Abstract
Purpose
To evaluate the safety and efficacy of percutaneous ultrasound-guided ‘three-step’ radiofrequency ablation (RFA) for the treatment of giant hepatic hemangioma.
Materials and methods
Patients with giant hepatic hemangioma who underwent percutaneous ultrasound-guided ‘three-step’ RFA (n = 52) and conventional RFA (n = 54) at our center from June 2013 to December 2017 were retrospectively analyzed. The ‘three-step’ RFA proceeds as follows. Step 1: Ablate the feeding artery of the hemangioma. Step 2: Aspirate blood from the tumor. Step 3: Ablation the lesion. Intraoperative information, postoperative recovery, therapeutic effects, and complications were compared between the two groups.
Results
The duration of RFA was significantly shorter (19.2 ± 0.8 min versus 44.5 ± 2.8 min, p < 0.001), the number of punctures was significantly lower (3.2 ± 0.1 versus 4.7 ± 0.3, p = 0.002), and the duration of hospital stay was significantly shorter (9.0 ± 0.5 versus 11.5 ± 0.7, p = 0.013) in the TS-RFA group than in the C-RFA group. The complete ablation rate (86.5% versus 40.7%), the maximum postoperative pain score (2.5 ± 1.3 versus 4.1 ± 2.0) and symptom relief were also significantly better in the TS-RFA group than in the C-RFA group (p < 0.05). No postoperative death occurred in either group. There were no grade III or higher complications in the TS-RFA group, but one patient in the C-RFA group developed the grade III complication of postoperative abdominal bleeding.
Conclusions
‘Three-step’ RFA is a safe and effective minimally invasive treatment for giant hepatic hemangioma. It is worthy of further promotion and application.
Introduction
Hepatic hemangioma is the most common benign tumor of the liver, with an incidence of 0.4–20% in the general population [Citation1,Citation2]. Regular follow-up is necessary for patients with small and asymptomatic hepatic hemangioma, but no therapeutic intervention is required [Citation3]. However, patients with giant hepatic hemangioma [Citation4] (diameter ≥ 5 cm) may have additional symptoms, such as abdominal pain, indigestion, jaundice, a rapid increase in lesion volume, or even spontaneous rupture and hemorrhage, which require active treatment [Citation5,Citation6]. Currently, surgical resection is the most effective method for the treatment of hepatic hemangioma, but the incidence and mortality associated with related complications have been reported to be as high as 27% and 3%, respectively [Citation7–Citation10]. Other therapeutic methods, such as transcatheter arterial embolization (TAE), microwave ablation, radiotherapy, and steroid therapy, have also been reported for the treatment of giant hepatic hemangioma, but their efficacy is not satisfactory [Citation11–Citation14].
Percutaneous ultrasound-guided radiofrequency ablation (RFA) is a safe and minimally invasive therapy with reliable efficacy. In recent years, this technique has been successfully applied to the treatment of hepatic hemangioma with a diameter of <5 cm [Citation15–Citation17]. For giant hepatic hemangioma, conventional RFA requires the use of an RFA electrode, which increases the volume of ablation or enhances the ablation power and subsequently prolongs the operation time, not only causing pain and discomfort in the patient but also easily damaging the adjacent organs, thereby causing complications such as bleeding, gastrointestinal tract perforation, acute renal failure, and other serious postoperative complications. We have previously reported [Citation18] the successful treatment of giant hepatic hemangiomas by ‘three-step’ RFA guided by percutaneous ultrasound. This new and standardized radiofrequency ablation technique for the treatment of giant hepatic hemangioma can significantly shorten the treatment time, reduce the complications of RFA and improve the success rate of RFA. However, no large-scale studies have reported the safety and efficacy of ‘three-step’ RFA for the treatment of giant hepatic hemangioma.
Therefore, the purpose of this study was to evaluate the safety and efficacy of a new RFA technique (‘three-step’ RFA) for the treatment of giant hepatic hemangioma.
Materials and methods
Ethics statement
This study was reviewed and approved by the Southwest Hospital of Third Military Medical University Institutional Review Board and the requirement for written informed consent was waived. All procedures were conducted in accordance with the Declaration of Helsinki.
Patients
The medical records of patients with giant hepatic hemangiomas (5–15 cm in diameter) who had undergone radiofrequency ablation at our center from June 2013 to December 2017 were retrieved and reviewed (). In total, 106 patients with giant hepatic hemangiomas (5–15 cm in diameter) were retrospectively analyzed in this study. From June 2013 to April 2015, 54 of these patients with giant hepatic hemangioma received conventional radiofrequency ablation, and from May 2015 to December 2017, our center innovatively used the ‘three-step’ RFA to treat 52 of these patients.
