Abstract
Purpose
To evaluate the clinical safety and efficacy of percutaneous radiofrequency ablation (RFA) using multitined expandable electrodes under magnetic resonance imaging (MRI) guidance in the treatment of small hepatocellular carcinomas (HCCs) in the hepatic dome.
Materials and methods
The data of 49 patients with 50 HCC lesions in the hepatic dome who underwent MRI-guided RFA from April 2010 to January 2018 were retrospectively analyzed. Planning, targeting, and controlling were performed under MR-guidance during the procedure. The complications after RFA were observed. Follow-up MRI was performed to evaluate the curative effect. The local progression-free survival, recurrence-free survival, and overall survival rates were calculated using the Kaplan-Meier survival curve.
Results
The procedures were successfully accomplished in all patients without major complications. The mean follow-up time was 36.9 ± 25.8 months (range, 3–99 months). Technical success was 100% after one RFA session with MRI assessment after 1 month. Local tumor progression was observed in one patient (2%) with the lesion located in the hepatic dome at 4 months on a subsequent follow-up MRI. The progression-free survival time was 25.0 ± 22.7 months (median, 17.0 months). The 1-,3-, and 5-year local tumor progression-free survival rates were all 98.0%. The 1-,3-, and 5-year recurrence-free survival rates were 68.1%, 39.9%, and 28.5%, respectively, and the estimated overall survival rates were 93.7%, 76.3%, and 54.3%, respectively.
Conclusion
Planning, targeting, and controlling of RFA were well supported by MRI with acceptable time. MRI-guided RFA for small HCCs in the hepatic dome is safe and effective with fewer RF sessions.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, with the fifth highest incidence rate and the third highest mortality rate in the world [Citation1]. Radiofrequency ablation (RFA) has been widely used in the treatment of HCC due to its minimal invasiveness and good curative effect [Citation2,Citation3]. RFA is proposed as a first-line treatment for small HCCs due to the similar efficacy with liver resection and minor complications [Citation4,Citation5]. RFA of HCCs located in the hepatic dome shows a substantial risk of diaphragmatic injury, even perforation, and has not been well accepted in the past [Citation6]. Presently, the main modes of image-guided ablation of HCC are ultrasound (US) and computed tomography (CT). Due to the gas interference at the bottom of the lungs, HCCs in the hepatic dome often become a blind spot under US guidance [Citation7]. Moreover, unenhanced US and CT often show poor visualization for smaller HCCs in the hepatic dome in patients with cirrhosis [Citation8,Citation9]. Magnetic resonance imaging (MRI) has the advantages of excellent soft tissue contrast, multi-parameter and multiplanar capabilities, and sensitivity to thermal effects during the entire RFA procedure [Citation10,Citation11]. The detection rate of small HCCs of MRI is higher than that of US and CT, particularly in patients with cirrhosis [Citation9]. Compared to US or CT guidance, fewer studies of RFA for HCC under MRI guidance have been reported, particularly with the lesion located in the hepatic dome [Citation12,Citation13].
The purpose of our study was to evaluate the clinical safety and efficacy of percutaneous RFA using expandable multitined electrodes guided by 1.5-T closed-bore MRI in the treatment of small HCCs (≤3 cm) in the hepatic dome.
Materials and methods
Patients
This retrospective, single-center study was approved by our ethical review board, and the need for written informed consent was waived. All patients were discussed by a multidisciplinary team before the RFA procedure.
Between April 2010 to January 2018, 532 consecutive patients with hepatic malignant tumors were underwent percutaneous RFA guided by 1.5 T MRI. The study inclusion criteria were as follows: (a) HCC located in the hepatic dome within 5 mm from the diaphragm; (b) a maximum diameter of the HCC nodules of up to 3.0 cm and fewer than three HCC nodules without macrovascular invasion or extrahepatic metastasis; and (c) liver function Child-Pugh grade A or B, prothrombin activity ≥40%, platelets ≥50 × 109/L, and Eastern Cooperative Oncology Group performance status ≤2 points.
A total of 49 patients with 50 HCC lesions in the hepatic dome who underwent 1.5 T MR-guided RFA were enrolled in the study (). The patients’ baseline characteristics are summarized in . The clinical diagnosis of HCC was confirmed based on the guideline proposed by the Ministry of Health of the People’s Republic of China [Citation14]. A positive finding indicative of HCC using dynamic CT/MRI or CEUS is an arterial hyperenhancement with washout seen on the portal or delayed-phase images. Imaging features of tumors in hepatic dome are summarized in .
