Abstract
Background
The aim of this study was to assess the feasibility, safety and efficacy of computed tomography (CT)-guided percutaneous microwave ablation with artificial ascites for problematic hepatocellular tumors.
Methods
Forty-eight patients with 61 problematic hepatocellular carcinomas who underwent CT-guided percutaneous microwave ablation with artificial ascites were reviewed retrospectively. Lesions less than 5 mm away from the gastrointestinal system, diaphragm, pericardium or kidney were defined as problematic tumors with the potential risk of thermal damage. Microwave ablation was performed after artificial ascites was established between tumors and the adjacent high-risk organs. The technical effectiveness of microwave ablation, local tumor progression and complications was assessed.
Results
Microwave ablation with artificial ascites was successfully performed in all 61 tumors. The technical effectiveness rate was 100% with contrast-enhanced CT performed immediately after the ablation procedure. Local tumor progression occurred in three (6%) of the 48 patients during the follow-up period (mean, 15 months; range, 6–24 months). No major complications related to the procedure occurred.
Conclusion
CT-guided percutaneous microwave ablation with artificial ascites is a feasible, safe and effective choice for treating problematic hepatocellular tumors, avoiding potential thermal damage to the adjacent high-risk organs.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide [Citation1]. Approximately, 50% of all cases of HCC are reported in China in addition to high risks of chronic hepatitis B and aflatoxin infections [Citation2]. Curative treatment of HCC includes surgical procedures, such as surgical resection or liver transplantation [Citation3]. Benefit from surgery is limited for most patients with HCC because of cirrhosis with portal hypertension, local advanced disease, extrahepatic extension or severe comorbidities affecting the cardiac, respiratory, central nervous system and renal functions [Citation4,Citation5]. Benefit from liver transplantation is also limited because of the shortage of donor organs [Citation6].
Locoregional treatment with different ablation modalities may be an option for curative treatment of early HCC tumors smaller than 2 cm [Citation7]. Ablation techniques are of two types: chemical, including the use of ethanol or acetic acid; and thermal, including the use of radiofrequency, cryoablation, microwave or laser. Image-guided local thermal ablation is widely performed owing to its ease of use, relatively low cost, short hospitalization time and minimal invasiveness [Citation8–11]. Complete ablation of liver lesions with adequate ablation margin is the primary goal of thermal ablation [Citation12,Citation13]. In early HCCs, the outcomes of ablation and surgical resection are comparable [Citation7,Citation9,Citation14].
However, thermal ablation may damage to the adjacent vital organs, leading to serious complications, such as intestinal perforation [Citation15]. Strategies to overcome this drawback include balloon catheter interposition, gas (carbon dioxide) instillation and artificial ascites to separate the tumors from the adjacent organs [Citation16–20].
Artificial ascites is an effective and safe method that separates the target organ from the adjacent structures. Under imaging guidance, 100–1750 ml saline or 5% glucose are administered into the peritoneal cavity via a needle, a catheter or an angiosheath [Citation21,Citation22]. Shallow breathing of the patient is necessary during the procedure. Artificial ascites usually disappears within a few days, and when it persists for more than 5 days, postoperative diuretics may be used [Citation23]. Artificial ascites used in liver tumors treated with thermal ablation separates the liver from the adjacent organs, preventing thermal injury and alleviating periprocedural pain [Citation24].
Radiofrequency ablation (RFA) has been the most common treatment modality for early HCC in the past 20 years [Citation9,Citation14,Citation25,Citation26]. It achieved a similar overall survival as surgical resection for patients with HCC smaller than 2 cm and liver function of Child–Pugh grade A [Citation7]. A propensity-score matching analysis comparing chemoembolization plus ablation versus surgical resection for single, medium-sized HCCs revealed similar outcomes for both; however, chemoembolization plus ablation was associated with shorter hospital stays [Citation27].
Cryoablation is less common than RFA for liver tumors [Citation28]. It requires placement of multiple applicators, has a longer ablation protocol, is more expensive, and increases the risk of bleeding [Citation29].
In recent years, microwave ablation has drawn more attention as another thermal ablation method [Citation30–32]. The efficacy of microwave ablation in the treatment of liver cancer has been confirmed [Citation33,Citation34]. Compared to RFA, microwave ablation has a faster temperature-rising time, larger ablation range and lower heat sink effect. These characteristics of microwave ablation may increase the risk of thermal damage to the surrounding organs during ablation of hepatic tumors [Citation35].
