were as follows: (1) primary CRC and CRLM were treated simultaneously during one surgery; (2) patients with ≤3 CRLM tumors; (3) maximal tumor diameter ≦5 cm; and (4) no extrahepatic metastasis detected before surgery. The exclusion criteria of the study were as follows: (1) emergency or laparotomy surgery; (2) metachronous CRLMs; (3) recurrent CRLM after primary treatment; (4) palliative treatment; and (5) other kinds of malignancies or severe comorbidities. Preoperative images were retrospectively viewed to confirm the presence of technically resectable CRLMs that were feasible for complete resection to maintain at least 30% of the future liver remnant.
Outcomes
The following demographic, clinical and pathological information were extracted from the database: age, gender, body mass index (BMI), comorbidities, American Society of Anesthesiologists (ASA) score, pre-operative Child–Pugh stage, operative duration, surgical site, neoadjuvant chemotherapy history, T stage and preoperative serum carcinoembryonic antigen (CEA) levels. The outcomes of interest were the length of hospital stay (LOS), expense and rates of postoperative complications, which included anastomotic leakage, hemorrhage, surgical site infection (SSIs), postoperative ileus, respiratory/urinary infection, deep vein thrombosis (DVT) and others. The long-term prognosis data were collected via the follow-up database. The overall survival (OS), disease-free survival (DFS) and intra-hepatic recurrent-free survival (RFS) rates were evaluated.
Procedures
The liver was examined by laparoscopic camera, followed by intraoperative ultrasonography to identify CRLM. The extent of hepatic resection was determined by the quantity, diameter and locations of the tumors, and lobectomy, segmentectomy, or limited resection was adopted accordingly. Liver transection was performed with a cavitron ultrasonic surgical aspirator (CUSA system, Soring, Quickborn, Germany) and energy devices such as Thunderbeat® (Olympus, Tokyo, Japan) and Harmonic® (Ethicon US, Cincinnati, OH, USA). Small vessels were ligated with CUSA electrocautery or laparoscopic clip devices. The main glissonian pedicle and hepatic vein were ligated with an endo-GIA stapler. The preserved margin during parenchymal dissection was ≥ 5 mm.
Ablation procedure
Thermal ablation was performed intra-operatively after CRC resection. Ablation was performed using a cool-tip RFA instrument (Valleylab, Mansfield, MA, USA) with a maximal output power of 200 W and single-pole, internally cooled ablation needles with 3-cm tips. The MyLab Twice and MyLab ClassC ultrasound machine (Esoate, Genoa, Italy) with CnTI contrast-specific imaging was used for the ablation procedure. Convex probes CA541 and CA431 were used for ultrasound scanning and guidance.
All ablations were performed by interventional doctors with at least 5 years of experience in liver tumor ablation. The cool-tip radiofrequency electrode was punctured percutaneously or directly into the center of each tumor. A boundary of >10 mm around the tumor was covered to achieve a safety margin. According to the size of the tumor, multiple overlapping ablations were performed as needed. The artificial ascites technique was used when tumors were located near the diaphragmatic muscle, gallbladder, heart, or other organs. The electrode path was cauterized while retracting the electrode to minimize the risk of post-ablation bleeding and tumor seeding.
Statistical analysis
Frequencies are presented for categorical variables, and mean ± standard deviation are given for continuous variables. Pearson’s χ2 or Fisher’s exact tests were used to analyze categorical variables. Student’s T-tests were used for analyzing normally distributed data; otherwise, Mann–Whitney U tests were used for continuous variables. Survival rates were estimated using the Kaplan–Meier method and median survival was compared using the log-rank test. All data analyses were performed using Statistical Package for the Social Sciences software, version 22 (IBM Corp, Armonk, NY, USA).
Results
A total of 68 patients (23 in the resection group and 45 in the ablation group) who underwent ablation or surgical resection of CRLM combined with primary CRC resection between 2011 and 2016 were enrolled in the current study, as shown in . The average age was 58 ± 12.12 and 61.62 ± 12.22 in the resection and ablation group, respectively. Most of the patients were male and were 69.57% and 68.89% in resection and ablation group. The BMI was similar in resection and ablation groups (21.60 ± 3.32 and 22.83 ± 3.26). However, there were more patients with rectal cancer in the ablation group than in the resection group (64.44% vs. 39.13%). In the resection group, two patients with rectal cancer underwent neoadjuvant chemotherapy (three cycles of CapeOX and mFOLFOX6 individually). In the ablation group, four patients with colon cancer and eight with rectal cancer underwent neoadjuvant chemotherapy and either CapeOX (seven patients) or mFOLFOX6 (five patients) was prescribed for three or four cycles. None of the enrolled patients underwent neoadjuvant or adjuvant radiation therapy.