Table 1. Patients’ characteristics.
Table 2. Imaging features of tumors in hepatic dome.
Seven patients had undergone partial hepatectomy, and 28 had undergone hepatectomy combined with trans-arterial embolization. Tumor recurrence in the hepatic dome was observed in 35 patients after hepatectomy. RFA was performed as the first-line therapy in 14 patients. Additional nine HCCs not located in the hepatic dome were ablated simultaneously in seven patients. Liver biopsies with pathologic confirmation were performed in the five patients who did not meet the noninvasive diagnostic criteria.
MRI equipment and MR-compatible RFA system
A 1.5 T dual gradient MRI scanner (GE Signa Infinity Twinspeed, USA) with a closed bore (inner-diameter, 60 cm) was used. The MR signal was received by a Torso body coil with two rectangular square holes to facilitate interventional operation. Respiratory and ECG gating sensors were placed around the chest wall and finger, respectively.
The RFA procedures were performed using MRI-compatible monopolar of expandable multitined perfusion electrode (StarBurst MRI, RITA, 14 G, shaft length 10/15 cm) which had a rigid trocar that included nine expandable hooks to a maximum dimension of 5 cm. To keep the MRI-incompatible RF generator (Model 1500×, RITA, Mountain View, CA) away from the magnet, a 25-ft MRI connecting extension cable was utilized. The algorithm of thermal deposition was based on the manufacturer’s guidelines for power and duration settings, and included the temperature-controlled mode.
RFA procedures
Preoperative preparation
During the 2 weeks before the operation, CE-MRI was performed to confirm the size, location, and number of lesions. The patients were intramuscularly injected with 100 mg of bucinnazine before the procedure.
The heart rate, respiration, and blood pressure were monitored during the procedure. The MR-incompatible RF generator was placed outside the magnet room and was connected by the extension cable. The patients were placed on the MR table in either the supine or left lateral position. Due to the limitation of the magnet bore, the patient’s body needed to be moved to the opposite side in order to facilitate the RF electrode insertion more easily. The return grounding pads were placed on the patients’ bilateral thighs. A radiologist with over 5-year experience in hepatobiliary interventions and RFA performed the RFA procedures.
Planning
The procedural imaging protocol included scanning sequences and parameters, as follows: [Citation1] fat suppression fast flipping fast spin echo T2-weighted image (fs FRFSE T2WI): slice thickness 5.0 mm, interval 1.0 mm, FOV 38 cm × 28 cm, scan time 50–70 s; [Citation2] 3D dynamic T1-weighted image (3D Dyn T1WI): slice thickness 3.0 mm, FOV 38 cm × 28 cm, scanning time 10–14 s; [Citation3] Diffusion-weighted imaging (DWI): slice thickness 5.0 mm, interval 1.0 mm, b value 50,800 s/mm2, scan time 25–40 s. The breath-hold scan was used for the 3D Dyn T1WI sequence. Respiratory gating trigger scan was used for the fsFRFSE T2WI and DWI sequences.
The fsFRFSE T2WI and 3D Dyn T1WI images were acquired using vitamin E pills that were pasted on the body skin as markers. Multiplanar scanning (transverse, coronal, sagittal) was performed for planning the RF electrode trajectory from the entry point to the target lesion to avoid critical structures. In order to avoid lung and thoracic injury, caudocranial tilting of the puncture path was planned. The planned trajectory should be punctured indirectly by going through partial normal liver parenchyma. The skin entry point was marked and the puncture angle and depth were measured according to the plan.
Targeting
The patient was routinely disinfected and draped in a sterile fashion including the Torso coil. An injection of 5 mL of 1% lidocaine was performed for local anesthesia. The RF electrode was inserted step-by-step, guided by the multiplanar 3D Dyn T1WI or fs-T2WI sequences, while slightly breath-holding, based on the respiratory gating control. During the RF electrode placement, scanning was performed several times to confirm that it was being placed in the right direction. Sometimes we used oblique coronal, oblique sagittal, or oblique axial planes in order to parallel to the RF electrode trajectory with a full-length display of the electrode (). When the RF electrode reached the edge of the lesion in the hepatic dome, the inner expandable multitined electrodes were expanded 2–4 cm to the lesion, based on the lesion’s size (). Repeated scanning was performed to confirm the relationship between the multitined electrode and the lesion, as well as the critical structures, such as the diaphragm and pericardium, making sure that the multitined electrodes overlapped the lesion without penetrating the diaphragm ().
