Abstract
Background
Image-guided radiofrequency ablation (RFA) of large solid benign thyroid nodules (BTNs) usually require a high amount of energy. Injection of ethanol into a benign thyroid nodule before RFA can lower the procedural time and patient discomfort.
Objective
To investigate the efficacy and safety of ethanol ablation (EA) combined with RFA in the treatment of solid BTNs (>10 ml) and to compare this modified method with RFA treatment alone.
Methods
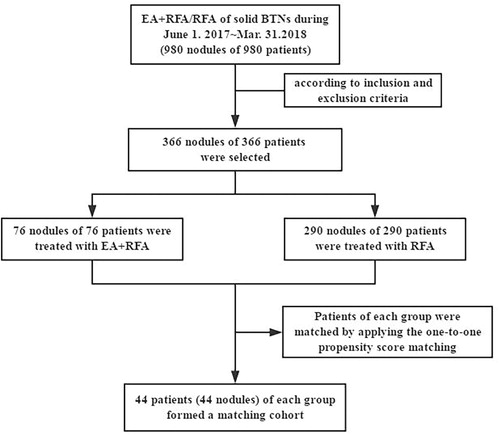
A total of 366 nodules in 366 patients were treated from June 2017 to Mar. 2018, 76 (M:F = 32:44, age 46 ± 14 years) were treated with EA + RFA and 290 (M:F = 99:191, age 49 ± 14 years) were treated with RFA. 44 patients (44 nodules) of each group formed a matched cohort after adjustment with propensity score matching. The average time, energy and power of the RFA procedure were retrospectively compared between the two groups. The postoperative nodule volume reduction ratio (VRR), compressive symptoms, cosmetic concerns, comprehensive satisfaction score, thyroid function and complications were retrospectively compared within and between the two groups after 6 months after treatment.
Results
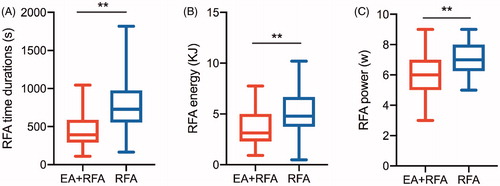
No significant differences were observed in the baseline characteristics between groups after propensity score matching adjustment. The mean RFA time (454.7 s (interquartile range (IQR), 290.8–589.0 s vs. 796.0 s (IQR, 554.0–976.30 s), p < .001), energy (3.69 ± 1.99 kJ vs. 5.10 ± 2.15 kJ, p = .009) and power (6.17 ± 1.38 W vs. 7.21 ± 1.29 W, p < .001) in the EA + RFA group were significantly lower than those in the RFA group. Mean nodule reduction at 6 months in the EA + RFA group and in the RFA group was 69.81 ± 11.48% vs. 67.43 ± 12.13% (10-30 ml, p = .454) and 62.75 ± 11.41% vs. 59.82 ± 10.53% (>30 ml, p = .456), respectively. The Medium nodules shrunk more than the large nodules (all p < .001), and the pressure symptoms and cosmetic signs significantly improved in the large nodules in both groups (all p < .05). Patients in the EA + RFA group had the highest satisfaction.
Conclusions
EA combined with RFA shortened the ablation time of RFA, reduced the total energy and power required and improved patient satisfaction. This modified RFA approach may be considered a low-risk and high-efficacy alternative to treat solid BTNs greater than 10 ml in size.
Introduction
Thyroid nodules (TNs) are very common, with 20–76% [Citation1] of nodules being detected by ultrasonography (US). Most TNs are benign, asymptomatic and only require active surveillance [Citation2]. However, approximately 20% of benign thyroid nodules (BTNs) display progressive growth, requiring intervention for symptomatic growth, cosmetic problems, or a possibility of malignant transformation [Citation3]. Although thyroid surgery is widely practiced and is typically safe, the procedure carries a risk of complications, such as large incision, slow postoperative recovery and esthetic problems, and may not be appropriate for high-risk surgical patients or for those who refuse surgical management [Citation4]. Therefore, minimally invasive nonsurgical techniques have been proposed to treat BTNs [Citation5].
Image-guided thermal ablations (IGTAs) are commonly performed in clinical practice and have been proposed to treat BTNs [Citation6,Citation7]. IGTAs are not only indicated in patients at high surgical risk but can also be proposed as first-choice treatment options [Citation2,Citation8]. Ultrasound-guided (US-g) thermal ablation with radiofrequency energy is a highly recommended treatment method in multiple guidelines and by expert consensus for patients with benign solid and predominantly solid TNs who complain of symptomatic or cosmetic problems [Citation9–11]. Many studies have demonstrated the efficacy and safety of US-g radiofrequency ablation (RFA) (with follow-up periods of six months to four years), which was performed by an experienced radiologist [Citation12–16].
