?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Waon therapy (WT) is the predominant thermal therapy for chronic heart failure in Japan, involving use of a far-infrared dry sauna. As sauna therapy requires certain equipment not readily available in hospitals, we tested the use of whole-body hot pack thermal therapy (HPTT). We compared the magnitude of skin vasodilation post-HPTT with that post-WT.
Methods
We recruited 19 healthy men (age [mean ± S.D.]: 26.8 ± 4.6 years) and employed a simple randomized crossover design. The HPTT required subjects to remain in a supine position on a bed for at least 10 min. Hot packs were then applied on the back, lower abdomen, and popliteal regions for 15 min (warming phase). Participants continued bed rest for 30 min (heat-retention phase) after removal of the hot pack. WT was performed as previously described. Blood pressure (BP), heart rate (HR), tympanic temperature (TT), and peak and average flow velocity of the right radial artery (PFV and AFV, respectively) and right brachial artery (BA) diameter were measured during HPTT and WT.
Results
HR, TT, PFV, and AFV persistently and significantly increased during warming and heat-retention phases of HPTT. In WT, HR and TT significantly increased during warming but decreased and plateaued during heat-retention. BP did not change significantly after either therapy; however, BA was dilated equally in both (HPTT: 3.70 ± 0.57 ⇒ 4.05 ± 0.59 mm, p = .001; WT: 3.63 ± 0.63 ⇒ 3.93 ± 0.61 mm, p < .001).
Conclusion
HPTT may be equivalent to WT with respect to vasodilation response of the skin.
1. Introduction
Heart failure is characterized by the heart’s inability to pump an adequate supply of blood to the organs of the body, resulting in disruptions of major bodily functions. Heart failure is a condition or a collection of symptoms that weakens the heart [Citation1].
Heart failure is a chronic disease with a generally poor prognosis. A previous study reported that the readmission rate of heart failure patients after diagnosis was 27% during the first 6 months after discharge and 35% during the year after discharge [Citation1]. Moreover, the mortality rate due to heart failure (classes III or IV of New York Association classification) was about 50% [Citation2]. Therefore, patients with heart failure require lifelong management [Citation3]. The management for chronic heart failure includes medications (beta-blocker, angiotensin-converting enzyme inhibitors, aldosterone receptor blockers, and diuretics), exercise therapy (continuous aerobic training and resistance training), and diet modifications (calorie control and low sodium-containing food) [Citation4]. Additionally, Waon therapy (WT), which includes sauna therapy at 60 °C, is used as a thermal therapy for chronic heart failure in Japan [Citation5,Citation6].
The effects of dry sauna use include a reduction in the cardiac load and improvements in cardiac contractility [Citation7]. Repeated dry sauna therapy use over two weeks was reported to improve endothelial and cardiac functions [Citation8], exercise capacity [Citation9], autonomic functions, and prognoses [Citation10].
However, dry sauna facilities are expensive and require a location and staffing, among other items. Because of these needs, sauna therapy has not become popular as a therapy for heart failure, and the benefit of thermal therapy cannot be provided to the majority of heart failure patients. In contrast, hot packs are widely used in physical therapy to reduce pain and improve muscle flexibility and joint stiffness [Citation11,Citation12]. However, providing therapeutic heat using hot packs is a topical method of treatment [Citation13] and studies have not evaluated the effects of warming the whole body using hot packs. Therefore, we tested the use of whole-body hot pack thermal therapy (HPTT) and aimed to elucidate its cardiovascular effects.
2. Materials and methods
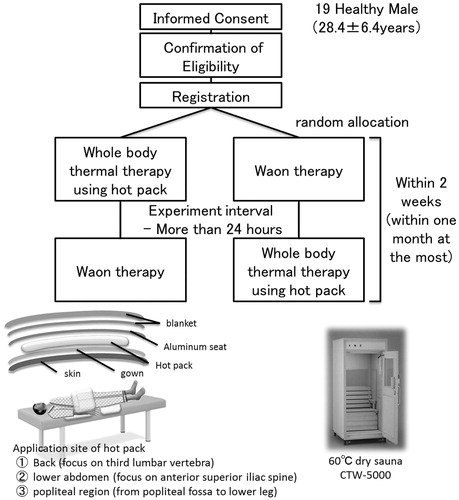
In this randomized crossover study, 19 healthy men (age [mean ± SD]: 28.4 ± 6.4 years) were recruited. The participants underwent either HPTT or WT in a random sequence. HPTT and WT were separated by a minimum of 1 day and a maximum of 1 month.
