Abstract
Purpose
To summarize the published literature on thermal ablation for primary hyperparathyroidism (PHPT) and to evaluate the effectiveness and safety of thermal ablation as a novel treatment strategy.
Materials and methods
Two authors carried out the literature search using four databases independently, including PubMed, Embase, Cochrane, and Web of Science. The meta-analysis included prospective and retrospective data that compared post-ablative outcomes to pre-ablative values. The primary outcomes were parathyroid hormone (PTH), serum calcium and volume of the parathyroid gland (VPG).
Results
From the 184 original articles, five studies (4 retrospective studies and 1 prospective study) examining 84 patients met the inclusion criteria. The meta-analysis showed significant reduction of PTH at 3 (standardized mean difference (SMD) = −1.09, 95% confidence index (CI) = −1.42 to −0.76, p < 0.001) and 6 months (SMD = −1.13, 95% CI = −1.46 to −0.80, p < 0.001) after thermal ablation. Serum calcium level was significantly reduced at 3 (mean difference (MD) = −0.31, 95% CI = −0.50 to −0.12, p = 0.001) and 6 months (MD = −0.31, 95% CI = −0.46 to −0.17, p < 0.001) after thermal ablation. There was no significant difference between pre-ablative VPG and that of 6 months after ablation (MD = −0.30, 95% CI = −0.70 to 0.09, p = 0.13). The most common complications were transient dysphonia and subcutaneous edema. No major complications or death occurred.
Conclusion
Thermal ablation is effective and safe for treatment of PHPT. PTH and calcium levels were reduced significantly at 3 and 6 months after thermal ablation.
Introduction
Primary hyperparathyroidism (PHPT) is a primary disease of the parathyroid, with a prevalence of 0.1%∼0.4%, being more frequently in female patients. It is caused by excessive synthesis and secretion of parathyroid hormone (PTH) by one or more of the four parathyroid glands and characterized by hypercalcemia and elevated or inappropriately normal concentrations of PTH [Citation1]. The most common manifestation of PHPT is asymptomatic hypercalcemia detected during routine biochemical testing [Citation2]. However, the disease has the potential to become symptomatic, resulting in complications that are mainly presented as skeletal, kidney and gastrointestinal involvement [Citation3]. It has been reported that PHPT is responsible for renal, skeletal and cardiovascular damage and increased risk of nephrolithiasis, osteoporosis, bone fractures, hypertension, arrhythmias, ventricular hypertrophy, vascular calcification, and mortality [Citation4–11].
Surgical treatment with extirpation of pathologic parathyroid tissue still remains the main curative treatment of PHPT [Citation12]. Although parathyroidectomy is recommended for patients who meet the indications of surgery in the clinic, it is considered to be high risk and associated with several complications, such as wound infection, post-operative hemorrhage, recurrent laryngeal nerve injury, persistent hypoparathyroidism and hypocalcemia [Citation13]. Though with improved surgical techniques, there are patients who either refuse surgical treatment or are ineligible for surgery. Senile patients suffer from risk to receive general anesthesia and full neck exploration [Citation14], and young female patients are anxious about the possibility of scar formation on their necks after surgery. In addition, parathyroidectomy requires initial image localization techniques to identify the adenoma, which are less successful for investigation of patients with mild hypercalcemia and in identification of multiple glands [Citation15]. Therefore, it has been warranted to identify therapeutic alternatives to surgical treatment.
Thermal ablation has been an important minimally invasive technique, and its appearance has expanded the selection range of non-surgical minimally invasive treatment. It has been proved to be safe and effective for the treatment of liver cancer, renal cancer and thyroid nodules, and has become an alternative of surgery in these fields [Citation16–18]. Up to now, there has been several options of thermal ablation such as microwave ablation (MWA), radiofrequency ablation (RFA), laser ablation (LA), and high-intensity focused ultrasound (HIFU) [Citation16,Citation19–22]. Compared to surgical treatment, local ablation remains to be a relatively simple procedure, easily tolerant for patients, with fewer complications, a shorter hospitalization and convalescence period [Citation23]. After more than a decade of clinical application since the first publication by Bennedbaek et al. [Citation24], thermal ablation technique has been demonstrated to be effective to destroy parathyroid gland tissue and normalize serum PTH and calcium [Citation25–31]. Nevertheless, no comprehensive review of thermal ablation in the treatment of PHPT has been reported so far. Therefore, the purpose of this systematic review and meta-analysis was to summarize the published literature about the effectiveness and safety of thermal ablation for the treatment of PHPT.