However, we encountered some benign nodules that were difficult to treat with RFA. These nodules were more than 4 cm and were solid or mainly solid with abundant blood flow. In these nodules, the heat sink effect that arises from blood vessels may decrease RFA efficacy. The carbonization of thyroid tissues may reduce absorption, and carbonized tissues may adhere to RFA needles, which affects ablation. Thus, solid large BTNs may be incompletely ablated, have excessive residue and easily recur, all of which are problems to solve.
Increasing the ablation energy and ablation power could be a solution. However, additional energy and power may increase the probability of complications, such as permanent hoarseness or Horner’s syndrome, due to thermal damage of the recurrent laryngeal nerve or cervical sympathetic ganglion [Citation11]. These increases in energy may also cause nodular hemorrhage and nodular rupture, which are serious complications [Citation11]. In addition, raising the ablation energy and power can also cause discomfort to patients (neck extension for a long time and neck pain due to thermal radiation). In addition, instead of using increased power and time with RFA, other strategies can be used, such as laser ablation (LA) with multiple needle insertions or microwave ablation (MWA) [Citation17,Citation18], but RFA was used in our study because it is the standard technique used in our center. Therefore, we urgently need an improved RFA method that is safe and effective and can avoid the above problems.
Recently, a method that features an ethanol injection prior to RFA was introduced for the treatment of hepatocellular carcinomas [Citation19,Citation20]. However, for TNs, ethanol ablation (EA) is usually effective for cystic or predominantly cystic nodules [Citation21]; for partial cystic thyroid nodules, EA combined with RFA has achieved optimal results [Citation11]. For solid nodules, can EA combined with RFA be used safely and effectively? This study was designed to explore this hypothesis.
To minimize the effect of potential confounders or selection bias, patients from each group were matched by applying the one-to-one propensity score matching [Citation22,Citation23]. Our retrospective study aimed to investigate the efficacy and safety of EA combined with RFA in the treatment of solid BTNs (>10 ml) and to compare this modified method with RFA treatment alone.
Materials and methods
Patients
This retrospective study was approved by the Chinese PLA General Hospital Ethics Committee, and patient consent was waived. A total of 980 nodules from 980 patients were treated with image-guided thermal ablation from June 2017 to Mar. 2018. According to the inclusion and exclusion criteria, small nodules (≤10 ml) were mainly excluded, and 366 nodules from 366 patients were selected, including 76 (M:F = 32:44, age 46 ± 14 years) were treated with EA + RFA and 290 (M:F = 99:191, age 49 ± 14 years) were treated with RFA (). In particular, we provided patients with two options before treating solid BTNs in the clinical practice: EA + RFA therapy and routine RFA therapy alone. After we explained the differences between the two options in detail, patients chose the treatment according to their own wishes. During June 2017 to Mar. 2018, a total of 76 patients chose EA + RFA to treat solid BTNs. All patients fulfilled the following criteria: (a) solid TN (≤10% of fluid component); (b) ultrasound revealed a single or dominant nodule that can be clearly detected in a multinodular goiter; (c) nodules >10 ml; (d) benign lesions confirmed by two separate US-guided fine needle aspirations (FNAs) of the TNs (no more than 6 months before treatment); (e) complicated cosmetic and/or symptomatic problems (i.e., local swelling, compressive symptoms and foreign body sensation on swallowing); (f) TNs with a maximum diameter >2 cm that continuously grew over time (the maximum diameter increased by more than 20% per year, and the patients were willing to be treated); and (g) no history of neck radioiodine therapy or thermal ablation. The exclusion criteria were as follows: (a) serious cardiopulmonary disease that made the patient unable to tolerate RFA; (b) severe coagulation disorder; (c) pregnancy; (d) allergy to alcohol; (e) refusal to undergo RFA; and (f) Follow-up was less than 6 months.
To minimize the effect of potential confounders on selection bias, propensity scores were generated using binary logistic regression [Citation17] to estimate the probability that a patient would undergo RFA instead of EA + RFA. Independent variables that entered into the propensity model included sex, age and baseline nodule volume. 44 patients (44 nodules) of each group formed a matched cohort after adjustment with propensity score matching (). The demographic data of the patients and nodules before and after propensity score matching are summarized in and .
Table 1. Baseline characteristics in all study patients according to baseline nodule volume group before propensity score matching.
Table 2. Baseline characteristics in all study patients according to baseline nodule volume group after propensity score matching.