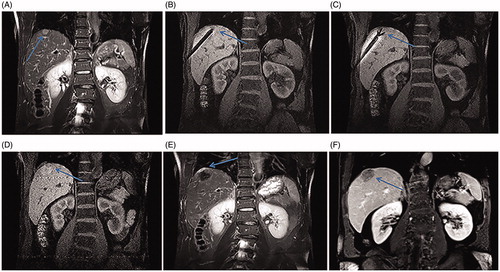
Figure 2. Recurrent HCC in the hepatic dome of a 59-year-old man treated with MR-guided RFA. (A) The recurrent nodule in segment VIII (arrow) is 15 mm in diameter and appears hyperintense in a coronal T2WI before RFA. (B–C) The RF electrode (arrow in B) is targeted gradually using the tilting of the puncture path under the coronal 3D-T1WI guidance. When the RF electrode reaches the edge of the nodule, the inner expandable multitined electrodes are expanded to 2.5 cm to overlap the nodule (arrow in C) without penetrating the diaphragm. (D–E) After RFA, the nodule is completely overlapped by the rim of hyperintensity on 3D-T1WI (arrow in D) and hypointensity on T2WI. Crescent-shaped effusions (arrow in E) are clearly displayed with the hyperintense signal in coronal T2WI between the liver capsule and the diaphragm. (F) The lesion (arrow) is completely ablated without a diaphragm injury at 3-month follow-up.
Monitoring
After a satisfactory distribution of the expandable multitined electrodes, wires of the RF electrode and return grounding pads were connected to the RF generator through the extension cable. The RFA system was based on the temperature measurement and tissue thermal conductivity impedance to confirm that the lesion was being ablated. The power of the RF generator and the target temperature were set at 150 W and 105 °C, respectively. The appropriate ablation procedure and duration were selected based on the different ablation ranges (2–4 cm/5–8 min). The local temperature and thermal conductivity impedance were monitored during the RFA procedure. During the RFA, 0.9% saline solution at room temperature was discontinuously injected into the RF electrode apertures based on the temperature and impedance in order to enhance the thermal conduction and reduce the carbonization of tissue. After the ablation, the power of the RF generator was turned off and the connecting wires were removed. Nonenhanced MRI was performed to assess the RF response and complications.
Controlling
Complete necrosis was assumed when the ablation zone completely overlapped with the rim of the hyperintense lesions on T1WI and hypointense lesions on T2WI, including a safety ablation margin 5–10 mm beyond ( and Citation3(G)). Otherwise, residual lesions were considered. The MRI finding of residual tumor was similar to the lesion before ablation (). If these cases, additional RFA was performed after repositioning of the electrodes (). After satisfactory tumor ablation, track ablation was performed before the electrode withdrawal. T2WI sequences scanning was repeatedly performed including the whole liver to evaluate for immediate complications.
Complication
Complications were assessed based on the guidelines of the Society of Interventional Radiology. The description of complications in this study follows the proposed standardization of terminology and reporting criteria [Citation15].
Follow-up and clinical outcome
Therapeutic effectiveness was assessed based on the evaluation of three-phases dynamic CE-MRI and serum tumor marker levels. Dynamic CE-MR images were repeated at 1-month and then at 3-month intervals in the first year after RFA and at 6-month intervals thereafter.
The technique efficacy was assessed 1 month after a single RFA session. Local tumor progression was determined on subsequent follow-up imaging. The progression-free survival (PFS) and 1-, 3-, and 5-year local tumor progression-free (LPFS) survival rates, overall recurrence-free survival rates as well as overall survival rates, were observed. The probability of recurrence-free survival was defined as the interval between RFA and the first date of any type of HCC recurrence—that was local and/or distant recurrence or the last follow-up date without recurrence.
Statistical evaluation
Statistical analysis was performed using SPSS software, version 22.0 (IBM, Chicago, USA) and the data were expressed as mean ± standard deviation (SD). Kaplan-Meier survival curves were used to calculated the 1-, 3-, and 5-year LPFS, overall recurrence-free survival, and overall survival.