2.1. Thermal therapy
The study protocols for both thermal therapies are summarized in . The participants avoided physical exercise, eating, and smoking for 3 h before the treatment, and they avoided caffeine consumption 6 h prior to the procedure on the day of therapy. This study was carried out in a quiet room at 28–30 °C. The participants wore hygroscopic gowns.
2.1.1. Whole-body hot pack thermal therapy (HPTT)
Hot packs, which had been warmed up to 80 °C, were used after wrapping a plastic bag and towel around them (Minatopack KPA1076 25 × 30 cm, hydro advertiser HC-6U, Minato Ikagaku Inc., Osaka, Japan).
The HPTT protocol involved a participant lying in the supine position on a bed for at least 10 min. The participant was covered with thin aluminum foil sheets (heat-retaining aluminum sheets JTH-1321 130 × 210 cm, IRIS OHYAMA, Sendai, Japan). Subsequently, the hot packs were applied to the participant’s back (on the third lumbar vertebra), lower abdomen (anterior superior iliac spine), and popliteal regions for 15 min (the warming phase). After 15 min, the hot packs were removed and the participants continued bed rest for 30 min (the heat-retention phase). During the warming and heat-retention phases, the participant remained covered with thin aluminum foil sheets and a blanket.
2.1.2. Waon therapy; WT
We used far-infrared dry sauna equipment for the WT (CTW-5000, Fukuda Densi, Tokyo, Japan). The WT protocol involved the patients lying in a supine position on a bed for at least 10 min. Subsequently, they were seated in the dry sauna for 15 min. During the heat-retention phase, they rested supine on the bed for 30 min wrapped in a blanket.
2.2. Physiological measurements
The variables measured included heart rate, blood pressure (BP), bodyweight (before and after thermal therapy), tympanic temperature (taken every minute), brachial artery (BA) diameter (at rest, at the end of warming, and 15 and 30 min into the heat-retention phase), and radial artery blood velocity and autonomic activity as reflected by heart rate variability (continuously measured).
2.2.1. Tympanic temperature
We continuously measured the tympanic temperature as an index of deep body temperature during both thermal therapies. In a previous study, the deep body temperature during WT was assessed by measuring the pulmonary artery temperature using a Swan-Ganz catheter [Citation7]. However, a good correlation between tympanic temperature and pulmonary artery temperature has been reported [Citation14].
Tympanic temperature was measured in the right ear [Citation15]. We inserted the thermistor probe in the right ear and confirmed its position when a participant reported a ‘scratch noise’ or ‘sharp pain’ caused when the head of the probe touched the tympanic membrane.
2.2.2. Brachial artery diameter
We used a 10-MHz linear array ultrasound transducer to measure the right BA diameter (UNEXEF 38 G, UNEX Corp., Nagoya, Japan). The proximal right BA diameter was measured in the supine position at least four times (at rest, just after warming, and after 15 and 30 min of heat-retention). To measure the BA diameter, the right upper arm was abducted and the forearm immobilized. The transducer was placed on the brachial artery and the position was marked using a permanent marker for repeated measurements in the same position. We practiced the process for accuracy and reproducibility according to the guidelines [Citation16].
Additionally, we calculated and the rate of change of the BA diameter using the following equations:
2.2.3. Radial artery blood velocity
We used an ultrasonic flow measurement system (ES-100V3, Hadeco Corp., Kawasaki, Japan) with an 8-MHz attachable transducer (BF8M15S8A, Hadeco Corp, Kawasaki, Japan) to continuously measure the blood velocity in the right radial artery. The right wrist was immobilized in approximately 15° of dorsiflexion. The transducer was placed on the exposed region of the radial artery, approximately 3 cm proximal to the palmar navicular bone and was fixed using a surgical tape. The wrist was covered with a wrist band for thermal protection and immobilization.