Pre-ablation assessment
The results of the US, FNA, and clinical examination, as well as the laboratory data of all patients, were evaluated prior to EA or RFA. The US and US-guided biopsy were performed by a single experienced radiologist (Y.K. L., who has more than 20 years of thyroid US experience) using the Siemens Acuson Sequoia 512 Ultrasound System with a 6L3 linear array probe. The volume of each nodule was calculated by using the following equation: V = πabc/6 (V is volume; a is the largest diameter; b and c are the two other perpendicular diameters). The TNs were categorized as intermediate nodules (10-20 ml) and large nodules (>20 ml) according to their baseline volume. The blood supply of each BTN was observed by CEUS before RFA by using a Siemens Acuson Sequoia 512 Ultrasound System with a 15L8W linear array transducer. The SonoVue contrast agent (2.4 ml, Bracco Imaging, Milan, Italy) was mixed with 5 ml of normal saline. When recording the patient information, a nodule-related symptom score was self-measured by patients using a 10-cm visual analog scale (0 − 10) [Citation11]. The cosmetic grading score was assessed by a physician (1–4 score; 1, no palpable mass; 2, a palpable mass but no cosmetic problem; 3, a cosmetic problem on swallowing only; 4, an easily detected cosmetic problem) [Citation11]. The laboratory examinations included tests for serum thyroid hormone (free T3, normal range, 2.76–6.30 pmol/l; free T4, normal range, 10.42–24.32 pmol/l;), serum thyrotropin (normal range, 0.35–5.50 mU/l), anti-thyroglobulin antibodies (normal range, 0–60 IU/ml), serum antithyroid peroxidase antibodies (normal range, 0–60 IU/ml), and serum calcitonin (normal range, 0–8.4 pg/ml) and blood coagulation tests (platelet count, prothrombin time, and activated partial thromboplastin time).
Procedure
EA and RFA were performed in the outpatient operating room, and the patient vital signs were monitored. All EA and RFA procedures were performed by an experienced radiologist (Y.K.L.) with more than 20 years of experience in thyroid interventional sonography. The patients were placed in a supine position with mild neck extension. After sterilizing the skin and administering anesthesia with 2% lidocaine at the puncture site and a thyroid anterior capsule, a hydrodissection was created if the distance between the tumor and the primary of cervical structures (including the trachea, carotid artery, internal jugular vein, esophagus, and recurrent laryngeal nerve) was less than 5 mm. The process involved the injection of 20–40 ml of normal saline using a 23-guage needle to form a distance of at least a 1 cm between the tumor and the critical structure to reduce the chance of thermal injury.
In the EA + RFA group, a 25-gauge PTC needle was inserted into the solid components of the target nodule through the isthmic area (known as the transisthmic approach) under US guidance. Minimal amounts of 99.9% ethanol (Chang-Hai Hospital, Shanghai, China, G510001) were injected at different sites (0.2 ml at each site) until most of the thyroid appeared with echogenicity (called intranodular echo-staining). After the injections, the needle was rapidly withdrawn, and we waited for 1–2 min until the hyperechoic signal was dispersed in most areas, and RFA was then performed. The RFA group was the control group and only underwent RFA.
RFA was performed with an Olympus Surgical Technologies (CelonLabPOWER, Europe, Germany, Hamburg) system and an 18-gauge bipolar radiofrequency (RF) applicator with a 15-mm active tip (CelonProSurge, micro 100-T15; Olympus Surgical Technologies, Europe, Germany, Hamburg) (). Under US guidance, we performed RFA using the transisthmic approach or direct nodule puncture approach [Citation6] and the ‘moving shot’ technique, as previously described by Baek and colleagues [Citation12] (). The RFA procedure was terminated when all of the conceptual units of the target nodule were converted to transient hyperechoic regions. However, notably, very few portions of the nodule near the important structures were retained to avoid causing thermal damage. The RFA power was set at 3 W. If the electrode tip did not achieve a high echo region within 5–10 s, the power was increased to 5–9 W. The exposure time was calculated mainly as the sum of the time of a transient hyperechoic area in each of the different zones undergoing ablation.
Figure 2. RFA equipment. (A) 18-gauge (diameter 1.3 mm) bipolar RFA needle (with a 15 mm active tip; (B) Olympus Surgical Technologies. RFA: radiofrequency ablation.
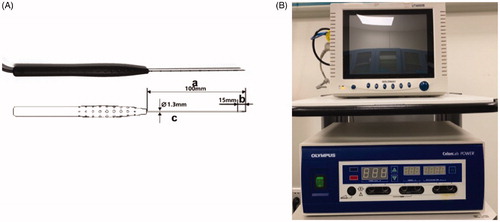
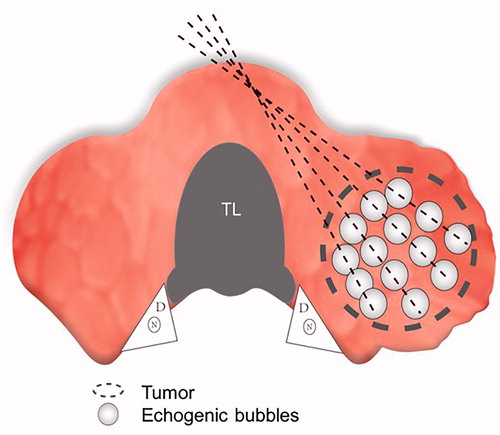
Figure 3. The ‘moving shot’ technique. The thyroid nodule (dashed oval) was divided into multiple small conceptual ablation units (small white ovals); the electrode (dashed line) was inserted into the deepest and most medial part of the nodule and was subsequently moved radially. Note the tracheal lumen (TL), dangerous triangle (D), and recurrent laryngeal nerve (N).