The safety and efficacy of thermal ablation with artificial ascites using radiofrequency for problematic hepatic tumors has been described by several authors [Citation17,Citation19,Citation21,Citation36]. A few clinical studies on microwave ablation with artificial ascites for problematic liver tumors have been published [Citation22,Citation23]. The aim of this study was to retrospectively assess the feasibility, safety and efficacy of computed tomography (CT)-guided percutaneous microwave ablation with artificial ascites for problematic hepatocellular tumors.
Materials and methods
Patients
This study was approved by the institutional review board of the Second Hospital of Shandong University. All patients signed an informed consent form before the start of the procedure. Inclusion criteria were as follows: 1) the diagnosis of HCC obtained using imaging or histopathology; 2) single nodular HCC measuring less than 5 cm or multinodular (less than three in number) HCC measuring less than 3 cm in the maximum diameter of each nodule; 3) Child–Pugh class A or B liver cirrhosis; 4) platelet count ≥50 × 109/L; and 5) inability to undergo surgery. Exclusion criteria were recent upper gastrointestinal bleeding caused by portal hypertension, severe coagulation disorders, bleeding tendencies, platelet count <50 × 109/L, Child–Pugh class C liver cirrhosis and uncontrolled infections. Lesions less than 5 mm away from the gastrointestinal system, diaphragm, pericardium and kidney were defined as problematic tumors with the potential risk of thermal damage.
From March 2016 to August 2017, percutaneous CT-guided microwave ablation with artificial ascites was performed for 89 problematic tumors of 62 patients. We excluded 14 patients who had a total of 28 liver metastases. Finally, 48 patients with 61 problematic tumors were included in this study. Among the 61 problematic tumors, 36, 20, four and one tumor was adjacent to the gastrointestinal system (ten to the stomach, 16 to the hepatic flexure of the colon, six to the transverse colon and four to the gallbladder), diaphragm, pericardium and kidney, respectively. Ten of the 48 patients had undergone tumor resection previously. Six of them underwent laparoscopy while four underwent laparotomy. The median recurrence time after surgery was 11.5 months (range, 5–26 months).
Forty patients had been treated previously with transcatheter arterial chemoembolization (TACE). Epirubicin and iodized oil were mixed and injected into the feeding arteries via a microcatheter. One TACE session was performed in 32 patients, and repeated TACE sessions in eight patients.
Chemotherapy (systemic or intravascular, TACE or port) was the first approach introduced to treat primary liver cancer and liver metastasis as a palliative method. It is successfully used in combination with ablation therapy. Microwave ablation is usually performed 7 days after TACE.
Baseline characteristics of the patients and tumors are summarized in .
Table 1. Baseline characteristics of the patients and tumors.
Artificial ascites and CT-guided microwave ablation
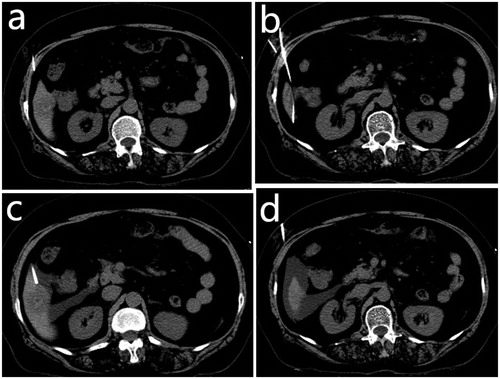
The procedure was performed under CT guidance. General anesthesia was induced with intravenous dexmedetomidine and local anesthesia with lidocaine. Artificial ascites was created with the administration of normal saline through a French sheath (Radifocus Introducer; Terumo, Tokyo, Japan). We punctured the peritoneal space between the lesion and the risk organs under CT guidance using an 18-gauge sheathed needle after a local anesthetic was administered into the skin at the puncture site, abdominal wall and peritoneum (). Subsequently, we removed the inner stylet of the sheath needle and inserted a 0.035-inch guidewire through the sheath (). A 5-French sheath was introduced into the peritoneal cavity over the guidewire (). Sufficient volume of 0.9% saline was injected through the sheath to separate the tumors from the risk organs by more than 0.5 cm (). After successful artificial ascites was produced, microwave ablation was performed. The solution was continuously introduced into the peritoneal cavity during the procedure. The circulation of continued instillation transferred the heat and minimized potential thermal injury of the non-target organs. This thermal energy pump effect of the fluid was a reason to select the hydrodissection technique rather than other techniques.