Results
Radiofrequency ablation procedures
Forty-nine RFA sessions were successfully performed under MR guidance in all patients. Good visualization was displayed in 49 lesions in the hepatic dome using the fs-T2WI and 3D-T1WI sequences with slightly hyperintense T1 combination with hyperintense T2-signals before the ablation (). Nevertheless, one lesion located in segment II of the liver adjacent to the cardiac was only identified in the DWI sequence with the slightly hyperintense signal (). Forty-nine MRI-compatible RF electrodes were successfully inserted into the 50 lesions step-by-step by scanning several times. Position adjustment was performed in five patients due to the narrow magnet’s bore during the initial targeting procedure. The RF electrode presented a signal void in all MRI sequences (). The multitined electrodes were clearly displayed as umbrella-shaped when expanding (). Multiplanar scanning, particularly a coronal combination with transverse 3D dynamic T1WI or fsFRFSE T2WI were performed to target the 49 lesions in the hepatic dome using caudocranial tilting of the puncture path ( and ). The lesion only identified in DWI was targeted by DWI-guidance (). The overall duration of the procedure was 70.5 ± 31.1 min (40–125 min).
Figure 3. Recurrent HCC after liver resection in the hepatic dome of a 64-year-old man treated with MR-guided RFA. (A–D) The recurrent nodule, 12 mm in diameter, in the hepatic dome of segment II appears hyperintense in T2WI (arrow in A). Conventional TACE was carried out, and typical tumor staining is seen (arrow in B). At 8-month follow-up, the lesion is still growing up to 21 mm in diameter (arrow in C) with a rim of arterial phase hyper-enhancement (arrow in D) before RFA. (E–F) The RF electrode is targeted under the axial 3D-T1WI combination with sagittal oblique 3D-T1WI guidance. The inner expandable multitined electrodes are expanded to 3.0 cm to overlap the nodule (arrow in F) without penetrating the diaphragm and pericardium. (G) A typical ‘target sign’ (arrow) is clearly shown in the ablative zone with the hypointense nodule completely overlapped by the hyperintensity on 3D-T1WI, with a safe margin immediately after RFA. (H, I) The ablative zone with the ‘target sign’ (arrow) assimilated and without enhancement in MRI after 16-month follow-up.
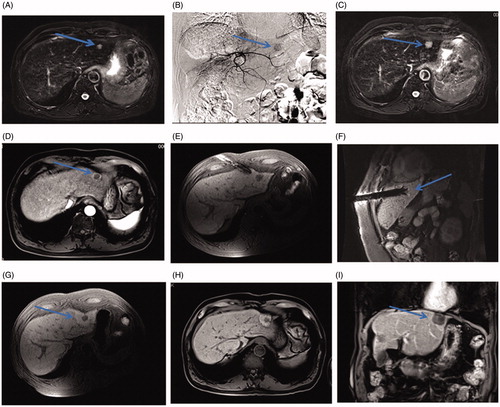
Figure 4. A small HCC in the hepatic dome of a 65-year-old woman with high serum AFP levels treated with MR-guided RFA using DWI. (A–D) The lesion in segment II, 13 mm in diameter, appears as an obvious enhancement in the arterial phase (arrow in C) and is hyperintense on the DWI (arrow, D) with isointensity on the T2WI and T1WI before RFA. (E–F) The RF electrode is targeted gradually using DWI-guidance (arrow in E) and clearly shows the placement of the expanded electrodes on the coronal 3D-T1WI. (G–I) Immediately after the RFA, the lesion is completely overlapped by the hyperintensity on 3D-T1WI and hypointensity on DWI (arrow in H) and T2WI (arrow in I).