Patients were intensively monitored during the RFA process. If the patient did not tolerate pain during ablation, the power was reduced or turned off for several seconds, or lidocaine was injected around the thyroid capsule. After RFA, the duration, total energy delivered, and power of the RFA was recorded. A contrast-enhanced ultrasound (CEUS) examination (Siemens Acuson Sequoia 512 Ultrasound System with a 15L8W linear array transducer) was used to observe the blood supply of the ablation area to ensure complete ablation. If there were residues, supplementary ablation was performed. Complications both during and after the procedure were evaluated according to clinical signs and symptoms. The patients were observed for approximately 2 h to check for possible immediate complications [Citation24]. Each nodule underwent a single treatment session and was followed up over time.
Follow-ups
After ablation, the patients were followed up at 1, 3, and 6 months and underwent periodic US examinations (radiologists experienced in ultrasound who were blinded to the study) and thyroid function tests. The CEUS examinations (Siemens Acuson Sequoia 512 Ultrasound System with a 15L8W linear array probe; Philips iU22 Ultrasound System with an L12-5 linear array probe; Mindray M9 Ultrasound System with an L12-4 linear array probe) were used during follow-up to evaluate the ablation volume and recurrence at each time point. The volume reduction ratio (VRR) was calculated as follows: VRR=([initial volume (ml) − final volume (ml)] x 100%)/initial volume (ml) [Citation13]. Regrowth and/or recurrence were defined as an increase in nodule volume >50% compared with that from the previous US examination [Citation13]. The symptom and cosmetic scores and complications of the two different treatment groups were evaluated at each follow-up. The treatment success rate was evaluated at 6 months post-ablation (VRR larger than 50%). We adopted the comprehensive satisfaction score (CSS) assessment questionnaire used in our center in recent years, which was designed based on patients’ feedback before and after ablation, including the following components () (1): radiofrequency ablation time was too long (2); significant pain during RFA (3); no compression symptom improvement (4); no cosmetic improvement; and (5) unwilling to choose EA + RFA/RFA again. An answer of ‘yes’ was 1 point, and an answer of ‘no’ was 0 points. The lower the score, the more satisfied the patients were with this microsurgical method.
Table 3. Comprehensive satisfaction score (CSS) evaluation questionnaire.
Statistical analysis
The results were analyzed by SPSS, version 22 (SPSS, Inc. Chicago, IL, USA). One-to-one matching between the treatment groups was accomplished by using the nearest-neighbor matching method [Citation22,Citation23]. Briefly, the distribution of propensity scores was evaluated by treatment group to make sure there was sufficient overlap among the groups to ensure comparability. Continuous variables were reported as the means ± SD with the range. Changes in VRR and thyroid function between every two follow-up periods and ablation energy, ablation power, symptom score (SS), cosmetic score (CS) and CSS between the EA + RFA and RFA groups were compared by independent two-sample t-tests. The ablation time was compared between the two groups with the Wilcoxon signed rank test. Chi-square tests were used to compare dichotomous variables (complications and therapeutic success rate) between the two groups. A p value less than .05 indicated a significant difference. Through a post hoc power analysis, a power of 85% was obtained in the test at a significance level of .05.
Results
Baseline characteristics before propensity score matching
The characteristics of the EA + RFA vs. RFA groups in all study patients according to baseline nodule volume before propensity score matching are summarized in . A total of 366 nodules from 366 patients received US-guided thermal ablation therapy. The EA + RFA group comprised 76 patients (mean age ± SD = 46 ± 14 years), and the RFA group comprised 290 patients (mean age ± SD = 49 ± 14 years). The baseline characteristics, such as sex and age, were similar between the two groups (p > .05). The mean total initial volume of nodules treated with EA + RFA did not show statistically significant differences compared with nodules treated with RFA (33.00 ± 18.72 vs. 35.81 ± 19.33 ml, respectively; p = .463). However, the mean baseline nodule volume of patients with nodules greater than 30 ml treated with RFA was significantly greater than that of patients treated with EA + RFA (51.38 ± 16.28 vs. 46.05 ± 15.11 ml, respectively; p = .047).
Baseline characteristics after one-to-one propensity score matching
We excluded 279 patients (EA + EFA, n = 33; RFA, n = 246) with a non-overlapping propensity score distribution (). Thus, the adjusted comparisons by propensity scores were based on data from 44 patients per treatment group. Confounding factors were well matched between the EA + RFA and RFA groups, especially the baseline volume of nodules in the large nodule group (44.55 ± 14.26 vs. 45.50 ± 14.07 ml, respectively; p = .851). In addition, the SS and CS were not significantly different between the two groups (p > .05). Therefore, after this matching, the resulting two patient groups had similar baseline characteristics.