The inclusion criteria for this study were as follows: (1) 18–70 years old; (2) single hepatic hemangioma with a diameter of 5–15 cm; (3) Child-Pugh grade A/B; (4) ICG15 ≤ 20%; (5) ECOG score of 0; (6) persistent hemangioma-related abdominal pain or discomfort and the exclusion of other gastrointestinal diseases capable of causing upper abdominal pain via gastroscopy ; (7) hemangioma increased by more than 2 cm in diameter within 1 year; (8) the distance between the hepatic hemangioma and the gallbladder, colon, stomach, and other internal organs was no less than 0.5 cm; and (9) the patient refused to receive surgical treatment but agreed to receive RFA. The exclusion criteria for this study were as follows: (1) multiple lesions or hepatic hemangioma with a diameter <5 cm or >15 cm; (2) severe bleeding tendency, a platelet count <50 × 109/L, or a prolonged prothrombin time >3 s; and (3) previous acceptance of other treatments for hepatic hemangioma.
Diagnosis of hepatic hemangioma
Currently, imaging examinations are the main methods used to diagnose hepatic hemangioma. In this study, a diagnosis of hepatic hemangioma was made based on at least two coincident radiologic findings using contrast-enhanced US, computed tomography (CT) and magnetic resonance imaging (MRI) [Citation19].
Preoperative preparation
All RFA procedures were performed by a hepatobiliary surgeon with more than 15 years of experience in RFA. All operations were performed under monitoring anesthesia, and puncture was performed percutaneously under the guidance of real-time ultrasound. An injection of fentanyl citrate (0.1–0.2 mg, Humanwell Pharmaceutical Co., Ltd., Yichang, China) was used for analgesia, an injection of dexmedetomidine hydrochloride (50–100 mg, Hengrui Medicine Co., Ltd., Jiangsu, China) was administered for sedation, and an injection of lidocaine hydrochloride (Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) was used for topical anesthesia. All ablations were performed using an RF electrode with a suction function (LDDJSl-0200200, Mianyang Lide Electronics Co., Ltd., Mianyang, China) and a multipole RF ablation system (LDRF-120S, Mianyang Lide Electronics Co., Ltd., Mianyang, China). The number of RF electrodes depends on the diameter of the tumor. For tumors with a diameter of <10 cm, one or two electrodes were used: for tumors with a diameter between 10 and 15 cm and three electrodes were used. Preoperative contrast-enhanced ultrasound was performed to identify the tumor site and tumor-feeding artery (). The optimal treatment position and needle insertion route (selected to avoid large blood vessels, bile ducts and adjacent organs) were selected, and the puncture point was marked on the skin.
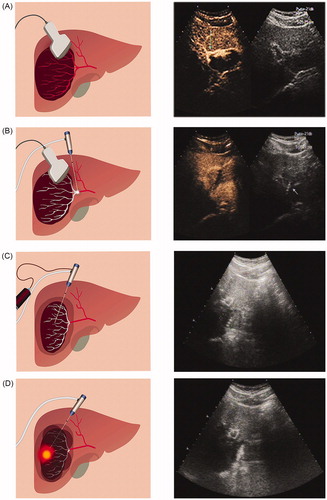
Figure 2. Percutaneous ultrasound-guided ‘three-step’ radiofrequency ablation of hepatic hemangioma located in the right lobe. (A) Contrast-enhanced ultrasonography is used to locate the tumor (the tumor was 7.66 cm in diameter) and its feeding artery. (B) The first step is to ablate the feeding artery. (C) The second step is to aspirate blood from the tumor (the diameter of the tumor after the blood was drawn was 4.11 cm). (D) The third step is to ablate the lesion.
