Abstract
The rise of immunotherapy (IT) in oncological treatment has greatly improved outcomes in a number of disease states. However, its use in tumors of the central nervous system (CNS) remains limited for multiple reasons related to the unique immunologic tumor microenvironment. As such, it is valuable to consider the intersection of IT with additional treatment methods that may improve access to the CNS and effectiveness of existing IT modalities. One such combination is the pairing of IT with localized hyperthermia (HT) generated through technologies such as laser interstitial thermal therapy (LITT). The wide-ranging immunomodulatory effects of localized and whole-body HT have been investigated for some time. Hyperthermia has demonstrated immunostimulatory effects at the level of tumor cells, immune cells, and the broader environment governing potential immune surveillance. A thorough understanding of these effects as well as the current and upcoming investigations of such in combination with IT is important in considering the future directions of neuro-oncology.
Introduction
While the emergence of effective immunotherapies has redrawn the landscape of modern oncology across various disciplines, their efficacy in the treatment of central nervous system (CNS) tumors such as glioblastoma (GBM) is limited. As such, a diverse range of synergistic treatment pathways has been employed to drive and sustain an adaptive antitumor response given the challenges presented by the unique immunologic microenvironment of the CNS. One such treatment paradigm involves the integration of two innovative modalities, laser interstitial thermal therapy (LITT) and immunotherapy (IT). This review seeks to examine the immunomodulating effects of LITT (and other thermal ablative schemas), and the rationale and future potential of concomitant IT.
Laser interstitial thermal therapy is a method of minimally invasive surgery first developed in the 1990s for deep-seated intracranial tumors. Since its inception, the use of LITT has expanded to a number of pathologies [Citation1–6]. The procedure involves the stereotactic placement of a laser probe tip within an identified target lesion that then generates a focused distribution of thermal energy to produce coagulative necrosis for lesion destruction [Citation7–11]. There are two commercial systems currently available for LITT, the NeuroBlate™ system from Monteris and the Visualase™ system from Medtronic, with some variation in their treatment protocols and construction. However, the overall technique and underlying mechanism are consistent across the marketplace [Citation12–14]. The probe is placed using preprocedural magnetic resonance imaging (MRI) trajectory planning. Once it is in position, light energy travels through a fiberoptic cable to the probe tip centered within the region of interest [Citation2,Citation4–6,Citation8,Citation10,Citation11,Citation15–20]. MRI software is then used to generate maps of thermal change and tumor necrosis using the Arrhenius thermal dose model to guide administration of the therapy over the targeted area [Citation21–24].
LITT utilization and mechanism of action
Over the past 30 years, studies have documented the use of LITT for a range of neurosurgical pathologies, including primary brain tumors, recurrent metastases, radiation necrosis, epidural spinal metastases, and epilepsy [Citation8,Citation9,Citation11,Citation25–49]. Though large-scale randomized trials comparing LITT to more conventional methods of treatment are currently lacking, several smaller studies have demonstrated successful outcomes in otherwise non-surgical candidate patients for a number of common LITT applications. Most of the existing LITT studies have focused on primary brain tumors, given their extremely grim prognosis after exhaustion of conventional therapies [Citation8,Citation11,Citation25–28,Citation31]. With current standard of care, GBM carries a dismal median survival of just under 21 months [Citation50–56].
Banerjee et al. reviewed the literature for LITT in neuro-oncology and found improved to comparable median overall survival of 20.9 months from diagnosis of recurrence in grade III/IV malignant gliomas relative to conventional treatments of chemotherapy, open surgery, high-dose brachytherapy, and resection [Citation3]. Barnett et al. conducted a systematic review and meta-analysis of LITT (n = 79) versus craniotomy (n = 1036) of high-grade gliomas near areas of eloquence and found improved extent of treated tissue with reduced complication rates (10% reduction in absolute risk difference, p < 0.0001) in the LITT patients [Citation57]. Alattar et al. [Citation58] showed local control of 80–100% of completely ablated lesions and median survivals between 5.8 and 19.8 months in patients receiving LITT for brain metastases after stereotactic radiosurgery. Ivan et al. [Citation59] examined cases of newly diagnosed high grade gliomas treated with LITT and found a median overall survival of 14.2 months and median progression-free survival of 5.1 months. These case series and reviews demonstrate the feasibility of LITT and value of further investigation into its applications.