Treatment characteristics
The treatment characteristics for our study patients are shown in . The mean RFA time was 454.7 s (interquartile range (IQR), 290.8–589.0 s) in the EA + RFA group and 796.0 s (IQR, 554.0–976.30 s) in the RFA group, which revealed that the duration was significantly shorter in the EA + RFA group than in the RFA group (p < .001). The mean RFA energy (EA + RFA: 3.69 ± 1.99 kJ and RFA: 5.10 ± 2.15 kJ, p = .009) and mean RFA power (EA + RFA: 6.17 ± 1.38 W and RFA: 7.21 ± 1.29 W, p < .001) were significantly lower in the EA + RFA group than in the RFA group.
VRR evaluation and therapeutic success rate
The VRRs at each follow-up after RFA are shown in and Citation6. In the follow-up periods, a significant amount of shrinkage was observed in the BTNs, and the VRRs showed similar outcomes between the two treatment groups. The VRR at 1, 3, and 6 months was 51.44 ± 9.44% vs. 51.00 ± 13.42% (p = .888), 62.69 ± 10.93% vs. 63.57 ± 11.65 (p = .772), and 69.81 ± 11.48% vs. 67.43 ± 12.13% (p = .454) in medium nodules of the EA + RFA vs. RFA group; and was 44.05 ± 8.53% vs. 41.57 ± 8.36% (p = .413), 53.05 ± 8.36 vs. 52.03 ± 8.39% (p = .733), and 62.75 ± 11.41 vs. 59.82 ± 10.53% (p = .456) in large nodules of the EA + RFA vs. RFA group. In both groups, the VRR of the large nodules was significantly lower than that of the medium nodules (p < .001). Specifically, at 6 months, nodules with volumes of 10 to 30 ml and nodules larger than 30 ml showed VRRs of 69.81 ± 11.48% and 62.75 ± 11.41% in the EA + RFA group (p < .001), respectively, and 67.43 ± 12.13% and 59.82 ± 10.53% in the RFA group (p < .001), respectively. The therapeutic success rate at the 6-month follow-up is shown in . There was no significant difference between the two groups. CEUS showed that during the follow-up period, no nodules regrew.
Figure 5. Changes in volume reduction ratio (VRR) of medium thyroid nodules and large thyroid nodules after RFA treatment at each follow-up. (A) VRR changes in medium nodules; (B) VRR changes in large nodules.
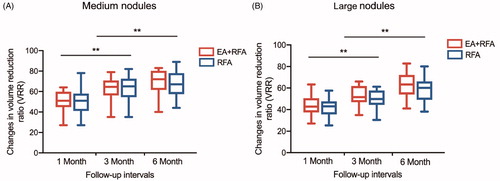
Figure 6. Images and appearance of the neck. A. Images of RFA treatment and follow-up of a thyroid nodule. (a) Conventional ultrasound image showed a solid thyroid nodule. (b) Color Doppler ultrasonography image showed abundant vascularity. (c) CEUS performed before RFA showed homogeneous hyperenhancement in the nodule (white arrows). (d) After a multisite injection of small amounts of ethanol (white arrows indicate echo-staining), RFA was performed using the moving shot technique. (e) CEUS was performed immediately after RFA and showed a complete lack of enhancement in the treated area (white arrows). (f) One month after RFA, the nodule volume (white arrows) was significantly reduced by 49.5%. (g) Three months after RFA, the nodule volume (white arrows) was significantly reduced by 76.3%. (h) Six months after RFA, the nodule volume (white arrows) was significantly reduced by 85.7%. The lesion margin is indicated by ‘+’. RFA, radiofrequency ablation; CEUS, contrast-enhanced ultrasound. B. Comparisons of the appearance of the neck before and after ablation. (a, b) A 27-year-old patient. Before RFA, a quail egg-sized nodule could be seen under the skin of the neck (black arrow), and tenderness could be felt. Three months after RFA, the thyroid nodule markedly shrunk, and no scars were left on the neck (black arrow). (c, d) A 33-year-old patient. A quail egg-sized nodule was visible under the skin of the neck (black arrow) prior to EA combined with RFA. Three months after RFA, the nodule almost disappeared, and no scars were left on the neck (black arrow).
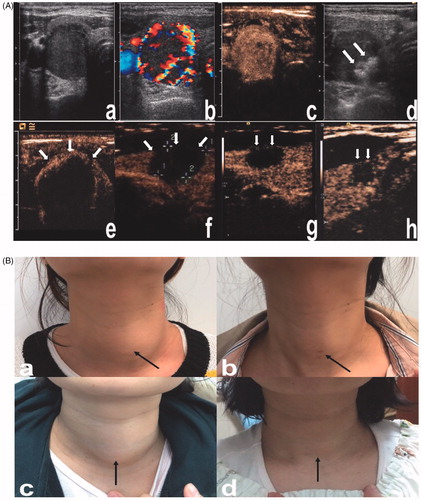
Table 4. The therapeutic success rate of the two treatment groups.