Significantly higher CRLM lesions (p = .042) were observed and located in multiple liver lobes (p = .041) in the ablation group. The ASA and Child–Pugh grades, smoking status, serum CEA, hemoglobin and albumin levels, pathological stages and comorbidities were all comparable between both groups ().
Table 1. Characteristics of patients in resection and ablation groups.
In the perioperative safety analysis, patients from both groups had similar surgical times (286.70 ± 78.33 vs. 313.67 ± 80.90 min) and conversion rates (2 vs. 0). One conversion was attributed to intraoperative hepatic bleeding and the other was associated with urethral injury. No R1 hepatic resection was performed according to the pathological result. Postoperative hospital stays (12.82 ± 9.25 vs. 8.40 ± 2.38 d) and transfusion rates (56.52% vs. 8.89%) were significantly lower in the ablation group than in the resection group. The total expenses (81.51 ± 20.20 vs. 82.21 ± 27.81 kRMB) were comparable between both groups. Although various complications occurred postoperatively, the surgical safety between both approaches was still similar. shows all the outcomes.
Table 2. Perioperative outcomes for resection and ablation groups.
All patients underwent postoperative chemotherapy according to the oncologists’ recommendations. Routine CapeOX or mFOLFOX6 regimens were administered initially. If recurrence was detected, second-line systemic (FOLFIRI) chemotherapy was prescribed with bevacizumab if necessary. In the resection group, 16 patients were diagnosed with tumor recurrence, and among these patients, two underwent ablation and one underwent additional resection. In the ablation group, 30 patients were diagnosed with tumor recurrence, and among these patients, 4 underwent additional ablation, 1 underwent transcatheter arterial chemoembolization (TACE) and 1 underwent resection. All the therapy schedules were decided by a multi-disciplinary team consisting of surgeons, oncologists, interventionists, radiologists and other medical professionals.
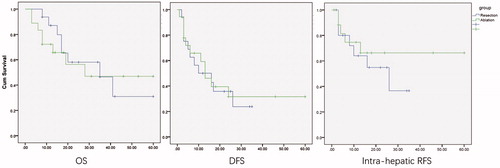
Patients were followed up after surgery and until their last visit in July 2018, and the maximal follow-up period was 92 months. Based on the Kaplan–Meier analyses, the median OS was 35 months in the resection group and 39 months in the ablation group (p = .714, ). Meanwhile, the median DFS was 10 months and 13 months in the resection and ablation groups, respectively (p = .680, ). Furthermore, the intra-hepatic RFS was estimated to further evaluate the radical intensity of both approaches, and the median survival was 26 and 37 months in the resection and ablation groups group (p = .496, ).
Figure 2. Overall survival, disease-free survival and intra-hepatic recurrent-free survival between ablation and resection groups.
Subgroup analysis
The number of CRLM lesions was associated with the prognosis and may alter the results in the baseline characteristics; therefore, solitary metastatic tumors were evaluated additionally.
In total, 34 patients were enrolled in the subgroup analysis with 16 in the resection subgroup and 18 in the ablation subgroup. The baseline features were comparable between both subgroups (). Consequently, patients who underwent ablation were associated with fewer transfusions (8 vs. 2, p = .023) and shorter postoperative stays (10.93 ± 4.26 vs. 7.39 ± 1.09 d, p = .005) than those who underwent resection, while overall and specific morbidity remained similar in both subgroups ().
Table 3. Characteristics of patients with solitary CRLM in resection and ablation subgroups.
Table 4. Perioperative outcomes for patients with solitary CRLM in resection and ablation subgroups.
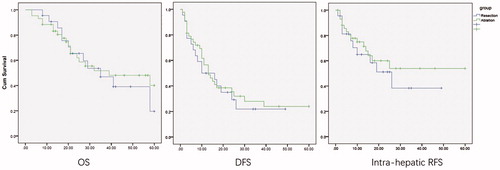
One patient in each group was lost to follow-up; therefore, only 15 and 17 patients were enrolled in resection and ablation groups, respectively. The OS (p = .817), DFS (p = .798) and intra-hepatic RFS (p = .436) were still comparable according to the Kaplan–Meier analyses ().