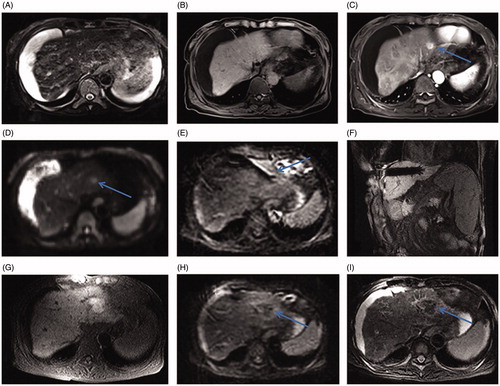
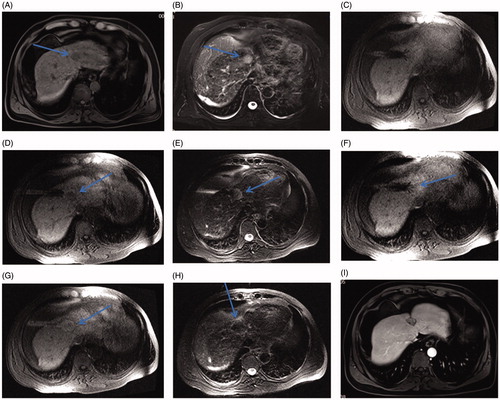
Figure 5. Small HCC in the hepatic dome of a 46-year-old man treated with MR-guided RFA. (A–B) The lesion in segment IV (arrow) is 21 mm in diameter and appears hypointense in T1WI (arrow, A) and hyperintense in T2WI (arrow, B) before RFA. (C) The RF multitined electrodes are expanded to overlap the lesion without penetrating the diaphragm and pericardium. (D–E) After the first RFA, the lesion is immediately overlapped partially by the rim of hyperintensity on 3D-T1WI and hypointensity on T2WI. The residual tumor of the left side still appears hypointense in T1WI (arrow in D) and hyperintense in T2WI (arrow in E) as pre-RFA. (F) A second ablation is performed after replacement of electrodes (arrow). (G, H) A typical ‘target sign’ (arrow in G) is clearly shown in the ablative zone on T1WI and appears hypointense in T2WI (arrow, H) immediately after RFA. (I) The ablative zone assimilated and without enhancement in MRI after 22-month follow-up.
Twenty-five patients developed radiating right shoulder pain during the ablation and five patients developed pain and discomfort in the precordial region, which improved after symptomatic analgesia treatment. Two patients had a decrease in heart rate during the ablation due to the vagus reflex. The ablation was stopped immediately and atropine at a 5 mg dose was injected intravenously. The ablation was resumed after the heart rate increased.
At the end of the initial RF procedures, 38 lesions in the hepatic dome completely overlapped with the rim of hyperintense signal on the 3D dynamic T1WI as well as hypointensity signal on the T2WI images, with a safety ablation margin of 5–10 mm beyond the lesions ( and ). The remaining 12 lesions showed an incomplete surrounding by the rim of hyperintense signal on the 3D dynamic T1WI as well as residual hyperintensity on the T2WI images; these were considered as incomplete ablations (). A second ablation was performed in order to acquire complete safety margin after repositioning of the expandable multitined electrodes (). After the second RF procedure, the remaining 12 lesions were completely ablated (). Crescent-shaped effusion related to the thermal reactivity was clearly displayed in the coronal T2WI between the liver capsule and diaphragm in 40 of the lesions (). In some cases, the phenomenon of a gradual increase of the signal intensity of the ablated lesions on the T1WI images was observed by dynamic scanning within 3 min to 30 min after the RFA. Additional nine lesions that were not located in the hepatic dome were also successfully ablated.
Tumor marker (AFP) changes
Among the 12 patients with a high level of AFP before the ablation, two (16.7%) displayed a negative result in the AFP assay, and five (41.6%) displayed a >50% decrease in the AFP level at 1 month after the ablation. Four (33.3%) of these patients displayed a < 50% decrease in the AFP level at 1 month after the ablation. In one (8.3%) patient, the AFP level increased at 1 month after the ablation.
Complications and side effects
No major complications were observed in our study. Fifteen patients (30.6%) experienced mild or moderate right shoulder pain which ranged in duration from 1 to 10 days. Minor complications occurred in nine patients (18.4%); 5 (10.2%) developed a small amount of pleural effusion after the ablation that did not require effusion drainage, and 4 (8.2%) patients developed a small amount of subcapsular hemorrhage without activity bleeding during the dynamic scanning. No cases of pneumothorax, diaphragmatic perforation, bronchial-biliary fistula, pericardial hemorrhage, liver failure, and RFA-related death occurred ().
Table 3. Incidence of complications related to RFA.
Follow-up and clinical outcome
The mean follow-up period was 36.9 ± 25.8 months (range, 3–99 months). The median follow-up was 31.0 months. Complete tumor ablation was achieved after one RF session in all 59 lesions according to the MRI assessment at 1 month after the RFA. The technical success was 100% after one RF session. Local tumor progression was observed in one patient (2%) with the lesion located in the hepatic dome at 4 months on subsequent follow-up MRI. The local tumor progression was successfully re-treated using MR-guided RFA. Secondary treatment effectiveness was 100% with a 16-month follow-up. New hepatic and extra-hepatic tumors appeared in 25 (51.0%) and 6 (12.2%) patients, respectively. During the follow-up period, no tumor seeding was observed.