SS, CS and CSS evaluations
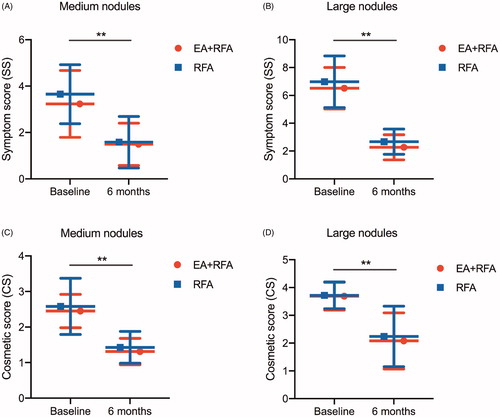
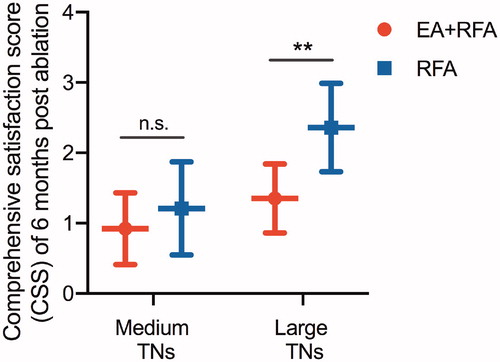
Compared with the baseline values, both the local compressive symptoms and cosmetic concerns improved significantly at 6 months in the medium (SS: EA + EFA, 3.23 ± 1.44 vs. 1.49 ± 0.91, p = .000; RFA, 3.65 ± 1.27 vs. 1.58 ± 1.11, p = .000; CS: EA + EFA, 2.45 ± 0.47 vs. 1.31 ± 0.37, p = .000; RFA, 2.58 ± 0.79 vs. 1.43 ± 0.45, p = .000) and large nodule subgroups (SS: EA + EFA, 6.51 ± 1.49 vs. 2.27 ± 0.90, p = .000; RFA, 6.98 ± 1.86 vs. 2.67 ± 0.91, p = .000; CS: EA + EFA, 3.69 ± 0.50 vs. 2.08 ± 1.01, p = .000; RFA, 3.72 ± 0.48 vs. 2.24 ± 1.09, p = .000) of both treatment groups (particularly for the large nodules, p < .05) (). At the 6-month follow-up after ablation, the CSS of patients with large nodules in the EA + RFA group was superior to that of the corresponding patients in the RFA group (p = .004) (). However, no significant difference was observed for patients with medium nodules between the two treatment groups ().
Hormone evaluation
The serum levels of thyroid hormones were normal in all patients during the follow-up period (1–6 months after the RFA) ().
Table 5. Comparison of thyroid functions between baseline and each follow-up in both treatment groups.
Complications and safety
The postinterventional complications were comparable between the groups (). There were no occurrences of hematoma, hypoparathyroidism or skin burns in the two groups. There were no significant differences in the occurrence of localized edema, voice change, fever and mild to moderate pain between the two groups. None of the patients experienced life-threatening or delayed complications during the follow-up period.
Table 6. Comparisons of the complications between two treatment groups.
Discussion
This retrospective study has demonstrated that injecting a small amount of anhydrous ethanol into benign solid TNs before RFA is effective and safe. Meanwhile, using ethanol before RFA can improve the technique of RFA by significantly shortening the duration and reducing the energy and power required for the procedure. In addition, we also found that a single session of RFA treatment was effective in reducing the nodule volume, particularly in the medium subgroup (small nodules were not involved in this study), and significantly relieving the symptoms of all patients. Moreover, the EA + RFA and RFA therapies showed nearly similar VRR in the follow-up periods and the comprehensive satisfaction of the patients with large nodules in the EA + RFA group was superior to that of the corresponding patients in the RFA group.
Previous studies have introduced stepwise combinations of EA and RFA to manage nodules with residual unablated areas and to resolve residual symptoms [Citation25], but no consensus has been published on the combined application of EA and RFA for solid BTNs.
In this study, a small amount of absolute ethanol was used as a ‘sensitizer’ for RFA to improve the efficacy of treating solid BTNs. Most of the solid BTNs that needed to be treated in clinical practice are large in size and have a rich blood supply. During RFA, most of the heat is carried away from the nodule by blood vessels. To achieve successful treatment, the energy and power of RFA should be increased. However, serious complications, such as nodular hemorrhage, nodular rupture, and thermal injury of the recurrent laryngeal nerve, may occur. In addition, although we tried to completely ablate large solid BTNs using RFA alone, some patients could not fully cooperate due to the long treatment time, resulting in a high residual rate and recurrence rate. Therefore, we created a modified treatment method (EA combined with RFA) based on the problems encountered during the treatment.
We used a fine needle to inject a small amount of anhydrous ethanol into the nodule (within 3-5 mm of the nodule capsule). The high pressure at the tip of the fine needle was conducive for diffusing ethanol in the tissue, which may enhance the therapeutic efficiency of RFA by rapidly destroying tumor vessels (coagulative necrosis, small-vessel thrombosis and hemorrhagic infarction), enhancing thermal conduction in the tumor tissues, reducing the energy requirement for coagulation per unit volume of tumor, and reducing the heat sink effect [Citation26,Citation27]. Moreover, ethanol heated by RFA may significantly extend the area of tissue necrosis [Citation26].