Laser interstitial thermal therapy leads to a cascade of enzyme induction, protein denaturation, melting of membrane lipids, vessel sclerosis, and coagulative necrosis, driving the intended and predictable thermal ablation [Citation19,Citation60]. Histologic examinations of treated lesions characterize the changes surrounding the laser probe into three primary regions: (1) a central coagulative necrosis; (2) a ring of macrophage-rich granulation tissue; and (3) a peripheral zone of vasogenic edema. Tissue viability increases radially away from the treatment foci as these regions absorb lower levels of the thermal load [Citation16,Citation20,Citation61–63]. A typical setup provides cooling of the laser probe to limit temperature at the tip of the probe to 90 °C to prevent charring, with heat dissipating over distance and establishing a temperature gradient [Citation64]. This temperature gradient is the root of the immunomodulation to be discussed through this review.
LITT and immunomodulation
While cytoreduction via thermal ablation within the targeted tumor is the primary tumoricidal effect of LITT, there is evidence that the resultant localized hyperthermia (HT) also modulates and enhances the innate antitumor immune response. Immunologic changes can be broadly categorized into three linked groups of effects: those that impact the tumor cells directly, those that modulate immune cell function and activation, and those that more grossly change the tumor microenvironment (TME). The impacts of localized HT on tumor and immune cells are summarized in . Specific changes, to be discussed in more detail below, including the release of tumor antigen-dense exosomes; immune-stimulating heat-shock proteins (HSPs); increased cytokine and chemokine production; enhanced antigen-presenting cell (APC), cytotoxic T cell, and natural killer (NK) cell activity; disruption of the blood–brain barrier (BBB); and vessel dilation with increased perfusion permitting greater immune surveillance [Citation42,Citation65–87]. The collective result is a combination of both immune-stimulating and immunosuppressive effects that modulate the body’s response to treatment and offer the potential to be therapeutically co-opted when HT is combined with IT.
Figure 1. Schematic representing integrated effects of hyperthermia on tumor and immune cells. Created with BioRender.com. APC: antigen-presenting cell; BBB: blood–brain barrier; HSP: heat shock protein; NK cell: natural killer cell.
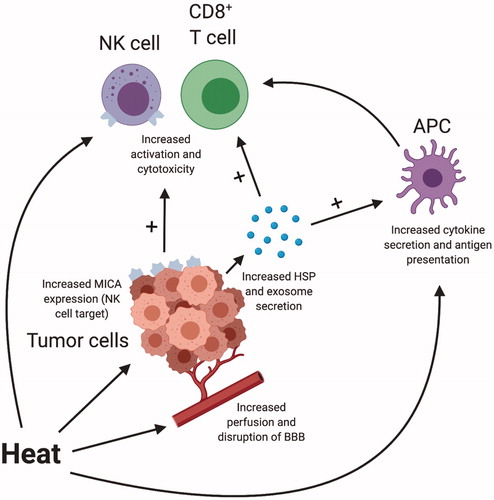
Impact on tumor cells
Significant work has been completed to characterize the impact of localized HT on tumor cells themselves. HT causes tumor cell production of heat shock proteins (HSP) and their release into the extracellular environment [Citation71]. HSPs are molecular chaperones that appear in response to heat exposure and have many immunologic functions, such as direct stimulation of NK cells to exert cytotoxic effects and APCs to enhance cytokine release and antigen presentation [Citation67,Citation88–90]. As these HSPs appear in the extracellular space, they are frequently bound to additional intracellular proteins, providing an avenue for cross-presentation of tumor neoantigens on MHC class I or traditional MHC class II presentation [Citation73,Citation91–93]. Udono et al. [Citation81] demonstrated that such cross-presentation, and the associated CD8+ T cell response, results in tumor-specific cytotoxicity. Suzue and Tamura both co-opted the same pathway through administration of HSPs from tumor cells to tumor-naïve mice, demonstrating inhibited progression of primary cancers, reduced metastases, and overall survival benefit [Citation77,Citation94]. Ostberg et al. demonstrated that exposure of in vitro tumor target cells to temperatures of 39.5 °C for 6 h resulted in increased expression of MICA, an NK cell target, resulting in enhanced cytotoxicity [Citation75]. Important to drawing inferences from these studies, Nikfarjam et al. [Citation74] showed that laser ablation in a murine model of colorectal liver metastases resulted in greater and more prolonged HSP levels compared to a control of ablated normal liver tissue.