Figure 1. (a) An 18-gauge sheathed needle was inserted into the peritoneal space between the lesion and the risk organs under computed tomography guidance. (b) The inner stylet of the sheath needle was removed, and a 0.035-inch guidewire was inserted through the sheath. (c) A 5-French sheath was introduced into the peritoneal cavity over the guidewire. (d) Sufficient volume of 0.9% saline solution was injected through the sheath to separate the tumors from the risk organs.
A water-circulating cooled microwave unit (ECO-100A1, ECO Medical, Nanjing, China) was used in this retrospective study. An adjustable power of 0–70 W could be produced using this microwave unit at 2450 MHz. The power output was generally set to 40–70 W. We punctured the tumor with an 18-gauge straight-needle electrode with a 0.5-cm exposed tip under CT guidance. Single or multiple electrodes were used during the procedure depending on the tumor size. Overlapping ablations were performed if the tumor was larger than 3 cm. The needle path was cauterized when the electrode was pulled out to reduce the risk of bleeding and needle tract metastasis. The ideal ablation area covered the tumor focus and surrounding 5–10 mm of normal liver tissue. Contrast-enhanced CT was performed immediately after ablation to evaluate the response to treatment (). The infused artificial ascites persisted in the peritoneal cavity after the procedure. Repeated microwave ablation was necessary for ten patients because of the residual enhancement of the target tumor during the initial procedure, indicating incomplete ablation.
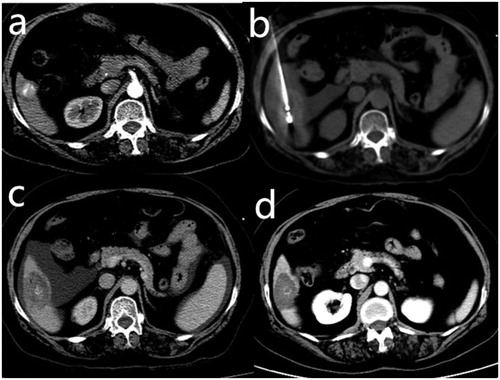
Figure 2. A 65-year-old woman with hepatocellular carcinoma in live segment VI. The patient was treated previously with transcatheter arterial chemoembolization. (a) Contrast-enhanced computed tomography (CT) scan before microwave ablation showed a slightly enhanced lesion with partial lipiodol deposition. The lesion was adjacent to the hepatic flexure of the colon. (b) The electrode needle was inserted into the lesion under CT guidance after the artificial ascites was infused. The lesion was separated from the colon. (c) Contrast-enhanced CT scans obtained immediately after microwave ablation showed complete ablation without enhanced zones in the lesion. (d) No enhancement in the target tumor or peripheral area was observed on the follow-up contrast-enhanced CT 1 month later.
Follow-up and study endpoints
We performed contrast-enhanced CT or magnetic resonance imaging (MRI) for all patients 1 month after the ablation procedure. Routine blood, tumor maker and liver function tests were also performed. Technical success was defined as complete ablation (no enhancement in the target tumor or peripheral area on contrast-enhanced CT or MRI) achieved immediately after the procedure and 1 month later. Routine contrast-enhanced CT or MRI and blood tests were repeated every 3 months. Local tumor progression was defined as the appearance of tumor enhancement within or at the edge of the previous complete ablation area [Citation37]. Remote intrahepatic tumor was defined as the appearance of a new lesion in the liver [Citation37]. Adverse events were classified as mild, moderate, severe, disabling and patient death according to the new adverse event classification proposed by the Society of Interventional Radiology [Citation38].
Statistical analysis
Quantitative variables are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS, version 19 (SPSS, Chicago, IL).
Results
The success rate of artificial ascites induction was 100%. The volume of the introduced solution was 503 ± 249.2 ml (range, 150–1200 ml). Microwave ablation with artificial ascites was successfully performed in all 61 tumors. The technical effectiveness rate was 100% with contrast-enhanced CT immediately after ablation. Complete ablation was achieved in all cases. Mild and moderate pain was reported in 25 (52%) patients, including four (8.3%) patients with pain in the right shoulder. Fever at 37.5–38.5 °C lasted for 1–7 days in 21 (43.7%) patients. These symptoms were alleviated with supportive treatment. No disabling events or deaths related to the procedure occurred. No severe complications, such as hemoperitoneum or infection, were observed after the procedure. summarizes the adverse events related to the procedure. Local tumor progression occurred in three (6.3%) patients with tumor diameters larger than 4 cm during the follow-up period (mean, 15 months; range, 6–24 months). Remote intrahepatic tumors were found in ten (20.8%) patients. No electrode needle track seeding was found during the follow-up period.