In addition to the above methods, other alternative therapies to treat mainly solid nodules include US-guided LA and MWA. Laser ablation has the advantages of accurate positioning, a low power requirement, minimal impact on the surrounding tissues and a favorable safety profile [Citation28–30]. Although laser ablation requires up to 4 fibers to treat large nodules, it does not increase invasiveness (even lower complications), and the costs in most of the countries are similar to those of RFA [Citation29–31]. We did not choose the LA technique because it is not a routine treatment in our center. The MWA technique may offer a larger ablation zone and less treatment time than RFA. However, MWA represents an emerging but still not fully evaluated and well established ablative method for BNTs [Citation18,Citation32,Citation33]. Therefore, in our study, EA + RFA may be an innovative approach in treating solid BTNs.
In this study, RFA was effective for treating BTNs and significantly reducing nodule volume, as evaluated at the 6-month follow-up. The efficacy of RFA was similar to that previously reported for a single session of RFA on mainly solid medium-sized nodules followed by a short follow-up period [Citation15,Citation34]. The initial nodule volume can affect the VRR. The VRR of medium nodules was better than that of large nodules in both the EA + RFA group and RFA groups, possibly because of a low deposition of energy inside the nodule. Recently, we confirmed this observation through an Italian multicenter study that utilized single RFA sessions [Citation34] and in the studies from Baek JH and Guang Y [Citation35,Citation36]. To prevent nodules from recurring, we applied the moving shot technique to completely ablate the nodules in this study. Studies have reported that a small number of residual nodules adjacent to important structures may recur within 1 to 2 years after RFA [Citation13,Citation37]. Recurrence usually occurs from viable tumor tissue that remains in the margin [Citation37]. However, no nodule recurrence was observed at 6 months post-ablation in either group in our study, which may be related to the short follow-up period.
The success rates of RFA with or without ethanol injection were 90.5% and 88.6%, respectively. Nine nodules failed to achieve therapeutic success (VRR less than 50% at the 6-month follow-up), including nodules with exogenous growth (in the direction of the upper or lower pole of the thyroid gland), nodules that were too large, and nodules that tended to be close to important structures, making complete ablation difficult to achieve. The possibility of this outcome was fully explained to the patients before performing RFA.
Compressive symptoms and cosmetic concerns were related to the nodule volume. In this study, the BTNs were categorized into medium and large subgroups in each group. We have indicated that RFA was able to rapidly improve the pressure symptoms and cosmetic concerns of the patients caused by medium or large nodules, and this improvement was stable until the end of the study period and has been demonstrated in many short- and mid-term follow-up reports [Citation38–40]. Notably, in most reports, multiple treatments were performed to obtain the largest possible shrinkage. In our clinical practice, a single treatment session was sufficient to attain the treatment goals. We found that the CSS of patients with large nodules in the EA + RFA group was better than that of the corresponding patients in the RFA group, which may be primarily because the alcohol injection before RFA reduced the heat sink effect, shortened the treatment time and alleviated pain.
All patients included in our study had normal preoperative thyroid function, which excludes the possibly that the functional state could affect the volumetric response to RFA treatment [Citation41]. All treated patients maintained their previous thyroid function levels. In contrast, surgical therapy for nodular thyroid disease imposes a substantial risk of hypothyroidism [Citation42]. Thyroid hormone replacement was required in approximately 9% to 49% of all thyroid disease patients treated by thyroid lobectomy [Citation43].
With respect to safety, this study showed no difference in the incidence of complications between the EA + RFA group and RFA groups, possibly because the procedures for both groups of patients were well tolerated and safe when performed by the same experienced radiologist. The most common complication of conventional EA was necrosis of the adjacent normal soft tissue and nerve tissue, which was due to leakages of the injected ethanol [Citation44]. However, we injected a small amount of alcohol into the solid part of the nodules, which can serve as an EA method, improve the efficacy of RFA, and prevent the leakage of alcohol. Some studies that evaluated the safety of combined EA and RFA performed by an experienced radiologist, whether in a stepwise or concomitant manner, have reported no procedure-related deaths or major complications [Citation19,Citation25]. In addition, 3 patients with transient hoarseness, mainly due to transient radiation damage caused by a thermal source close to the recurrent laryngeal nerve, recovered in 2 months. These findings indicate that EA combined with RFA can be a safe and effective method for treating solid BTNs.