Radiofrequency ablation procedure
The specific implementation steps used on the ‘three-step’ RFA group were as follows. Step 1 (): ablate the feeding artery of the hemangioma. Under ultrasound guidance, insert the needle into the area of the blood supply artery, damaging the feeding artery with an initial power of 50 W. Examine intratumoral blood flow by ultrasound. Step 2 (): aspirate blood from the tumor. Adjust the position of the needle under ultrasound guidance to allow the tip to enter and draw blood from the tumor. When negative pressure is achieved, retract the needle slightly, and adjust the needle position to puncture other parts of the tumor and continue to draw blood. Extract as much blood as possible from the tumor, and then monitor the volume of the tumor by real-time ultrasound. Remeasure the maximum diameter of the tumor after the blood draw. Step 3 (): ablation the lesion. To ensure safety and therapeutic effects, insert the tip of the needle into the tumor at least 1 cm from normal liver tissue. If multiple electrodes are used, they should be inserted in parallel into the tumor. In this research, the ablation strategy was determined according to real-time ultrasound. Using the pyramid-shaped three-dimensional ablation method, the tumor was destroyed, and no blind area was left. A fixed power (80 W) was used for ablation. The RFA system could continuously measure the tissue resistance, and the power output is stopped automatically if the resistance exceeds a specific limit (300 Ω). Therefore, the time of the procedure can change according to the situation. The ablation procedures were monitored by real-time US, and the ablation was not terminated until the transient hyperechoic cloud caused by the gas covered all units of the tumor. After ablation was completed, the electrode needle was removed in ‘needle tract ablation mode’ to prevent bleeding. Conventional RFA was performed in a similar manner, but one or more needle electrodes were inserted directly into the tumor for ablation.
Postoperative efficacy evaluation and follow-up
At 15 min after RFA completion, ultrasound contrast was reexamined immediately in the two groups. If the residual lesion was <3 cm, it was defined as technical success.
Conventional RFA was performed again in the presence of an enhancement lesion >3 cm in size. Complete ablation was defined as the finding of no enhancement area in the hemangioma by contrast-enhanced US, computed tomography (CT), and magnetic resonance imaging (MRI). Clinical success was defined as the improvement or disappearance of symptoms during follow-up. A visual analog scale (VAS) was used to assess postoperative pain on a scale of 0–10 (0, no pain; 10, worst imaginable pain). Postoperative complications were also assessed. Abdominal contrast-enhanced ultrasound, CT or MRI was performed at follow-up visits at 1, 6, and 12 months after RFA.
Statistical analyses
If a continuous variable was normally distributed, the mean ± standard error was used. If not, the median (range) was used. We used the two samples t-test to compare the mean difference for continuous variables between the two groups. Meanwhile, if the variance was not equal, the Mann–Whitney U test was used. The classification variables were evaluated by Pearson’s Chi-square test or Fisher’s exact test. Repeated measures ANOVA was used to compare biochemical indicators. p < 0.05 indicated that the difference was statistically significant. Version 25 (IBM SPSS Statistics for Windows, IBM Corporation, Armonk, NY) software was used for statistical analysis.
Results
Preoperative patient and tumor characteristics
The characteristics of the two groups of patients are summarized in . There were no significant differences between the two groups in terms of age, sex, concomitant liver disease, laboratory data, reasons for RFA, number of tumors, and location of tumors. The mean tumor diameter of the TS-RFA group was 7.3 ± 0.2 (5.0–11.2) cm, while that of the C-RFA group was 7.1 ± 0.2 (5.0–12.8) cm. The mean tumor diameter was comparable between the two groups (p = 0.112).
Table 1. Patient and tumor characteristics between the two study groups.
Intraoperative and postoperative results
The intraoperative information and postoperative results of the two groups of patients are shown in . In the TS-RFA group, the mean time to ablate the feeding artery was 3.8 ± 0.1 (1.4–6.4) min, and the mean volume of blood drawn from the tumor was 143.6 ± 10.3 (20–340) ml. After blood was drawn, the mean diameter of the tumor decreased to 5.3 ± 0.2 (2.8–9.7) cm, which was significantly smaller than either the original diameter (7.3 ± 0.2) or the diameter achieved in the C-RFA group (7.1 ± 0.2) (p < 0.001). Compared with patients in the C-RFA group, those in the TS-RFA group had significantly shorter RFA times (19.2 ± 0.8 min versus 44.5 ± 2.8 min, p < 0.001), puncture times (3.2 ± 0.1 versus 4.7 ± 0.3, p = 0.002), and hospital stays (9.0 ± 0.5 versus 11.5 ± 0.7, p = 0.013). According to the results of contrast-enhanced ultrasound, 45 patients (86.5%) in the TS-RFA group achieved complete ablation, while only 22 patients (40.7%) in the C-RFA group achieved complete ablation. The rate of complete ablation was, therefore, significantly higher in the TS-RFA group than in the C-RFA group (p < 0.001). The maximum pain score of patients was significantly lower after TS-RFA than after C-RFA (p < 0.001), and the number of symptoms that disappeared or improved was significantly higher in the TS-RFA group than in the C-RFA group (p = 0.04).