A total of 15 patients died during the follow-up period; nine died due to HCC recurrence and progression, three patients died of liver failure, two died of gastrointestinal bleeding due to portal hypertension, and one patient died from another disease, not related to the liver disease. The mean tumor progression-free survival (PFS) was 25.0 ± 22.7 months (median, 17.0 months). The 1-, 3-, and 5-year local tumor progression-free survival (LPFS) rates were all 98.0% (). The overall 1-, 3-, and 5-year recurrence-free survival rates were 68.1%, 39.9%, and 28.5%, respectively. The overall 1-, 3-, and 5-year survival rates were 93.7%, 76.3%, and 54.3%, respectively ().
Figure 6. Graph showing the probability of LPFS in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. The 1-, 3-, and 5-year LPFS rates are 98.0%.
![Figure 6. Graph showing the probability of LPFS in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. The 1-, 3-, and 5-year LPFS rates are 98.0%.](/cms/asset/c7f0292b-633f-4b24-be17-55f443a509b3/ihyt_a_1728397_f0006_c.jpg)
Figure 7. Probability of recurrence-free survival and estimated overall survival in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. (A) Graph showing overall recurrence-free survival. The estimated overall 1-, 3-, and 5-year recurrence-free survival rates are 68.1%, 39.9%, and 28.5%, respectively. (B) Graph showing overall survival estimation. The estimated overall 1-, 3-, and 5-year survival rates are 93.7%, 76.3%, and 54.3%, respectively.
![Figure 7. Probability of recurrence-free survival and estimated overall survival in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. (A) Graph showing overall recurrence-free survival. The estimated overall 1-, 3-, and 5-year recurrence-free survival rates are 68.1%, 39.9%, and 28.5%, respectively. (B) Graph showing overall survival estimation. The estimated overall 1-, 3-, and 5-year survival rates are 93.7%, 76.3%, and 54.3%, respectively.](/cms/asset/eeeddd4f-b175-4feb-b2c3-b664dc72440f/ihyt_a_1728397_f0007_c.jpg)
Discussion
Image-guided RFA of HCC in the hepatic dome was considered as a challenging treatment by operators. There have been reports of major complications, such as diaphragmatic perforation, pneumothorax, pneumonia, and arrhythmia after RFA of tumors located in the hepatic dome [Citation6,Citation16]. Accurate ablation under image-guidance is based on good visualization of the lesion. Due to the poor visualization using US and CT guidance in small HCCs located in the hepatic dome, particularly in patients with liver cirrhosis, image fusion, such as CT-US fusion or MRI-US fusion [Citation17,Citation18], artificial assisted techniques, such as artificial ascites or pleura effusions [Citation19,Citation20], an lipiodol marked by TACE were performed to improve the visualization of the tumors [Citation21]. Axial scanning was only performed in CT, and it was difficult to evaluate the needle angle when tilting caudocranially using CT-guidance without delayed multiplanar reconstruction, and the risk of complications was increased with the transthoracic puncture [Citation22].
Because of the excellent soft tissue contrast and multi-parameter imaging, MRI can easily distinguish between cirrhotic nodules and HCCs and enables good visualization of small lesions, particularly in the hepatic dome, where US and CT do not provide good visualization [Citation23]. In our study, a total of 49 lesions in the hepatic dome were clearly displayed on the conventional MRI T2WI and T1WI, with a slightly hyperintense T1WI and a slightly hyperintense T2WI. Only one smaller lesion in the hepatic dome of segment II showed an equal signal on the conventional T2WI and T1WI sequences, with a slightly hyperintense signal in the DWI sequence. Thus, DWI-guidance was performed to target this lesion. Under the guidance of oblique-coronal scanning combined with axial scanning in order to target the lesion in the hepatic dome step-by-step, the full length of the RF electrode and its relationship with the three-dimensional space of the lesion were clearly displayed due to the multiplanar capabilities of MRI. All lesions in the hepatic dome were targeted by caudocranial tilting of the puncture path to successfully avoid lung and thoracic injury, which could result in pneumothorax and hemothorax. The vessels could be visualized in the MR images without contrast enhancement due to the ‘flowing void effect’.