Materials and methods
Institutional review board approval was not required for this type of study at the authors’ institutions. This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (known as PRISMA) statement [Citation32].
Search strategy
Four databases, including PubMed, Embase, Cochrane, and Web of Science were systematically searched for relevant published articles with the following terms: (ablation) AND (thermal OR thermoablation OR interventional OR RFA OR radiofrequency OR laser OR MWA OR microwave OR HIFU) AND (hyperparathyroidism OR hyperplastic, parathyroid gland OR parathyroid hyperplasia OR parathyroid nodules OR parathyroid adenoma OR parathyroid neoplasia) AND (efficacy OR outcome OR survival). No beginning date limit was set. The literature search was continuously updated until the end of October 2019. The search was restricted to human subjects and English-language studies.
Eligibility criteria
The studies selected were required to meet the following inclusion criteria: (1) Original research papers that were written in English. (2) The study participants were human. (3) Randomized controlled trials (RCTs), prospective or retrospective studies. (4) Studies of PHPT patients who were found PTH levels higher than the upper limit of normal, serum calcium levels higher than the upper limit of normal or within normal range, normal renal function tests and parathyroid gland hyperplasia diagnosed by ultrasound or radionuclide imaging. (5) The patients underwent US-guided thermal ablation for therapy and the study compared pre-ablative and post-ablative clinical results such as PTH, serum calcium and volume of the parathyroid gland (VPG). (6) The study demonstrated the clinical value of thermal ablation for PHPT. (7) A follow-up period of at least 3 months was required after ablation.
The studies were excluded according to the following criteria: (1) Abstracts, case reports, case series, in vitro studies and animal studies were excluded. (2) Studies consisting of patients with secondary hyperparathyroidism or tertiary hyperparathyroidism. (3) Studies with a lack of relevant outcome data. If an author has more than one article, the articles that are not the most recent or with incomplete data are excluded. Disagreements on the inclusion of any study were resolved by discussion until a consensus was reached between the authors.
Data extraction and quality assessment
One author extracted data from eligible full-text articles with a standardized data extraction form, and this extraction was independently confirmed by two other authors. Any discrepancy was resolved by discussion until consensus was reached. The primary outcomes of this study included changes in serum PTH, calcium and VPG. Besides, the following data were collected: authors, publication year, study design, affiliation, follow-up period, number of patients, demographic data (gender and age), type of thermal ablation, major and minor complications. Major and minor complications were defined according to the Society of Interventional Radiology [Citation33]. The major complications are defined as events that may threaten the patient’s life, lead to permanent severe sequelae or long-term hospitalization if left untreated. Minor complications are defined as adverse consequences that need medication to be treated to relieve it. According to the above definition, in our study, major complications included severe bleeding and persistent hypocalcemia, while minor complications included transient dysphonia, subcutaneous edema, transitory hypocalcemia. The Cochrane assessment tool was used to assess the quality of the RCTs, whereas the Newcastle–Ottawa scale (NOS) was used to assess the quality of the non-randomized studies, including prospective and retrospective studies. Besides, each outcome was assessed using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system of rating quality of evidence. GRADE provides explicit criteria for rating the quality of evidence that include study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect [Citation34].
Statistical analysis
The meta-analysis utilized a post-ablation to pre-ablation (serve as baseline) comparison, with all subjects serving as their own controls. Each treatment outcome at the available time points was assessed and recorded as an absolute value. Serum PTH, calcium and VPG were continuous variables; thus, the standardized mean difference (SMD)/mean difference (MD) in change from baseline and the 95% confidence interval (CI) were analyzed in the meta-analysis. The magnitude of SMD/MD was quantified based on Cohen’s Criteria, using Cohen’s d. The effect sizes were defined as ‘small, 0.2 ≤ d < 0.5,’ ‘medium, 0.5 ≤ d < 0.8,’ and ‘large, d ≥ 0.8’ [Citation35]. Additionally, subgroup analyses were performed to explore the source of heterogeneity when there was moderate or high heterogeneity.