Table 2. Adverse events related to microwave ablation assisted with artificial ascites.
Discussion
Thermal ablation is a minimally invasive and effective treatment of liver tumors [Citation7,Citation14,Citation26]. Ablative margin 5–10 mm beyond the border of tumor is required to achieve curative ablation [Citation39]. Thermal injury of the critical non-target structures is a main consideration in complete ablation [Citation15].
Separation of the tumors from the adjacent vital organs could be achieved by producing artificial ascites, which builds a layer of fluid to insulate heat transmission and reduce hepatic ambient temperature. In a previous retrospective study including 151 malignant hepatic tumors treated with microwave ablation, the hydrodissection technique was applied to convert the untreatable tumors to treatable cases in 4% of patients [Citation23]. We retrospectively assessed the feasibility, safety and efficacy of CT-guided percutaneous microwave ablation with artificial ascites for problematic hepatocellular tumors in this study. Results demonstrated that this method may not only protect organs from thermal damage but also achieve complete ablation.
In the present study, we achieved a technical effectiveness rate of 100%, which was similar to previous studies reported by Kitchin et al. (100%) and Zhang et al. (96.9%) [Citation22,Citation23]. A sufficient ablation zone is the key to achieving success. With the assistance of artificial ascites, the tumors were separated from the risk organs to be ablated. The rate of local tumor progression was 6.3% in this study, which was an encouraging result. Kitchin et al. and Zhang et al. performed artificial ascites-assisted microwave ablation under ultrasound guidance, and the results of local tumor progression were 17.2% and 16.1%, respectively [Citation22,Citation23]. Two main factors related to local tumor progression were incomplete ablation and large tumor size [Citation40]. Our procedures were performed under CT guidance, which showed favorable spatial and tissue resolutions. Other factors, such as previous laparotomy and gastrointestinal gas interference, had little effect on the quality of CT images and ablation.
A larger tumor is more likely to be incompletely ablated, leading to local tumor progression [Citation40–42]. This finding was consistent in our study. The three patients who had local tumor progression during the follow-up period in our study had tumor diameters larger than 4 cm. Sequential chemoembolization and microwave ablation were performed in these patients.
TACE was performed previously in most patients included in this study. We assumed that the synergistic effect between TACE and ablation may be a reason for the low local tumor progression rate. Blood flow to the tumor was reduced using a chemoembolization agent, which could decrease the heat-sink effect of ablation. Simultaneously, the effect of chemotherapeutic drugs injected into the blood vessels of tumors was enhanced with ablation [Citation43]. It is better to combine chemoembolization with ablation than to perform ablation alone [Citation44].
We did not observe severe complications in the present study. This result was similar to those of previous studies in which microwave ablation with artificial ascites had been performed [Citation23,Citation45]. The risk organs were protected from potential thermal injuries by the artificial ascites [Citation20]. We scorched the needle tract in the process of withdrawing the electrode needle to reduce the risks of abdominal bleeding and needle metastasis [Citation46].
Despite promising outcomes, there were several limitations to this study. First, there were inherent limitations due to the retrospective nature without a control group. The true benefit could not be assessed because a control group could not be set-up at the risk of serious complications. Second, this study had a small series with a short follow-up period. Third, most patients had a history of TACE, which was a confounding factor for the efficacy. Finally, this was a single-center study. Bias may exist in the decision for therapy and operating experience. Thus, multicenter studies with a large sample and long-term follow-up are required to confirm the safety and effectiveness of this method in the future.
In conclusion, CT-guided percutaneous microwave ablation with artificial ascites is a feasible, safe and effective choice for treating problematic hepatocellular tumors, avoiding potential thermal damage to the risk organs. However, large multicenter studies are necessary to confirm this point.
Acknowledgments
We thank Yancu Hertzanu, M.D professor Emeritus of Radiology, Ben Gurion University of the Negev, Beer Sheva, Israel for his advice in editing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. e1261.
- Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790–799; discussion 799–800.
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–524.
- Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(5):S248–S260.