The indices of radial artery velocity measured were peak flow velocity (peak value of flow pattern) and average flow velocity (at an average of 70 Hz sampling data) [Citation17]. We recorded these parameters and calculated the mean sampling values every 5 min.
2.2.4. Autonomic activity
We used heart rate variability to estimate the autonomic activity. The locations of the electrodes were based on CM5 [Citation18,Citation19] and adjusted to record the R waves clearly. R wave interval data were stored at a sampling rate of 1,000 Hz using an LRR 03 (Crosswell, Yokohama, Japan) during both thermal therapies, and the RR intervals were automatically calculated using the Reflex Meijin (Crosswell, Yokohama, Japan). We used low-frequency (LF) and high-frequency (HF) components to evaluate the autonomic activities. The LF/HF ratio is associated with sympathetic activity, and HF activity is associated with parasympathetic activity [Citation20].
2.3. Statistical analysis
We used G*power ver.3 for calculating sample size. The sample size calculated by G*power ver.3 (ANOVA, effect size f: 0.25, α err prob:0.05, power (1-β err prob):0.8, number of measurement:10) was fourteen. Therefore, we established that the estimated sample size was fifteen.
Data are presented as mean ± standard deviation. Statistical analyses were performed with Statistics for Excel 2012 (Social Survey Research Information Co., Tokyo). We used data recorded every 5 min for tympanic temperature and systolic and diastolic BP and average data of every 5 min for autonomic activity (LF/HF and HF components) and radial artery blood velocity. BA diameters were measured four times as reported in Section 2.2.2 above. Statistical analyses included two-way analysis of variance (ANOVA) and post hoc Bonferroni correction. Significance was defined as p<.05. Body weights before and after the therapies were compared using paired t-test with significance defined as p<.05.
2.4. Ethical considerations
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by the ethical committee of Hakodate National Hospital (approval number: H28-1114006). Informed consent was obtained from all participants for their participation in the study and for the publication of this report. The authors confirm that all participants cannot be identified via the paper and that they have been fully anonymized. Furthermore, the authors affirm that all mandatory health and safety procedures were complied within the course of conducting any experimental work reported in the paper.
3. Results
In this study, no adverse events, such as low-temperature burn, tympanic injury, and postural hypotension were observed. Significant differences in baseline measurements, such as bodyweight, blood pressure, heart rate, radial artery blood velocity, brachial artery diameter, and autonomic activity were not observed between HPTT and WT.
3.1. Bodyweight
Bodyweight was significantly reduced in both thermal therapies (HPTT: from 66.03 ± 10.53 to 65.79 ± 10.57 kg; WT: from 66.02 ± 10.72 to 65.78 ± 10.70 kg; p < .001). The mean decrease in bodyweight was approximately 0.25 kg in both therapies and was not significantly different between the two (HPTT: 0.24 ± 0.18 vs. WT: 0.25 ± 0.12 kg, p = .87).
3.2. Tympanic temperature
In HPTT, the tympanic temperature rose continuously and was significantly higher after 5 min of heat-retention when compared with that at rest (). On the other hand, the tympanic temperature during WT dramatically rose during the warming phase and remained significantly elevated during the heat-retention phase.
Figure 2. Tympanic temperature trends. This figure shows the trends of the tympanic temperatures every 5 min during both thermal therapies. vs. rest †p<.01; vs. WT §p<.01.
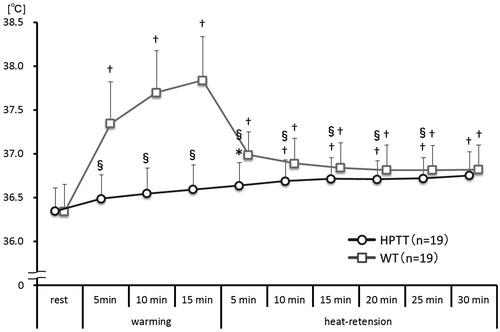
The tympanic temperature was significantly higher in WT than that in HPTT between 5 min of warming and 25 min of heat-retention; however, it was not significantly different at 30 min of heat-retention.