Heterogeneity among the studies were explored by using the χ2 test and quantified with inconsistency factor (I2). Heterogeneity was defined as a p value less than 0.10 or I2 greater than 50% [Citation36]. A fixed-effects model was applied when there was no or low heterogeneity (I2<50%), while a random-effects model was applied when there was moderate or high heterogeneity (I2>50%) [Citation37,Citation38]. Sensitivity analysis was conducted by omitting one study by turn to test the robustness of the primary outcomes [Citation36,Citation39]. Potential publication bias was assessed using funnel’s plot and quantified by Egger’s test [Citation40,Citation41].
p-values were considered significant if less than 0.05. All analyses were performed by using Review Manager, version 5.3 (Nordic Cochrane Center, Oxford, England) and Stata 12.0 (StataCorp, College Station, Tex).
Results
Literature search
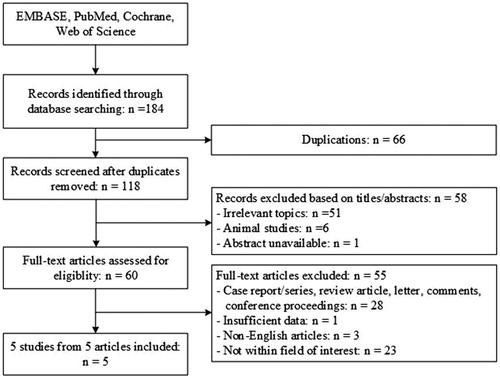
The primary literature search provided a total of 184 potentially relevant studies (). After discarding the duplicate studies and reading the tiles and abstracts of the articles, 124 publications were excluded. The remaining studies were further assessed for eligibility based on the full text articles. Five articles were included in the final analysis after reviewing. One prospective study and 4 retrospective studies were included in this meta-analysis [Citation25,Citation27,Citation42–44]. In the study by Liu et al. [Citation44], patients in another group received parathyroidectomy for PHPT. We extracted the data of patients in the MWA group only.
Study characteristics and risk of bias
The selected studies included 84 cases. The follow-up period ranged from 1 month to 36 months. The basic characteristics of the included studies are shown in . The included five studies were not RCTs. Thus, based on the NOS, the risk of bias was assessed. All of them were rated 7 stars, which is considered as relatively high-quality (selection of subjects: 3 stars; comparability of groups: 2 stars; assessment of outcome: 2 stars).
Table 1. Characteristics of the included studies.
Meta-analysis results
PTH level
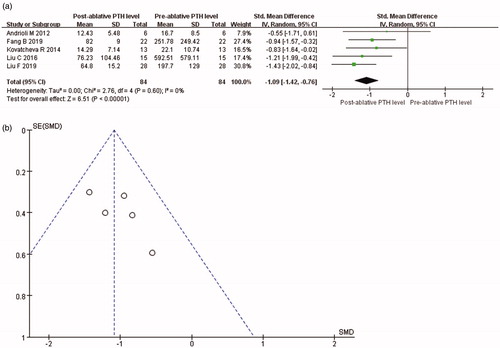
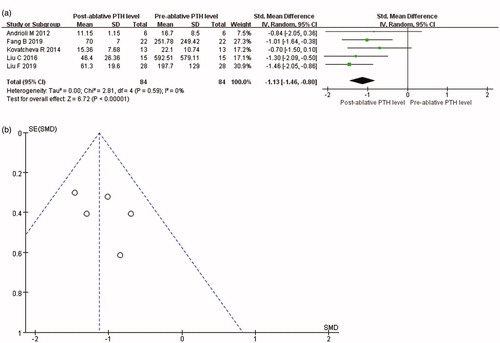
Data about PTH levels were reported in all included articles. It was showed that PTH level was significantly reduced at 3 months after thermal ablation for PHPT (SMD = −1.09, 95% CI = −1.42 to −0.76, p < 0.001; ). The value of Cohen’s d was 1.11, and the effect size was defined as large. In addition, PTH level was significantly reduced at 6 months after thermal ablation (SMD = −1.13, 95% CI = −1.46 to −0.80, p < 0.001; ). The value of Cohen’s d was 1.16, and the effect size was defined as large. As the PTH levels at baseline were considerably varied, the random-effects model was used in both analyses.