In addition to the upregulation of HSPs, tumor HT also increases the concentration of released tumor exosomes into the TME. Exosomes are small membrane vesicles containing chemokines and concentrated tumor antigens that can be subsequently presented through APCs to stimulate further tumor-specific T cell responses [Citation84,Citation95]. Dai et al. [Citation96] demonstrated that such tumor-derived exosomes can significantly induce dendritic cell (DC) maturation, as well as prime tumor-specific cytotoxic T cells for anti-tumor immunity. Guo et al. found that exosomes from heat-stressed tumor cells stimulated DCs to secrete cytokines converting regulatory T cells (Tregs) into Th17 cells and inhibited tumor growth in a murine colon adenocarcinoma model [Citation68]. When considering these findings in the context of applied HT therapies, an important question arises around optimization of the duration and intensity of heat. Notably, many of the results previously described have been identified in the range of normal fever temperatures, generally below 42 °C [Citation65]. There is concern, then, that killing cells prematurely at the higher temperatures that LITT incurs may achieve the cytoreductive goal but fail to generate the desired immunostimulatory response. Understanding the immunologic changes that occur at temperatures upwards of 50 °C will be an important goal moving forward.
Direct impact on immune cells
Beyond the tumor cells, HT therapies also have direct impact on immune cells themselves, including APCs, NK cells, T cells, and macrophages. Improved cytotoxicity of CD8+ T cells and NK cells has been demonstrated under HT conditions, as well as increased tumor antigen-specific IFN-γ production [Citation72,Citation75]. Other groups showed that heating DCs to mild fever ranges results in enhanced maturation, antigen uptake, IL-12 production, migration, upregulation of both MHC class I and class II, and T cell stimulation [Citation69,Citation76,Citation80,Citation86]. Van Bruggen et al. [Citation82] showed an analogous activation of macrophages with similar temperature ranges. Isbert et al. [Citation97] demonstrated in vivo that LITT compared to resection in rat intrahepatic tumors reduced peritoneal spread and increased expression of CD8 and co-stimulatory molecules. Rats with liver adenocarcinomas treated with laser thermotherapy demonstrated resistance to tumor re-challenge and absence of tumor spread with increased CD8+ T cells compared to resections [Citation98]. Heat treatment by magnetite cationic liposomes in murine glioma subcutaneous flank tumor models resulted in resolution of the treated tumor as well as an untreated tumor on the opposite side, with increased CD8+ and CD4+ T cell tumor activity and infiltration at both locations [Citation99]. Regarding human patients, the immune-modulating impact of LITT therapy has been previously demonstrated in cases on non-CNS tumors. For example, patients with liver metastases of colorectal cancer treated with LITT had increased tumor-specific cytotoxic T cell stimulation with increased cytolytic activity in in vitro assays [Citation83].