Table 2. Intraoperative information and postoperative outcomes.
In our study, seven patients in the TS-RFA group had residual lesions (diameter <3 cm), no obvious symptoms, and no obvious hemangioma enlargement during the 1-year follow-up period and received no further treatment. In the C-RFA group, 32 patients did not exhibit complete ablation (diameter <3 cm); among these, 6 patients received RFA treatment again because their symptoms did not improve or the diameter of the residual tumors increased during the follow-up period. Finally, all 6 patients exhibited complete ablation and were discharged successfully, while the remaining 26 patients were not treated again.
Liver function, renal function, and hemoglobin (HGB) indexes were compared between the two groups, and TBil, ALT and AST were found to be significantly higher in the C-RFA group than in the TS-RFA group on the first day after RFA (p < 0.05). Compared with preoperative levels, postoperative TBil, ALT and AST levels were significantly higher in both groups on day 1 after RFA (p < 0.05) and significantly lower on days 3 and 7 after liver function protection and symptomatic treatment (p < 0.05) ()). In addition, Cr was significantly higher in the C-RFA group than in the TS-RFA group on the first day after RFA (p < 0.05), and no difference was found on postoperative days 3 and 7 ().
Figure 3. Comparison of preoperative and postoperative liver function, renal function and hemoglobin indexes.
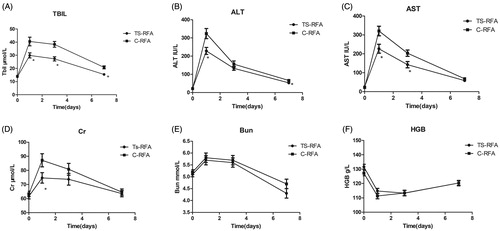
Postoperative Bun and HGB were compared, but no differences were found between the two groups at any time point ().
Postoperative complications and follow-up
No treatment-related deaths occurred in the study. Patients in both groups exhibited fever, pleural effusion, hemoglobinuria, and acute renal insufficiency. All patients returned to normal within 2–7 days after conservative or symptomatic treatment, with no significant differences observed between the two groups (p > 0.05). Compared with the C-RFA group, in the TS-RFA group, the number of jaundice patients decreased significantly (p < 0.05), and this condition resolved 2–4 days after symptomatic treatment. Two patients in the C-RFA group developed moderate anemia (grade II) and recovered after transfusion and symptomatic treatment. In addition, one patient developed a serious complication (grade III) and was diagnosed with non-coagulation by puncture of the peritoneal cavity. After laparoscopic exploration, hemostasis and symptomatic treatment, the patient recovered ().
Table 3. Postoperative morbidity.
Discussion
For patients who have large hepatic hemangiomas with symptoms or rapidly growing lesions, surgical resection is a radical therapy option [Citation20,Citation21]. However, regardless of laparotomy or laparoscopic surgery, patients receiving this highly invasive treatment often require a long recovery period and experience many complications [Citation22,Citation23]. A large number of studies have reported on the minimally invasive nature and good effects of RFA as a treatment method for giant hepatic hemangioma, and it has been shown that RFA can be regarded as a first-line treatment approach [Citation24,Citation25]. However, conventional RFA involves the direct insertion of the electrode into the tumor for ablation, and its ablation effect is not good in this patient population due to the large tumor volume and the ‘heat-sink’ effect [Citation26]. Moreover, as tumor volume increases and ablation time lengthens, larger numbers of red blood cells are destroyed, and patients become more prone to serious complications, such as bile duct injury, acute renal insufficiency, and irreversible liver failure [Citation27,Citation28].
A digital subtraction angiography (DSA) study of hepatic hemangioma showed that the main feeding artery of most hemangiomas is the hepatic artery [Citation29]. Firouznia et al. [Citation30] reported that after TAE treatment of 20 giant hepatic hemangiomas via hepatic arteries, the mean diameter of the tumors decreased from 97.00 mm (range: 25–200, SD: 47.85) to 88.95 mm (range: 23–195 SD: 43.27) (p = 0.004). Previous studies [Citation31] have also reported that combination therapy with TAE blocking the blood supply to tumors prior to radiofrequency ablation will help us successfully eliminate giant hepatic hemangiomas while avoiding ablation-related complications. After TAE treatment of 15 giant hepatic hemangiomas, all patients underwent only one radiofrequency ablation, 14 (93.3%) of the hemangiomas were completely ablated, and only 4 patients (26.7%) experienced ablation-related mild complications. Therefore, we proposed replacing TAE with ultrasound-guided RFA to reduce the blood supply of giant hepatic hemangioma. Moreover, the volume of hemangioma can be further reduced by extracting the blood that has accumulated in the tumor, which can also significantly shorten the ablation time and reduce complications.