Calcium level
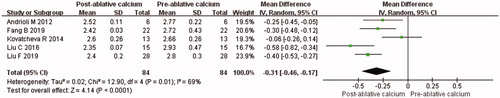
Data about calcium levels were reported in all included articles. It was showed that serum calcium level was significantly reduced at 3 months after thermal ablation for PHPT (MD = −0.31, 95% CI = −0.50 to −0.12, p = 0.001; ). The value of Cohen’s d was 1.13, and the effect size was defined as large. There was significant heterogeneity among the studies (p < 0.001, I2 = 80%), the random-effects model was used for meta-analysis. To explore the factors of heterogeneity, subgroup analysis was performed according to the pre-ablative VPG (mean VPG <2 ml and mean VPG ≥ 2 ml). Mean VPG at baseline was 0.43 ± 0.33 ml, 0.55 ± 0.28 ml, 2.08 ± 2.89 ml, 2.62 ± 3.32 ml and 3.21 ± 0.43 ml from the study of Andrioli et al., Kovatcheva et al., Fan et al., Liu et al. and Liu et al., respectively. After subgroup analysis, I2 was less than 50% in both mean VPG <2 ml group (I2 = 0%, MD = −0.09, 95% CI = −0.24 to 0.06, p = 0.22) and mean VPG ≥ 2 ml group (I2 = 43%, MD = −0.45, 95% CI = −0.55 to −0.35, p < 0.001).
Figure 4. Forest plot, meta-analysis of comparison between serum calcium levels at 3 months after ablation and that of pre-ablation.
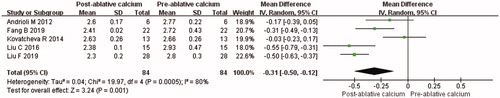
Serum calcium was significantly reduced at 6 months after thermal ablation on the PHPT (MD = −0.31, 95% CI = −0.46 to −0.17, p < 0.001; ). The value of Cohen’s d was 1.19, and the effect size was defined as large. There was significant heterogeneity among the studies (p = 0.01, I2 = 69%), the random-effects model was used for the meta-analysis. Similarly, subgroup analysis was performed according to the pre-ablative VPG (mean VPG <2 ml and mean VPG ≥2 ml). After subgroup analysis, I2 was less than 50% in both mean VPG <2 ml group (I2 = 49%, MD = −0.15, 95% CI = −0.28 to −0.01, p = 0.03) and mean VPG ≥ 2 ml group (I2 = 40%, MD = −0.40, 95% CI = −0.50 to −0.30, p < 0.001).
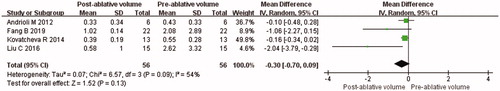
Volume of parathyroid gland
Data about change of VPG were reported in four studies. There was no significant difference between pre-ablative VPG and that of 6 months after ablation (MD = −0.30, 95% CI = −0.70 to 0.09, p = 0.13; ). The value of Cohen’s d was 0.42, and the effect size was defined as medium. There was significant heterogeneity among the studies (p = 0.09, I2 = 54%), the random-effects model was used for the meta-analysis. Similarly, subgroup analysis was performed according to the pre-ablative VPG (mean VPG <2 ml and mean VPG ≥ 2 ml). After subgroup analysis, I2 was less than 50% in both mean VPG <2 ml group (I2 = 0%, MD = −0.15, 95% CI = −0.31 to 0.02, p = 0.08) and mean VPG ≥ 2 ml group (I2 = 0%, MD = −1.19, 95% CI = −2.21 to −0.17, p = 0.02).
Complication
Transient dysphonia caused by impaired mobility of the ipsilateral vocal cord was reported in all included studies. Besides, in the study by Kovatcheva et al. [Citation42], patients complained of subcutaneous edema, which disappeared in 1–4 weeks. Fan et al. [Citation43] reported needle tract bleeding and transitory hypocalcemia after ablation, which were managed by manual compression and adjusting the dosage of oral calcium preparations, respectively. In the studies by Liu et al. [Citation44], transitory hypocalcemia was reported, which was relieved by the use of calcitriol and calcium. None of these incidents resulted in permanent severe sequelae, long-term hospitalization or death.