- Vodkin I, Kuo A. Extended criteria donors in liver transplantation. Clin Liver Dis. 2017;21(2):289–301.
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729.
- Breen DJ, Lencioni R. Image-guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol. 2015;12(3):175–186.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61(5):1579–1590.
- Lee LH, Hwang JI, Cheng YC, et al. Comparable outcomes of ultrasound versus computed tomography in the guidance of radiofrequency ablation for hepatocellular carcinoma. PLoS One. 2017;12(1):e0169655.
- Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54(8):1151–1156.
- Sala M, Llovet JM, Vilana R, for the Barcelona Cly'nic Liver Cancer (BCLC) Group, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40(6):1352–1360.
- Lam VW, Ng KK, Chok KS, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008;15(3):782–790.
- Chen X, Chen Y, Li Q, et al. Radiofrequency ablation versus surgical resection for intrahepatic hepatocellular carcinoma recurrence: a meta-analysis. J Surg Res. 2015;195(1):166–174.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Yamakado K, Nakatsuka A, Akeboshi M, et al. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14(9):1183–1186.
- Kondo Y, Yoshida H, Shiina S, et al. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006;93(10):1277–1282.
- Kariya S, Tanigawa N, Kojima H, et al. Radiofrequency ablation combined with CO2 injection for treatment of retroperitoneal tumor: protecting surrounding organs against thermal injury. AJR Am J Roentgenol. 2005;185(4):890–893.
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19(11):2630–2640.
- Bhagavatula SK, Chick JF, Chauhan NR, et al. Artificial ascites and pneumoperitoneum to facilitate thermal ablation of liver tumors: a pictorial essay. Abdom Radiol. 2017;42(2):620–630.
- Lee EJ, Rhim H, Lim HK, et al. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: experimental study with a porcine model. AJR Am J Roentgenol. 2008;190(6):1659–1664.
- Zhang M, Liang P, Cheng ZG, et al. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia. 2014;30(2):134–141.
- Kitchin D, Lubner M, Ziemlewicz T, et al. Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia. 2014;30(5):299–305.
- Hinshaw JL, Laeseke PF, Winter TC, 3rd, et al. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol. 2006;186(5 Suppl):S306–S310.
- Chen R, Gan Y, Ge N, et al. Transarterial chemoembolization versus radiofrequency ablation for recurrent hepatocellular carcinoma after resection within Barcelona Clinic Liver Cancer Stage 0/A: a retrospective comparative study. J Vasc Interv Radiol. 2016;27(12):1829–1836.
- Koh PS, Chan AC, Cheung TT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford). 2016;18(1):72–78.
- Lee HJ, Kim JW, Hur YH, et al. Conventional chemoembolization plus radiofrequency ablation versus surgical resection for single, medium-sized hepatocellular carcinoma: propensity-score matching analysis. J Vasc Interv Radiol. 2019;30(3):284–292. e281.
- Li B, Liu C, Xu XX, et al. Clinical application of artificial ascites in assisting CT-guided percutaneous cryoablation of hepatic tumors adjacent to the gastrointestinal tract. Sci Rep. 2017;7(1):16689.
- Dunne RM, Shyn PB, Sung JC, et al. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. Eur J Radiol. 2014;83(4):632–638.
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–139.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6 Part 1):2967–2973.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22(9):1983–1990.
- Livraghi T, Meloni F, Solbiati L, For the Collaborative Italian Group using AMICA system, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35(4):868–874.
- Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61(7):1100–1101.
- van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–1446.
- Uehara T, Hirooka M, Ishida K, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007;42(4):306–311.
- Ahmed M, Solbiati L, Brace CL, for the International Working Group on Image-guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, and the Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28(10):1432–1437. e1433.
- Koda M, Tokunaga S, Miyoshi K, et al. Ablative margin states by magnetic resonance imaging with ferucarbotran in radiofrequency ablation for hepatocellular carcinoma can predict local tumor progression. J Gastroenterol. 2013;48(11):1283–1292.
- Kim YS, Rhim H, Cho OK, et al. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59(3):432–441.
- Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38(10):977–981.
- Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97(5):1253–1262.
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252(3):905–913.
- Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81(3):e189–193.
- Liu SR, Liang P, Yu XL, et al. Percutaneous microwave ablation for liver tumours adjacent to the marginal angle. Int J Hyperthermia. 2014;30(5):306–311.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.