Impact on immune infiltration
The last category to consider in the impact of HT therapies is the broader changes that affect immune cell localization. Heating tumors drives vascular adaptation, as studies in rats showed inducing mild HT to 42.5 °C for 30 min increased the diameter of local arterioles by 35%, blood flow by 50%, and pO2 by 50% [Citation100–102]. It also created shifts in IL-6 signaling and subsequently ICAM expression that resulted in increased T cell trafficking into tumors [Citation79]. Localized HT has also been shown to disrupt the BBB, potentially permitting enhanced trafficking of immune cells into the tumor itself [Citation42,Citation103–105]. Leuthardt et al. [Citation104] demonstrated that LITT to recurrent GBMs lead to a disruption of the peritumoral BBB that peaked within 1–2 weeks and resolved after 4–6 weeks. This finding is further supported by evidence that the permeability of BBB is dependent on brain temperature and increases from 38.5 °C before plateauing at 41–42 °C [Citation106]. Morris et al. [Citation42] demonstrated in human epilepsy patients that LITT resulted in disruption of the BBB for up to 8 months after treatment, with one patient developing a delayed optic neuritis. Multiple IT methods including antibodies, targeted toxins, bi-specific T cell engagers (BiTEs), and checkpoint blockade inhibitors have demonstrated issues with tumor infiltration [Citation107–110]. Thus, localized HT may allow increased access to traditional IT modalities to the CNS tumor site, potentially improving their efficacy.
Combining LITT with IT
Given the range of immunomodulatory impact with localized HT treatments, integration with conventional oncologic treatments was a reasonable next step for the field. The potential of such combinatorial therapies has been demonstrated in preclinical studies finding increased tumor cell lethality for chemotherapy and radiotherapy with HT treatments, and such pairings in human patients have already resulted in improved survival rates [Citation111–113]. With the addition of IT as a treatment modality, the current goal is to use localized HT to flip the tumor environment from an immunosuppressed ‘cold’ state to a ‘hot’ state that is more responsive to checkpoint blockade or adoptive T cell therapies (ACT) [Citation114].
Evidence for the rationale of utilizing HT therapies and IT has been published in pre-clinical studies. Bear et al. demonstrated in a murine metastatic melanoma model that thermo-ablative therapy using gold nano-shells promoted the expression of pro-inflammatory cytokines and chemokines and induced the maturation of DCs within tumor-draining lymph nodes. When combined with the transfer of tumor-specific pmel T-cells, this also prevented primary tumor recurrence as well as inhibiting metastatic tumor growth sites [Citation115]. den Brok et al. showed that adoptive splenocyte transfer from donor mice with tumors heated to ablative temperatures resulted in improved antitumor responses for previously tumor-naïve recipients. Additionally, the same group showed that pairing ablation with an anti-CTLA-4 antibody resulted in protection against tumor re-challenge [Citation94]. Wang et al. showed the combination of local photothermal ablation using single-walled carbon nanotubes with anti-CTLA-4 therapy prevented the development of distant tumor metastases in a murine lung cancer model, along with prolonged animal survival [Citation116]. Han et al. demonstrated that thermal ablation of murine flank colorectal tumors followed by administration of toll-like-receptor agonists and anti-CTLA-4 therapies resulted in destruction of tumors at distant sites with a significant increases in their CD8+ T cell to Treg ratio, as well as long-term resistance to tumor re-challenge [Citation117]. Luo et al. showed that tumor ablation followed by PD-1 blockade in murine breast and lung cancer models resulted in primary tumor resolution as well as a systemic immune response that suppressed metastatic lesions and re-challenge [Citation118]. Similar results have been demonstrated in murine models of neuroblastoma, colon cancer, and breast cancer [Citation119,Citation120]. Applications of combined HT and IT in preclinical malignant glioma models are currently limited in the literature. One study utilized a murine flank GBM model to test the pair. This group developed a novel method to generate gold nanoparticles that selectively accumulated in tumors and amplified the effect of light-based photothermal ablation in a treatment mechanism analogous to LITT. When paired with anti-PD-L1 antibodies, the combined treatment group demonstrated reduced tumor growth and improved survival relative to controls and each therapy in isolation, as well as lasting immunologic memory that rejected tumor re-challenge [Citation121]. These results support further investigation into the integration of thermal therapy and IT, and the potentially synergistic effect of the two on local and metastatic lesions as well as long-term antitumor immunologic memory.