Sensitivity analyses, publication bias and GRADE assessment
Sensitivity analyses for change of PTH and serum calcium levels at 3 and 6 months after thermal ablation and VPG at 6 months after ablation were used to judge the dependability of the results. One study was deleted at a time; the heterogeneity and the results still showed no difference.
The publication bias analyses were conducted for the changes of PTH, serum calcium and VPG at each evaluation time-point in the included studies. Publication bias analyses were quantified using Egger’s test () and assessed by conducting funnel plots with PTH change at 3 and 6 months after ablation. The graphical funnel plot seemed to be symmetrical ( and Citation3(b)). All of the p values were more than 0.05. Therefore, statistically significant publication bias was unlikely to occur.
Table 2. Publication bias analyses with Egger’s test based on primary outcomes.
GRADE assessment was provided for each outcome. PTH levels change at 3 and 6 months after thermal ablation were rated as moderate (+++). Calcium levels change at 3 and 6 months after thermal ablation were rated as moderate (+++). VPG change at 6 months after thermal ablation was rated as low (++).
Discussion
To the best of our knowledge, the present study was the first meta-analysis that evaluated the effectiveness of US-guided thermal ablation on the PHPT. For the meta-analysis, five studies were included. The post-ablative change of serum PTH, calcium and VPG from baseline, which were measured before ablation, were analyzed. The overall outcome showed that PTH and serum calcium levels were reduced at 3 and 6 months after thermal ablation.
In PHPT, approximately 80% of patients have a single parathyroid adenoma, 10%∼11% have more than one adenoma, and less than 10% have hyperplasia of all four glands [Citation45]. Parathyroid carcinoma count for less than 1% cases of PHPT [Citation1,Citation46]. In the clinical practice guidelines [Citation1], parathyroidectomy was recommended for patients who meet the surgical indications. However, there are patients who are unsuitable for or refuse surgery. The rationale of thermal ablation for PHPT is to induce necrosis in as much of the hyperfunctioning parathyroid gland as possible with thermal energy. Thus, the goal to treatment for PHPT is to lower the levels of PTH and serum calcium to avoid potential deleterious effects of long-term hypercalcemia. US-guided percutaneous thermal ablation has several advantages including minimal invasiveness, optimal temperature to induce cell death and larger ablation volume [Citation47–49].
In this meta-analysis, decrease of PTH levels could be observed at 3 and 6 months after thermal ablation. This may be a result of the destruction of the hyperplasia parathyroid gland by thermal ablation, and the total release of PTH was reduced. Besides, based on Cohen’s criteria, the effect sizes of SMD were large at both 3 and 6 months after ablation. Effect size of SMD was interpreted as the percent of nonoverlap of the post-ablative value with those of the pre-ablative one [Citation35]. Therefore, an effect size of 1.11 indicated that a nonoverlap of 58.9% in the two distributions of pre-ablative and post-ablative, so as an effect size of 1.16. Calcium homeostasis is a complex process that depends on the synergistic effect of three kinds of hormones including PTH, calcitonin and 1,25-dihydroxycalciferol. Although PTH and calcium levels were reduced significantly at 3 and 6 months after thermal ablation in this meta-analysis, Fan et al. [Citation43] found that PTH rapidly reduced at 1 month after ablation, but serum calcium levels gradually decreased and reached obvious reduction at 3 months after ablation. One reason may be that the feedback caused by serum calcium reductions would still reduce the secretion of calcitonin and increase the synthesis of 1,25-dihydroxycalciferol to minimize fluctuations in serum calcium level. Nevertheless, in this study, the early change of PTH and calcium after ablation could not be assessed due to insufficient data. Therefore, more studies should be needed to demonstrate the early change of PTH and calcium levels. Although there was moderate or high heterogeneity in meta-analysis, but I2<50% was achieved after subgroup analyses, which showed that the unbalanced pre-ablative VPG among the studies was one of the factors of heterogeneity. As reported in a previous study, it is well known that the nodule size could be an important factor that affect the efficacy [Citation50]. Besides, difference in experience among different operators in thermal ablation can be the cause of the perceived heterogeneity [Citation51]. Though it might be a significant issue, but it was not able to be analyzed in this meta-analysis. In the future, further studies with larger sample size that focus on this issue are still needed.