Discussion
CRLM is the main cause of cancer-related mortality of CRC, and 15–25% of patients are diagnosed with CRLM at the initial presentation globally [Citation2,Citation3]. Local hepatic treatment combined with systemic therapy remains the first option in the treatment of CRLM and could improve prognosis [Citation4]. The commonest curative approach for synchronous CRC and CRLM is a staged procedure with primary tumor resection first, followed by liver surgery at a later stage [Citation12]. Liver-first staged procedure, which aims to prevent the progression of CRLM during the interval between subsequent surgeries, was introduced recently [Citation13,Citation14]. However, consecutive procedures may aggravate the overall surgical risk due to adhesions, anatomical changes, or wound extension, and increase patients’ financial burden by rehospitalization [Citation15]. Furthermore, CRLM progression has been observed after removal of the primary tumor in some studies [Citation16,Citation17], indicating that undergoing an upfront primary colorectal resection may be an adverse prognostic variable [Citation18]. Thus, simultaneous surgery, in which primary CRC and CRLM are treated during the same operation, was established as another treatment option.
Simultaneous resection of both primary and metastatic tumors was found to be feasible with an acceptable complication rate compared to the colorectal-first approach [Citation6,Citation7,Citation19,Citation20]. However, the surgical approach was only suitable for patients with the good physical condition and limited CRLM burden, such as lesion size and amount [Citation21–23]. Standing at the era of minimally invasive surgery (MIS) and enhanced recovery after surgery (ERAS), a novel approach that could balance MIS/ERAS with equivalent surgical safety is greatly needed.
As an alternative to liver resection, ablation is associated with lower morbidity and mortality, and has comparable survival rates for metachronous CRLM patients in recent studies [Citation24,Citation25]. Improvements in ablation have facilitated the ablation of a spherical zone with a diameter of > 5 cm, which has enhanced its applicability [Citation26,Citation27]. Ablation seems to be the rational choice for MIS and ERAS.
Previous studies have suggested that tumor diameter and number are the most important factors that influence the effects of ablation. Primary lymph node status, timing of metastasis and CEA levels can also affect the survival and recurrence rates of patients [Citation28–30]. Thus, we specially defined the CRLM parameter in the inclusion criteria. Baseline characteristics were comparable between both groups with respect to the majority covariant, except the CRLM lesion number and hepatic lobe distribution. Patients in the ablation group had a higher liver-lesion burden; nevertheless, subsequent subgroup analysis could help eliminate such confounding.
Perioperative outcomes manifested with ablation were associated with lower LOS (12.82 ± 9.25 vs. 8.40 ± 2.38 d) and transfusion (56.52% vs. 8.89%) than those of traditional surgical approaches. Furthermore, these superior characteristics did not destabilize postoperative safety such as anastomotic fistula, surgical site infection, hepatic abscess, hemorrhage, ileus, infection and readmission within 30 days. Ablation was performed with a small puncture incision, without additional surgical wound and drainage after surgery, which ameliorated the recovery of patients in the ablation group. Local disease progression was reported as 9–48% in the ablation group compared with 2–9% in resection CRLM in previous studies [Citation31–33]. Unlike surgical resection, the pathology of the lesion margin is unevaluable in the ablation operation. Hence, local recurrence is the main dispute on ablation for CRLM. In the present study, long-term OS, DFS and intrahepatic RFS were all comparable between both groups. This finding may have several explanations. First, the safe ablative margin was defined as more than 1 cm in this study, and moreover, detailed pre- and intra-operative imaging evaluations helped avoid ‘undetectable lesions’ for recurrence. Second, the patients’ CRLM load was strictly defined in the current study to avoid tumor burden interference. Third, improvements in ablation have facilitated completed response of ablation, even for lesions with a diameter of >5 cm. Fourth, 14 patients underwent neoadjuvant chemotherapy before surgery, which helped eliminate undetected CRLM lesions and resulted in better prognosis; moreover, a shorter LOS in the ablation group suggested an early recovery and earlier initiation of systematic therapy, which may improve the DFS and OS directly.