The limitations of this study must be taken into account. First, we recognize that the sample size was relatively small and that additional data from multi-center studies are required to adequately assess the utility of EA combined with RFA in the treatment of solid BTNs. Second, the duration of our follow-up period was 6 months, which is a relatively short period to collect follow-up data and to evaluate the long-term recurrence of the treated nodules. Third, an additional ethanol injection was added to the operation, which demands more from the operators. In terms of CSS evaluation, this questionnaire was not an internationally common scale, making the results difficult to compare with other studies, but it was an important feedback component at our center in recent years based on the postoperative follow-up of patients. Four, in the past five years, our research center has been using the Olympus Surgical Technologies (CelonLabPOWER, Europe, Germany, Hamburg) system and an 18-gauge bipolar radiofrequency applicator with a 15-mm active tip (CelonProSurge, micro 100-T15; Olympus Surgical Technologies, Europe, Germany, Hamburg). Unlike the internally cooled electrodes used by other overseas research centers such as Baek’, the type of radiofrequency needles we used may be increasing the heat during the thermal ablation process; therefore, other methods such as ethanol injection are needed to help solve this problem and provide reference methods for other research centers across the country that are experiencing the same problem. Finally, although most of the hyperechogenic signals faded within a few minutes after the ethanol injection, a small area still remained, which does not affect the ability of an experienced physician to clearly distinguish the location of the needle tip; however, a less experienced radiologist may be less confident in this process.
In conclusion, injecting very small amounts of absolute ethanol into multiple sites of a nodule prior to a single RFA session can significantly shorten the ablation time of RFA, reduce the total energy and power required, induce a substantial volume reduction, improve compressive symptoms and esthetic concerns, improve patient satisfaction, and does not affect normal thyroid function. This modified RFA approach should be considered a low-risk and high-efficacy alternative to treat BTNs larger than 10 ml in size.
Disclosure statement
All authors have no potential conflicts of interest to disclose.
Additional information
Funding
References
- Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest. 2010;33(5):287–291.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(Supplement 1):1–639.
- Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138(4):315–318.
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667–673.
- Hegedus L. Therapy: a new nonsurgical therapy option for benign thyroid nodules? Nat Rev Endocrinol. 2009;5:476–478.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dietrich CF, Muller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Mh G, X D. Expert consensus on thermal abaltion for thyroid benign nodes, microcarcinoma and metastatic cervical lymph nodes (2015 edition). Chin J General Surg. 2016;25:944–946.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13(2):117–125.
- Kim JH, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219–225.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653–3659.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.
- Yang YL, Chen CZ, Zhang XH. Microwave ablation of benign thyroid nodules. Future Oncol. 2014;10(6):1007–1014.
- Zhang YJ, Liang HH, Chen MS, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology. 2007;244(2):599–607.
- Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370(1):78–84.
- Hahn SY, Shin JH, Na DG, et al. Ethanol Ablation of the Thyroid Nodules: 2018 Consensus Statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2019;20(4):609–620.
- McDonald RJ, McDonald JS, Kallmes DF, et al. Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology. 2013;269(3):640–645.
- Haukoos JS, Lewis RJ. The Propensity Score. JAMA. 2015;314(15):1637–1638.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Park HS, Baek JH, Choi YJ, et al. Innovative techniques for image-guided ablation of benign thyroid nodules: combined ethanol and radiofrequency ablation. Korean J Radiol. 2017;18(3):461–469.
- Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68(7):1524–1530.
- Crescenzi A, Papini E, Pacella CM, et al. Morphological changes in a hyperfunctioning thyroid adenoma after percutaneous ethanol injection: histological, enzymatic and sub-microscopical alterations. J Endocrinol Invest. 1996;19(6):371–376.
- Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab. 2014;99(7):E1283–1286.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and Risk Factors for Complications of Laser Ablation for Thyroid Nodules: a Multicenter Study on 1531 Patients. J Clin Endocrinol Metab. 2015;100(10):3903–3910.
- Park HS, Baek JH, Na DG. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;33:953–954.
- Dossing H, Bennedbaek FN, Hegedus L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165:123–128.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–16.
- Deandrea M, Sung JY, Limone P, et al. Efficacy and Safety of Radiofrequency Ablation Versus Observation for Nonfunctioning Benign Thyroid Nodules: A Randomized Controlled International Collaborative Trial. Thyroid. 2015;25(8):890–896.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. Am J Roentgenol. 2010;194(4):1137–1142.
- Guang Y, He W, Luo Y, et al. Patient satisfaction of radiofrequency ablation for symptomatic benign solid thyroid nodules: our experience for 2-year follow up. BMC Cancer. 2019;19(1):147.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33:905–910.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217(3):673–677.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25(1):112–117.
- Verloop H, Louwerens M, Schoones JW, et al. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243–2255.
- Park HK, Kim DW, Ha TK, et al. Factors associated with postoperative hypothyroidism after lobectomy in papillary thyroid microcarcinoma patients. Endocrine Res. 2015;40(1):49–53.
- Mauz PS, Maassen MM, Braun B, et al. How safe is percutaneous ethanol injection for treatment of thyroid nodule? Report of a case of severe toxic necrosis of the larynx and adjacent skin. Acta Oto-Laryngologica. 2004;124(10):1226–1230.