Regarding complications and adverse effects after thermal ablation for PHPT, in five studies included in this analysis, a total of 17 out of 84 patients (20.2%) complained of transient dysphonia after ablation, which was self-limited and disappeared within 1 month after the procedure. This may be explained by the recurrent laryngeal nerve reaction to heat stress and temporarily impaired mobility of the vocal cord [Citation43]. As the recurrent laryngeal nerve is anatomically close to the parathyroid gland, protection of it is extremely important in ablation procedure. Subcutaneous edema, needle tract bleeding and transitory hypocalcemia was seen in 5 (6.0%), 2 (2.4%), and 1 (1.2%) out of 84 patients, respectively. These events were classified as minor complications that did not significantly prolong hospitalization and only needed medication to relieve it. No major complication or death related to ablation procedure for parathyroid occurred. Therefore, thermal ablation for PHPT appears to be safely applied without major complications and devastating adverse effects. Theoretically, as parathyroid gland is anatomically adjacent to the thyroid, the complications of ablation for both parathyroid gland and thyroid were similar. A previous meta-analysis showed that a pooled proportion of 2.38% for overall complications and 1.35% for major complications after RFA for thyroid nodules. Major complications included voice change, nodule rupture, permanent hypothyroidism and brachial plexus injury, and minor complications included pain, hematoma, vomiting, skin burns, and transient thyroiditis [Citation52]. From the current status, in comparison to ablation for thyroid nodules, ablation for PHPT is more prone to complications because of the complexity of parathyroid gland and neck anatomy. Therefore, some techniques to protect the adjacent tissue and organs in order to lower the rate of complications are needed in the future studies. On the other hand, the comparison of safety between thermal ablation and parathyroidectomy for PHPT still remains rare. Only one study [Citation44] included in this meta-analysis made a comparison, and showed that the incidence of side effects and complications were comparable between these two treatments, but less blood loss and shorter surgical time were achieved in patients who received thermal ablation. However, the sample size in this study was relatively small and the conclusion should be further verified in RCTs and meta-analysis with larger sample size. Comparison between thermal ablation and parathyroidectomy for secondary hyperparathyroidism (SHPT) had been summarized in a recent meta-analysis [Citation53]. It came to a conclusion that thermal ablation reduced the risk of hypocalcemia but increased the risk of SHPT persistence or recurrence compared with parathyroidectomy. Residual parathyroid glands can result in persistent or recurrent hyperparathyroidism. The reason may be that technology of thermal ablation is relatively immature and thermal ablation could not be performed under direct observation. In brief, whether this conclusion can be extended to PHPT still needs further verification.
There are some limitations to this article. First, it was limited due to the relatively small number of included studies, sample size and clinical outcome. Thus, the change of other clinically relevant indicators from baseline, such as post-ablative symptom relief, serum phosphorus and bone density, could not be analyzed. In the future, meta-analysis with a larger number of studies would be warranted. Second, efficacy at 1 year after ablation were not evaluated, because some studies did not focus on it, resulting in insufficient information. Further studies with long follow-up period were needed. Last, RCTs were not included in the analysis. After a thorough search of the database, no related RCTs were found. However, demonstrating the efficacy of thermal ablation for PHPT was a new field. Thus, RCTs are in an urgent need to be prompted in the future.
Conclusions
In conclusion, thermal ablation is effective and safe for treatment of PHPT. PTH and calcium levels were reduced significantly at 3 and 6 months after thermal ablation. Self-limited transient dysphonia was the most common complication. No related major complication or death occurred.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178.
- Machado NN, Wilhelm SM. Diagnosis and evaluation of primary hyperparathyroidism. Surg Clin North Am. 2019;99(4):649–666.
- Minisola S, Gianotti L, Bhadada S, et al. Classical complications of primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(6):791–803.
- Walker MD, Silverberg SJ. Cardiovascular aspects of primary hyperparathyroidism. J Endocrinol Invest. 2008;31(10):925–931.
- Lind L, Hvarfner A, Palmer M, et al. Hypertension in primary hyperparathyroidism in relation to histopathology. Eur J Surg. 1991;157(8):457–459.
- Vestergaard P. Primary hyperparathyroidism and nephrolithiasis. Ann Endocrinol (Paris). 2015;76(2):116–119.