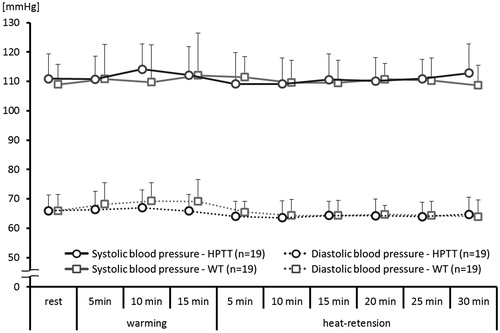
3.3. Blood pressure
Both systolic and diastolic BPs did not change significantly during either thermal therapy: Systolic BP; in HPTT: 110.9 ± 8.5 to 112.8 ± 9.9 mmHg; in WT: 109.0 ± 6.9 to 108.6 ± 6.8 mmHg (p = 0.668). Diastolic BP; in HPTT: 66.0 ± 5.4 to 64.7 ± 5.8 mmHg; in WT: 66.0 ± 5.5 to 63.9 ± 5.6 mmHg (p = .205) ().
3.4. Heart rate
The heart rate during HPTT rose continuously and was significantly higher after 10 min of heat-retention when compared with the heart rate at rest (). On the other hand, the heart rate during WT rose immediately during the warming phase until the warming was completed; however, during the heat-retention phase of WT, the heart rate declined and then plateaued.
Figure 4. Heart rate trends. This figure shows the average trend of heart rates taken every 5 mins during both thermal therapies. vs. rest *p<.05; †p<.01; vs. WT §p<.01.
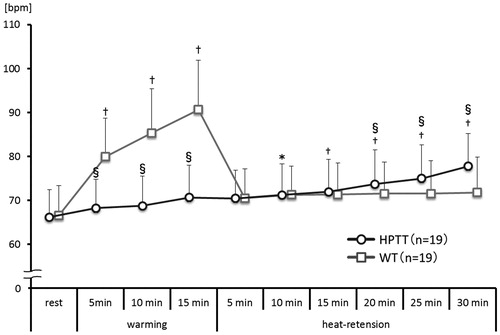
The heart rate in WT was significantly higher than that in HPTT during warming; however, after 20 min of heat-retention, the heart rate in HPTT was significantly higher than that in WT.
3.5. Radial artery blood velocity
In HPTT, the radial artery peak blood velocity rose continuously and was significantly higher after 5 min of heat-retention (). The peak value of radial artery blood velocity was observed toward the end of heat-retention. The mean radial artery blood velocity significantly rose after 15 min of warming, and its peak value was observed at the end of heat-retention.
Figure 5. Right radial artery blood velocity trends. This figure shows the average trends of right radial artery peak and mean blood velocity taken every 5 minutes. vs. rest *p<.05; †p<.01; vs. WT ‡p<.05; §p<.01.
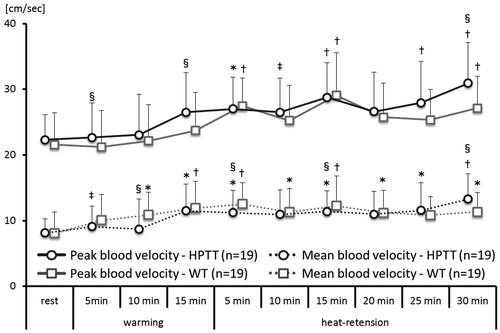
In WT, the radial artery peak blood velocity significantly rose during heat-retention. Its peak value was observed after 15 min of heat-retention and gradually reduced toward the end of heat-retention. The radial artery mean blood velocity significantly rose after 10 min of warming and remained elevated until the end of heat-retention. The peak value of radial artery mean blood velocity was observed at 5 min of heat-retention and gradually reduced toward the end of heat-retention.