Using multitined expandable RFA electrodes can generate spheroid necrotic zones [Citation24], and in our study most of the HCCs were spheroid. MRI-compatible RF multitined expandable electrodes were used in all cases in our study. After the multitined electrodes were expanded, the spatial position relationship between each multitined electrode and the lesion, as well as the diaphragm, were clearly shown by the multiplanar capabilities of MRI. In our study, crescent-shaped effusions between the liver capsule and the diaphragm related to the thermal reactivity, which separated the lesions from the diaphragm, reducing the thermal injury of the diaphragm, were clearly displayed in the coronal T2WI in 40 lesions. None of the patients in our study developed diaphragmatic perforation, fistula, or hemothorax during the follow-up.
The assessment of the immediate treatment response after RFA is critical to determine the efficacy of the procedure and reduce the rate of tumor residuals. Monitoring of the treatment response under US-guidance is restricted because of a hyperechogenic response which is generated due to the air bubbles induced by the vaporization during RFA. Moreover, this echogenic area may sustain for 30 min and up to 6 h to disturb the precise evaluation because the echogenic response does not demonstrate coagulation necrosis [Citation25]. Therefore, the hyperechogenic response should be viewed only as a rough approximation of the area of induced tissue necrosis. RFA under US-guidance frequently proceeds more than one session due to the inaccurate assessment of the immediate treatment response [Citation7]. The delineation of the tumor tissue and induced coagulation on the CT images is often limited, as is using CT-guided RFA. Monitoring of the thermal response during the RFA procedure is the major advantage of MR-guidance because of the favorable correlation between the MRI finding and the pathology [Citation10]. The zone of coagulative necrosis is characterized by a decreased signal intensity on the T2WI and an increased signal intensity on the T1WI [Citation26]. Koda et al. [Citation27] considered that the non-contrast MRI can accurately assess the immediate treatment response after RFA. In contrast to the US and CT guidance, the ablated lesions were still well visualized as hypointense nodules on the T1WI. “Target sign” displayed with the rim of hyperintense surrounding ablated lesion is usually shown on T1WI sequence. Due to the typical MRI finding after RFA, complete necrosis was assumed when the lesions completely overlapped by the ablation zone with the rim of hyperintense on T1WI as well as hypointense on T2WI, including a safety ablation margin of 5–10 mm beyond. Otherwise, reposition of the electrodes to the residual lesion and additional RFA was performed. Meng Li et al. [Citation7] achieved thermal ablation for liver cancers adjacent to the diaphragm in average 1.2 sessions under US-guidance. Hence, MRI provides superior monitoring of the immediate treatment response and complete ablation may be achieved in fewer sessions than with the US and CT guidance [Citation13,Citation28]. In our study, 58 lesions, including 49 lesions in the hepatic dome, showed a typical ‘target sign’ on T1WI and decreased signal intensity on T2WI immediately after the first or second RFA. The technical success was 100% after one RF session according to the MRI assessment at 1 month after the procedure. Local tumor progression was observed in one patient (2%) at 4 months on subsequent follow-up MRI and was retreated using RFA.
The tumor progression-free survival was 25.0 ± 22.7 months (median, 17.0 months). The overall 1-, 3-, and 5-year recurrence-free survival rates were 68.1%, 39.9%, and 28.5%, respectively. The overall 1-, 3-, and 5-year survival rates were 93.7%, 76.3% and 54.3%, respectively, which was comparable to those reported in most of the literature [Citation29,Citation30].
This study and MR-guidance had some limitations. First, MR-guided RFA is more time-consuming than US and CT-guidance and the limited closed-bore increased the difficulty of the procedure. Second, this was a single-operator, single-center study with a small number of cases. A multicenter study with a larger population size and a prolonged observation time is required to further access the curative effect. Third, thermal injury of the diaphragm was assessed only according to the symptomatology and MRI finding; it was not based on pathological proof. MR thermometry was not used to monitor the distribution of the thermal field during RFA in our study. These limitations should be improved in further studies.
In conclusion, the planning, targeting, and controlling of RFA were well supported by the capabilities of MRI. MR-guided RFA is safe and effective for the treatment of small HCCs up to 3 cm in the hepatic dome, with fewer RF sessions, a higher complete ablation rate and a lower local tumor progression rate.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Seror O, N’Kontchou G, Nault JC, et al. Hepatocellular carcinoma within milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology. 2016;280(2):611–621.