To further erase the baseline bias rooted in both groups, patients with only one CRLM were analyzed specifically in both groups. The demographic features of the patients turned out to be similar. The results remained stable as ablation was associated with analogous surgical safety. Furthermore, the advantage of ablation as a lower transfusion rate and shorter postoperative hospital stays remained significant in subgroup analysis. Long-term OS, DFS and intra-hepatic RFS were also non-significantly different, suggesting a comparable prognosis between ablation and surgical resection for solitary CRLM and proved the stability of the overall results.
All these results demonstrated the feasibility of thermal ablation for CRLM. Patients who underwent simultaneous treatment of CRLM ablation and CRC resection had fewer transfusions and shorter hospital stays, without compromising on surgical safety and long-term prognosis. With good quality control for safety margins and evaluation, simultaneous ablation may be the optimal choice for CRLM.
There are some limitations to the present study. First, as a retrospective trial, patients could not be randomized, although the subsequent matching and subgroup analysis may help reduce the bias and help evaluate the results. Second, the sample size was relatively small because only those patients with resectable CRLM and who underwent synchronous treatment were enrolled. Third, the difference in postoperative adjuvant therapy, or a patient’s refusal to receive chemotherapy, resulted in a selection bias. Thus, we look forward to future well-designed randomized control trials to validate our results.
Conclusion
Ablation was associated with less blood loss and shorter hospital stay than surgical resection, without compromising the surgical safety and long-term prognosis with respect to the simultaneous treatment of both CRLM and primary CRC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer Oxf Engl 1990. 2013;49:1374–1403.
- Hayashi M, Inoue Y, Komeda K, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27.
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
- Adams RB, Aloia TA, Loyer E, et al.; Americas Hepato-Pancreato-Biliary Association, Society of Surgical Oncology, Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91–103.
- Weber JC, Bachellier P, Oussoultzoglou E, et al. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg. 2003;90:956–962.
- Vallance AE, van der Meulen J, Kuryba A, et al. The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: a population-based study of current practice and survival. Colorectal Dis. 2018;20:486–495.
- Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg. 2013;148:597–601.
- Hof J, Wertenbroek M, Peeters P, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103:1055–1062.
- Eltawil KM, Boame N, Mimeault R, et al. Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol. 2014;110:734–738.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol off J Eur Soc Med Oncol. 2016;27:1386–1422.
- Mayo SC, Pulitano C, Marques H, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013; 216:707–716.
- Mentha G, Roth AD, Terraz S, et al. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435.
- Jegatheeswaran S, Mason JM, Hancock HC, et al. The liver-first approach to the management of colorectal cancer with synchronous hepatic metastases: a systematic review. JAMA Surg. 2013;148:385–391.
- Peeters C, de Waal RMW, Wobbes T, et al. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer. 2006; 119:1249–1253.
- Scheer MGW, Stollman TH, Vogel WV, et al. Increased metabolic activity of indolent liver metastases after resection of a primary colorectal tumor. J Nucl Med. 2008;49:887–891.
- van der Wal GE, Gouw ASH, Kamps J, et al. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg. 2012; 255:86–94.
- Slesser AA, Khan F, Chau I, et al. The effect of a primary tumour resection on the progression of synchronous colorectal liver metastases: an exploratory study. Eur J Surg Oncol. 2015;41:484–492.
- Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491.
- Muangkaew P, Cho JY, Han H-S, et al. Outcomes of simultaneous major liver resection and colorectal surgery for colorectal liver metastases. J Gastrointest Surg. 2016;20:554–563.
- Nanji S, Mackillop WJ, Wei X, et al. Simultaneous resection of primary colorectal cancer and synchronous liver metastases: a population-based study. Can J Surg. 2017;60:122–128.
- Tzeng CWD, Cooper AB, Vauthey JN, et al. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford). 2014; 16:459–468.
- Sato M, Tateishi R, Yasunaga H, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol. 2012;47:1125–1133.
- Oshowo A, Gillams AR, Lees WR, et al. Radiofrequency ablation extends the scope of surgery in colorectal liver metastases. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2003;29:244–247.
- Evrard S, Becouarn Y, Fonck M, et al. Surgical treatment of liver metastases by radiofrequency ablation, resection, or in combination. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2004;30:399–406.
- Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol. 2011;18:1947–1954.
- Nielsen K, van Tilborg A, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37:1340–1347.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968.
- Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948–956.
- Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30:567–574.
- Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. Discussion 825–827
- de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.