3.6. BA diameter
In both therapies, the right BA diameter significantly expanded at the end of the warming phase, and at 15 and 30 min of the heat-retention phase when compared with the diameter at rest (). The differences in right BA diameter in HPTT were 0.18 ± 0.14 at the end of warming, 0.29 ± 0.15 at 15 min of heat-retention, and 0.34 ± 0.17 mm at the end of heat-retention (p < .001), whereas those in WT were 0.20 ± 0.14, 0.29 ± 0.15, and 0.29 ± 0.14 mm (p<.001). The ratios of change in the BA diameter in HPTT were 4.82 ± 3.16, 8.06 ± 4.35, and 9.58 ± 4.89%, whereas those in WT were 5.90 ± 4.49, 8.50 ± 5.19, and 8.25 ± 4.68%.
Figure 6. Right brachial artery diameter trends. This figure shows the trends of right brachial artery diameters during both thermal therapies. vs. rest †p<.01.
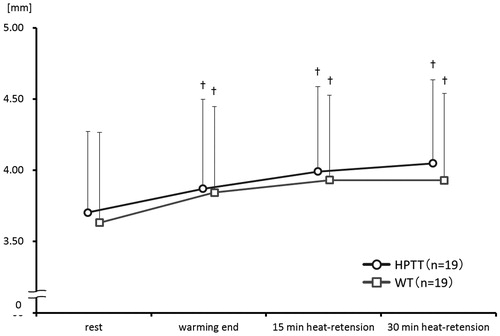
Between the groups, the right BA diameter was not significantly different at the end of the warming phase, or at 15 and 30 min of the heat-retention phase.
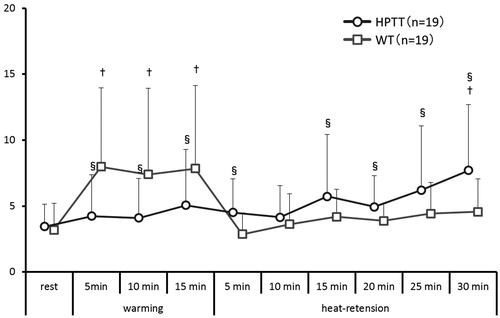
3.7. Autonomic activity
In HPTT, the LF/HF ratio demonstrated significantly higher sympathetic activity at 30 min of heat-retention when compared with that at rest (). In WT, the LF/HF ratio during warming was significantly higher compared with that at rest; however, during the heat-retention phase, the LF/HF ratio declined and then plateaued. Between the groups, the LF/HF ratio in WT was significantly higher than that in HPTT; however, after 15 min of heat-retention, the LF/HF ratio in HPTT was significantly higher than that in WT.
4. Discussion
In this study, we compared the changes in cardiovascular and skin vascular parameters following HPTT and WT. The heart rate, tympanic temperature, and autonomic activity demonstrated significantly different responses between the groups, while BP, radial artery blood velocity, and BA diameter demonstrated similar reactions. Afterload reduction following WT in chronic heart failure results in peripheral vasodilatation due to elevation of the deep body temperature [Citation7,Citation21–23]. However, in this study, the deep body temperature following HPTT was significantly lower than that following WT (). Nevertheless, the increases in the radial artery velocity and brachial artery diameter were comparable between WT and HPTT. Therefore, our results suggest that the afterload reduction by peripheral WT and HPTT may also be comparable, although we do not have direct data on the central hemodynamics. The failure of the BP to decrease after either WT or HPTT in our study may be due to the fact that our subjects were normal healthy subjects, in whom peripheral vasodilation may be counteracted by systemic sympathetic vasoconstriction. In addition, the hearts of normal subjects, unlike those of CHF patients, are not under excessive afterload (afterload mismatch).