- Dong Ho L, Jeong Min L, Jae Young L, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909.
- Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2017;287:461–472.
- Alessandro C, Fabio P, Matteo C, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Kim YS, Rhim H, Sung JH, et al. Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Intervent Radiol. 2005;16(3):407–410.
- Li M ,Yu XL ,Liang P, et al. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia. 2012;28(3):218–226.
- Asvadi NH, Anvari A, Uppot RN, et al. CT-guided percutaneous microwave ablation of tumors in the hepatic dome: assessment of efficacy and safety. J Vasc Intervent Radiol. 2016;27(4):496–502.
- Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol H. 2011;9(2):161–167.
- Stephan C, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging. 2010;27(2):421–433.
- Hoffmann R, Rempp H, Kessler DE, et al. MR-guided microwave ablation in hepatic tumours: initial results in clinical routine. Eur Radiol. 2017;27(4):1467–1476.
- Terraz S, Cernicanu A, Lepetit-Coiffé M, et al. Radiofrequency ablation of small liver malignancies under magnetic resonance guidance: progress in targeting and preliminary observations with temperature monitoring. Eur Radiol. 2010;20(4):886–897.
- Rempp H, Waibel L, Hoffmann R, et al. MR-guided radiofrequency ablation using a wide-bore 1.5-T MR system: clinical results of 213 treated liver lesions. Eur Radiol. 2012;22(9):1972–1982.
- Medical and Health Care Bureau of the Health and Family Planning Commission of the People’s Republic of China. Criteria for diagnosis and treatment of primary liver cancer (2017 edition). Inform Infect Dis. 2017;16:705–720.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update: supplement to the consensus document. Radiology. 2014;273(1):241–260.
- Tito L, Luigi S, M Franca M, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Song KD, Lee MW, Rhim H, et al. Percutaneous US/MRI fusion-guided radiofrequency ablation for recurrent subcentimeter hepatocellular carcinoma: technical feasibility and therapeutic outcomes. Radiology. 2018;288:878–886.
- Lee MW, Rhim H, Cha DI, et al. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1–3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013;24(7):958–965.
- Yu NS, Hyunchul R, Tae Wook K, et al. Percutaneous radiofrequency ablation for hepatic tumors abutting the diaphragm: clinical assessment of the heat-sink effect of artificial ascites. AJR Am J Roentgenol. 2010;194(2):227–231.
- Zhang D, Liang P, Yu X, et al. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperther. 2013;29(7):663–670.
- Tang C, Shen J, Feng W, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization for unresectable hepatocellular carcinoma: a retrospective study. Medicine. 2016;95(20):e3754.
- Shibata T, Shibata T, Maetani Y, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Intervent Radiol. 2004;15(11):1323–1327.
- Weiss J, Hoffmann R, Rempp H, et al. Feasibility, efficacy, and safety of percutaneous MR-guided ablation of small (≤12 mm) hepatic malignancies. J Magn Reson Imaging. 2019;49(2):374–381.
- Ku CY, Hyunchul R, Sik AY, et al. Percutaneous radiofrequency ablation therapy of hepatocellular carcinoma using multitined expandable electrodes: comparison of subcapsular and nonsubcapsular tumors. AJR Am J Roentgenol. 2006;186(5 Suppl):269–274.
- Choi D, Lim HK, Kim SH, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent in evaluating therapeutic response-preliminary results. Radiology. 2000;217(2):558–563.
- Lin Z-Y, Chen J, Deng X-F. Treatment of hepatocellular carcinoma adjacent to large blood vessels using 1.5T MRI-guided percutaneous radiofrequency ablation combined with iodine-125 radioactive seed implantation. Eur J Radiol. 2012;81(11):3079–3083.
- Koda M, Tokunaga S, Miyoshi K, et al. Assessment of ablative margin by unenhanced magnetic resonance imaging after radiofrequency ablation for hepatocellular carcinoma. Eur J Radiol. 2012;81(10):2730–2736.
- Stephan C, Hansj Rg R, Rüdiger H, et al. Image-guided radiofrequency ablation of hepatocellular carcinoma (HCC): is MR guidance more effective than CT guidance? Eur J Radiol. 2014;83(1):111–116.
- Sandro R, Valentina R, Laura R, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53(1):136–147.
- Francica G, Saviano A, Sio ID, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis. 2013;45(4):336–341.