In our study, the average diameter of giant hepatic hemangioma was 7.3 ± 0.2 (5.0–11.2) cm, and the average ablation time required by the ‘three-step’ RFA was 19.2 min, which was significantly shorter than the average ablation time of conventional RFA (44.5 ± 2.8 min). TS-RFA reduced the mean diameters of 5–15 cm tumors from 7.3 ± 0.2 cm to 0.4 ± 0.1 cm and the mean diameters of hemangiomas <10 cm from 7.0 ± 0.1 cm to 0.2 ± 0.1 cm. The complete ablation rate was as high as 86.5% (45/52). None of the patients required surgical intervention, and the length of hospital stay was significantly shortened. Moreover, TS-RFA was also an effective method to eliminate or relieve symptoms. All 22 patients who originally presented with symptoms improved or their symptoms disappeared completely. The clinical success rate reached 100%. A total of 6 patients (11.5%) treated with TS-RFA developed 5 types of grade I and II complications and eventually recovered completely. However, previous studies [Citation32] reported that the average ablation time of microwave ablation for treating giant hepatic hemangiomas with an average diameter of 7.02 ± 1.55 (4.1–10.8) cm was 19.5 min, and the clinical success rate was 95% (38/40). Therefore, the ‘three-step’ RFA shortens the time to treat giant hepatic hemangiomas compared with microwave ablation, and has a higher clinical success rate.
For benign tumors, we believe that the goal of treating giant hepatic hemangioma should be different from the approach used for malignant tumors. The ultimate goal is to strengthen disease control and improve quality of life. If hemangiomas occurring in high-risk areas (such as near the mediastinum, hepatic portal, or gallbladder) have a small residual presence after RFA completion, it is not necessary to pursue complete ablation. If the diameter of the residual tumor increases rapidly during the follow-up period or the patient’s symptoms do not improve, RFA treatment may be considered again.
Our study has some limitations. First, because this is a retrospective study, a suitable number of cases should be included in prospective studies to verify these results. Second, due to the small number of cases of hepatic hemangioma ≥10 cm, we analyzed these cases together with lesions ≥5 cm and <10 cm, which may have generated some bias. Therefore, the efficacy of ‘three-step’ RFA for hepatic hemangioma ≥10 cm requires more data and further analyses. Finally, the main blood supply of hepatic hemangioma remains controversial. Some studies have found that most of the blood supply of the hemangioma [Citation33–Citation35] comes mainly from the hepatic artery, with the peripheral collateral of the portal vein the main draining vein. However, in a few hemangiomas, the portal vein is the main source of blood supply. Thus, some hemangiomas do not shrink in size significantly after ablation of their main feeding artery and subsequent blood withdrawal from the tumor, making further ablation more difficult. Therefore, compared with conventional RFA, the ‘three-step’ RFA has increased difficulty in locating and destroying the main feeding vessel of the tumor. Hence, for complicated hepatic hemangioma cases with multiple lesions, a large tumor size or complex blood vessels, TAE combined with ‘three-step’ RFA can be employed.
In conclusion, ‘three-step’ radiofrequency ablation has good safety and effectiveness. For the treatment of giant hepatic hemangioma, ‘three-step’ RFA can be used as an alternative to surgery.
Acknowledgments
The authors thank all the medical staff at the Clinical Research Center of Southwest Hospital, the Medical Record Library of Southwest Hospital, and the anonymous reviewers for their excellent advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- EASL. Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386–398.
- Starzl TE, Koep LJ, Weil RR, et al. Excisional treatment of cavernous hemangioma of the liver. Ann Surg. 1980;192:25–27.
- Bahirwani R, Reddy KR. Review article: evaluation of solitary liver masses. Aliment Pharm Ther. 2008;28:953–965.
- Gao J, Fan RF, Yang JY, et al. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23:7077–7086.
- Hoekstra LT, Bieze M, Erdogan D, et al. Management of giant liver hemangiomas: an update. Expert Rev Gastroenterol Hepatol. 2013;7:263–268.
- Farges O, Daradkeh S, Bismuth H. Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg. 1995;19:19–24.