4.1. Differences in cardiovascular reactions between HPTT and WT
4.1.1. Changes in deep body temperature
In this study, we used dry hot packs. In a previous study, dry hot packs, in comparison with wet hot packs, were demonstrated to have lower thermal conductivity, lower osmosis of warmth, and were considered unsuitable for increasing the blood volume and raising the subcutaneous temperature [Citation24]. Furthermore, hot pack therapy is a local thermal therapy, making it difficult to raise the whole body temperature if they are used alone. Therefore, in this study, we used thin aluminum foil sheets so as not to lose the radiant heat of the hot pack. In this type of HPTT, the tympanic temperature continuously rose between the warming and heat-retention phases; finally, the rise was equivalent to that with WT (15 min of warming: + 0.3 °C from rest; 30 min of heat-retention: + 0.4 ± 0.2 °C from rest). The rising tympanic temperature following HPTT was not the result of direct local heating effects, whereas that following WT was undoubtedly affected by the hot environment in the sauna. However, with WT, the tympanic temperature quickly rose during warming (+ 1.5 ± 0.3 °C from rest and at 15 min warming) and gradually fell during heat-retention (+ 0.5 ± 0.2 °C from rest at 30 min heat-retention). Accordingly, the pattern of the changes in deep body temperature between HPTT and WT was different. This result was because of the HPTT warming using dry hot packs with low-energy storage and transmission of heat as opposed to the 60 °C dry sauna in WT. Furthermore, during the heat-phase of HPTT, we believe that the gradual rise in tympanic temperature was because of the continued warming by the thin aluminum foil sheets, in contrast with the blanket used in WT.
4.1.2. Changes in radial artery blood velocity and BA diameter
The BA blood velocity following HPTT was significantly higher than that following WT after 15 min of warming. We believe that the increase in blood velocity after HPTT was strongly associated with the peripheral vasodilation due to the thermal stimulus as we observed no change in the LF/HF ratio, HF component, or heart rate. Increased shear stress or blood velocity triggers vasodilation; hence, we believe that the vasodilation at the end of warming after HPTT was strongly associated with the increase in blood velocity [Citation25].
In contrast, after WT, the peak blood velocity did not significantly change after warming; however, the mean blood velocity was significantly higher. Whether the expanding arterial diameter at the end of warming in WT was triggered by an increase in shear stress during warming or an increase in blood volume (increased venous return) after the change in the position from sitting to supine was not clear.
4.2. Vasodilatory effects of WT and HPTT
In a previous study, time-dependent changes in vasodilation after WT and HPTT were not clarified [Citation7]. Our results demonstrated that significant vasodilation occurred and was equal in magnitude after both thermal therapies.
Various factors, including nitric oxide from endothelial cells, cause vasodilation by their actions on the vascular smooth muscles. Vasodilation by the transient receptor potential (TRP) channels in endothelial cells is involved in the first vasodilatory reaction in thermal therapy [Citation26,Citation27].
In this study, we did not use any medication that could evoke vasodilatation. Therefore, with either HPTT or WT, we suggest that heat stress for the TRP channel evokes vasodilation and increased blood flow.
4.3. Differences in the body positions in HPTT and WT
WT required moving from the sauna (warming phase) to a bed (heat-retention phase), changing the participant position from sitting to supine. Remaining seated reduces venous return reduction, and a change in position from sitting to supine temporally increases the venous return. Therefore, a change in position during WT can possibly reduce the LF/HF ratio by temporarily increasing the intracardiac pressure (, from 15 min of warming to 5 min after heat-retention in WT). On the other hand, in HPTT, the participants remained supine. Furthermore, HPTT can be performed at the bedside and does not require additional setup. Our study showed that HPTT had similar effects on afterload reduction as WT did, even though it was different with respect to the type of heat stimuli, positioning during heating, and the heat-retention method.
These results suggest that it is possible to provide the benefits of thermal therapy to patients who cannot remain seated or have an aversion to closed spaces.
4.4. Limitations of this study and future studies
The present study has some limitations. We prescribed the participants to avoid physical exercise for 3 h before the study. However, the possibility of the influence of exercise performed before that period cannot be excluded [Citation29,Citation29]. It is unclear whether the benefits of HPTT are equal to those of WT in patients with chronic heart failure because the participants included in this study were limited to healthy males. Nevertheless, this study demonstrated that the rapid peripheral vascular effects of HPTT are comparable to those induced by WT. Therefore, studies must be conducted in the future to confirm whether HPTT can improve peripheral blood flow and simultaneously provide afterload reduction in patients with cardiovascular illnesses.