- Peacock M. Primary hyperparathyroidism and the kidney: biochemical and clinical spectrum. J Bone Miner Res. 2002;17 (Suppl 2):N87–94.
- Mazzuoli GF, D’Erasmo E, Pisani D. Primary hyperparathyroidism and osteoporosis. Aging Clin Exp Res). 1998;10(3):225–231.
- Makras P, Anastasilakis AD. Bone disease in primary hyperparathyroidism. Metabolism. 2018;80:57–65.
- Ejlsmark-Svensson H, Bislev LS, Lajlev S, et al. Prevalence and risk of vertebral fractures in primary hyperparathyroidism: a nested case-control study. J Bone Miner Res. 2018;33(9):1657–1664.
- Misiorowski W, Czajka-Oraniec I, Kochman M, et al. Osteitis fibrosa cystica-a forgotten radiological feature of primary hyperparathyroidism. Endocrine. 2017;58(2):380–385.
- Nilsson IL. Primary hyperparathyroidism: should surgery be performed on all patients? Current evidence and residual uncertainties. J Intern Med. 2019;285(2):149–164.
- Egan RJ, Scott-Coombes DM. The surgical management of sporadic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(6):847–859.
- Strom C, Rasmussen LS, Sieber FE. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69 (Suppl 1):35–44.
- Katz SC, Wang GJ, Kramer EL, et al. Limitations of technetium 99m sestamibi scintigraphic localization for primary hyperparathyroidism associated with multiglandular disease. Am Surg. 2003;69(2):170–175.
- Head HW, Dodd GD, III. Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127(5):S167–S78.
- Ben Hamou A, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules? Int J Hyperthermia. 2019;36(1):666–676.
- Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation. JCO. 2018;36(36):3591–3600.
- Dobnig H, Amrein K. Value of monopolar and bipolar radiofrequency ablation for the treatment of benign thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2019;33(4):1–15.
- Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol. 2019;29(8):4026–4035.
- de Senneville BD, Moonen C, Ries M. MRI-guided HIFU methods for the ablation of liver and renal cancers. Adv Exp Med Biol. 2016;880:43–63.
- Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100(5):1903–1911.
- Feng B, Liang P. Local thermal ablation of renal cell carcinoma. Eur J Radiol. 2012;81(3):437–440.
- Bennedbaek FN, Karstrup S, Hegedüs L. Ultrasound guided laser ablation of a parathyroid adenoma. BJR. 2001;74(886):905–907.
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. Am J Roentgenol. 2012;199(5):1164–1168.
- Jiang TA, Chen F, Zhou X, et al. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J Endocrinol. 2015;2015:1–6.
- Liu C, Wu B, Huang PT, et al. US-guided percutaneous microwave ablation for primary hyperparathyroidism with parathyroid nodules: feasibility and safety study. J Vasc Intervent Radiol. 2016;27(6):867–875.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32(2):180–186.
- Sormaz IC, Poyanli A, Acar S, et al. CASE SERIES The Results of Ultrasonography-Guided Percutaneous Radiofrequency Ablation in Hyperparathyroid Patients in Whom Surgery Is Not Feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Wang G, Liu S, Liu X, et al. Microwave ablation: an effective treatment for mild-to-moderate secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2017;33(8):946–952.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282(2):576–584.
- Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Intervent Radiol. 2003;14(9):S199–S202.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 1–17.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Zwahlen M, Renehan A, Egger M. Meta-analysis in medical research: potentials and limitations. Urol Oncol. 2008;26(3):320–329.
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1(2):97–111.
- Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–262.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statist Med. 2006;25(20):3443–3457.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperther. 2019;36(1):835–840.
- Bilezikian JP. Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993–4004.
- Insogna KL. Primary Hyperparathyroidism. N Engl J Med. 2018;379(11):1050–1059.
- Regier M, Chun F. Thermal ablation of renal tumors: indications, techniques and results. Dtsch Arztebl Int. 2015;112(24):412–418.
- Vogl TJ, Farshid P, Naguib NN, et al. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119(7):451–461.
- Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914–1923.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;33(3):295–299.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Gong L, Tang W, Lu J, et al. Thermal ablation versus parathyroidectomy for secondary hyperparathyroidism: a meta-analysis. Int J Surg. 2019;70:13–18.