- Hanazaki K, Kajikawa S, Matsushita A, et al. Giant cavernous hemangioma of the liver: is tumor size a risk factor for hepatectomy? J Hepatobiliary Pancreat Surg. 1999;6:410–413.
- Yoon SS, Charny CK, Fong Y, et al. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg. 2003;197:392–402.
- Lerner SM, Hiatt JR, Salamandra J, et al. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139:818–821, 821–823.
- Chen L, Zhang L, Tian M, et al. Safety and effective of laparoscopic microwave ablation for giant hepatic hemangioma: a retrospective cohort study. Ann Med Surg. 2019;39:29–35.
- Park WC, Rhillips R. The role of radiation therapy in the management of hemangiomas of the liver. JAMA. 1970;212:1496–1498.
- Iyer CP, Stanley P, Mahour GH. Hepatic hemangiomas in infants and children: a review of 30 cases. Am Surg. 1996;62:356–360.
- Cui Y, Zhou LY, Dong MK, et al. Ultrasonography guided percutaneous radiofrequency ablation for hepatic cavernous hemangioma. WJG. 2003;9:2132–2134.
- J G, J K, Xm D, et al. Laparoscopic vs computerized tomography-guided radiofrequency ablation for large hepatic hemangiomas abutting the diaphragm. World J Gastroenterol. 2015;21:5941–5949.
- Hinshaw JL, Laeseke PJ, Weber SM, et al. Multiple-electrode radiofrequency ablation of symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol. 2007;189:W146–W149.
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89.
- Park SY, Tak WY, Jung MK, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54:559–565.
- Liu H, Zhang X, Tan YH, et al. Investigation of three-step radiofrequency ablation in treatment for giant hepatic hemangioma. Chin J Bases Clin General Surg. 2016;23:160–163. Chinese.
- Dietrich CF, Mertens JC, Braden B, et al. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139–1145.
- Liu X, Yang Z, Tan H, et al. Characteristics and operative treatment of extremely giant liver hemangioma >20 cm. Surgery. 2017;161:1514–1524.
- Jinhuan Y, Gang D, Binyao S, et al. Is laparoscopic hepatectomy suitable for giant hepatic hemangioma larger than 10 cm in diameter? Surg Endosc. 2019;5:e110–e112.
- Chen MF. Hepatic resection for benign tumours of the liver. J Gastroenterol Hepatol. 2000;15:587–592.
- Miura JT, Amini A, Schmocker R, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford). 2014;16:924–928.
- Sharpe ER, Dodd GR. Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: report of two cases and review of literature. J Vasc Interv Radiol. 2012;23:971–975.
- Gao J, Ding X, Ke S, et al. Radiofrequency ablation in the treatment of large hepatic hemangiomas: a comparison of multitined and internally cooled electrodes. J Clin Gastroenterol. 2014;48:540–547.
- Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78–85.
- van Tilborg AA, Nielsen K, Scheffer HJ, et al. Bipolar radiofrequency ablation for symptomatic giant (>10 cm) hepatic cavernous haemangiomas: initial clinical experience. Clin Radiol. 2013;68:e9–e14.
- van Tilborg AAJM, Dresselaars HF, Scheffer HJ, et al. RF ablation of giant hemangiomas inducing acute renal failure: a report of two cases. Cardiovasc Intervent Radiol. 2016;39:1644–1648.
- Sun JH, Nie CH, Zhang YL, et al. Transcatheter arterial embolization alone for giant hepatic hemangioma. Plos One. 2015;10:e135158.
- Firouznia K, Ghanaati H, Alavian SM, et al. Management of liver hemangioma using trans-catheter arterial embolization. Hepat Mon. 2014;14:e25788.
- Ji J, Gao J, Zhao L, et al. Computed tomography-guided radiofrequency ablation following transcatheter arterial embolization in treatment of large hepatic hemangiomas. Medicine (Baltimore). 2016;95:e3402.
- Liu F, Yu X, Liang P, et al. Ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous haemangiomas. Int J Hyperthermia. 2018;34:1061–1066.
- Hanafusa K, Ohashi I, Himeno Y, et al. Hepatic hemangioma: findings with two-phase CT. Radiology. 1995;196:465–469.
- Jang HJ, Choi BI, Kim TK, et al. Atypical small hemangiomas of the liver: “bright dot” sign at two-phase spiral CT. Radiology. 1998;208:543–548.
- Kim KW, Kim TK, Han JK, et al. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology. 2001;219:707–711.