5. Conclusions
We tested the use of HPTT and elucidated the cardiovascular effects of HPTT in normal healthy subjects. The vasodilatory response and afterload reduction following HPTT are similar to those following WT. This result suggests HPTT is potentially useful for heart failure patients.
Acknowledgements
We would like to thank Editage for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol. 2010;55(1):13–22.
- Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017;19(9):1095–1104.
- Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239.
- McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017;27(6):420–425.
- Miyata M, Kihara T, Kubozono T, et al. Beneficial effects of Waon therapy on patients with chronic heart failure: Results of a prospective multicenter study. J Cardiol. 2008;52(2):79–85.
- Tei C, Imamura T, Kinugawa K, WAON-CHF Study Investigators, et al. Waon therapy for managing chronic heart failure - results from a multicenter prospective randomized WAON-CHF study. Circ J. 2016;80(4):827–834.
- Tei C, Horikiri Y, Park JC, et al. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation. 1995;91(10):2582–2590.
- Kihara T, Biro S, Ikeda Y, et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J. 2004;68(12):1146–1151.
- Sobajima M, Nozawa T, Fukui Y, et al. Waon therapy improves quality of life as well as cardiac function and exercise capacity in patients with chronic heart failure. Int Heart J. 2015;56(2):203–208.
- Kihara T, Miyata M, Fukudome T, et al. Waon therapy improves the prognosis of patients with chronic heart failure. J. Cardiol. 2009;53(2):214–218.
- James WB, Susan LM, Thomas PN. Jr. Modalities for therapeutic intervention. 6th ed. Philadelphia: F. A. Davis Co. 2016.
- Michelle H, Cameron MD. Physical agents in rehabilitation: an evidence-based approach to practice. 5th ed. St. Lowis: Elsevier, 2017.
- Takahashi N, Nakamura T, Kanno N, et al. Local heat application to the leg reduces muscle sympathetic nerve activity in human. Eur J Appl Physiol. 2011;111(9):2203–2211.
- Barnason S, Williams J, Proehl J, et al. Emergency nursing resource: non-invasive temperature measurement in the emergency department. J Emerg Nurs. 2012;38(6):523–530.
- Brinnel H, Cabanac M. Tympanic temperature is a core temperature in humans. J Therm Biol. 1989;14(1):47–53.
- Corretti MC, Anderson TJ, Benjamin EJ, International Brachial Artery Reactivity Task Force, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265.
- Nobuoka S, Aono J, Nagashima J, et al. Influence of hot bathing on blood flow velocity pattern of peripheral artery. J Jpn Soc Balneol Climatol Phys Med. 2006;63:187–192.
- Dash PK. Electrocardiogram monitoring. Indian J Anaesth. 2002;46:251–260.
- Quyyumi AA, Crake T, Mockus LJ, et al. Value of the bipolar lead CM5 in electrocardiography. Br. Heart J. 1986;56(4):372–376.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381.
- Cui J, Arbab-Zadeh A, Prasad A, et al. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation. 2005;112(15):2286–2292.
- Cui J, Boehmer JP, Blaha C, et al. Chronic heart failure does not attenuate the total activity of sympathetic outflow to skin during whole-body heating. Circ Heart Fail. 2013;6(2):271–278.
- Crandall CG, MacLean DA. Cutaneous interstitial nitric oxide concentration does not increase during heat stress in humans. J Appl Physiol. 2001;90(3):1020–1024.
- Draper DO, Harris ST, Schulthies S, et al. Hot-pack and l-MHz ultrasound treatments have an additive effect on muscle temperature increase. J Athl Train. 1988;33:21–24.
- Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46(1):9–15.
- Hsu WL, Yoshioka T. Role of TRP channels in the induction of heat shock proteins (Hsps) by heating skin. Biophysics. 2015;11(0):25–32.
- Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol. 2011;203(1):99–116.
- Hagberg JM, Montain SJ, Martin WH. 3rd, Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1987;63(1):270–276.
- Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension. 1993;22(5):653–664.