Currently, there are few published data on pairing HT with IT in human patients (). In one small case series of just two patients, Paiva et al. [Citation122] showed extended survival with renal cell carcinoma metastases to the head and neck treated with laser-induced thermal therapy and IL-2. In a pilot study of patients with ovarian, pancreatic, gastric, colorectal, cervical, or endometrial cancer, 33 patients received local HT therapy plus ACT alone, or with either salvage chemotherapy or anti-PD-1 antibody. Seven out of 10 patients with local HT plus ACT, and 6 out of 11 patients treated additionally with anti-PD-1, had disease control. Overall, the study had an objective response rate of 30% with significantly increased cytokine markers among the clinical responders and a favorable toxicity profile among all groups [Citation123].
Table 1. Published hyperthermia (HT) with immunotherapy (IT) studies in human patients.
Beyond these results, there are several ongoing or upcoming phase I/II trials targeting CNS pathologies with LITT/HT in addition to IT regimens (). The available information indicates that these trials will investigate the pairing with checkpoint blockade therapy targeting the PD-1/PD-L1 axis with either pembrolizumab or avelumab in recurrent GBM as well as in CNS metastases from melanoma, non-small cell lung carcinoma (NSCLC), and renal cell carcinoma. A group out of Mount Sinai (NCT03341806) is currently enrolling 30 patients with recurrent GBM to receive LITT followed by IV avelumab every 2 weeks, compared to avelumab alone, with measured outcomes of dose limiting toxicity, objective response rate, progression-free survival, and overall response rate [Citation124]. An upcoming trial at the University of Florida (NCT04187872) will entail LITT followed by pembrolizumab every 3 weeks until recurrence for up to 2 years in patients with brain metastases from melanoma, non-small cell lung carcinoma, or renal carcinoma that have recurred after stereotactic radiosurgery [Citation125]. Lastly, a trial at Case Comprehensive Cancer Center (NCT03277638) is currently recruiting patients with recurrent GBM for LITT with administration of pembrolizumab 7 days before, 14 days after, or 35 days after treatment [Citation126]. Additional trials exploring the combination of HT and IT in extracranial disease are summarized in .
Table 2. Intracranial hyperthermia (HT) with immunotherapy (IT) clinical trials.
Table 3. Extracranial hyperthermia (HT) with immunotherapy (IT) clinical trials.
Conclusion
Ultimately, the integration of therapeutic modalities and expansion of our toolset in oncologic disease are important steps forward. The recent rise of IT has substantially shifted the context of these investigations. However, its success in tumors of the CNS remains limited and therefore combination with treatments that may improve access and effectiveness are a valuable avenue of research. The immunomodulatory impacts of localized HT have been known and investigated for some time, with demonstrated effects at multiple levels of the antitumor immune response. Given its immunostimulatory potential, pairing localized HT with the continually evolving IT strategies is a promising path with supportive preclinical data. As both HT and IT are pushed forward with ongoing independent research, the trials above represent the current next steps as we work toward characterization and optimization of their combination.
Disclosure statement
Ethan S. Srinivasan BS: none. Eric W. Sankey MD: none. Matthew M. Grabowski MD: none. Pakawat Chongsathidkiet MD: none. Peter E. Fecci MD, PhD: Speaker honoraria and grant support from Monteris Medical.
Additional information
Funding
References
- Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl. 1991;52:81–83.
- Sugiyama K, Sakai T, Fujishima I, et al. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg. 1990;54-55:501–505.
- Banerjee C, Snelling B, Berger MH, et al. The role of magnetic resonance-guided laser ablation in neurooncology. Br J Neurosurg. 2015;29(2):192–196.
- Prince E, Hakimian S, Ko AL, et al. Laser interstitial thermal therapy for epilepsy. Curr Neurol Neurosci Rep. 2017;17(9):63.
- Silva D, Sharma M, Juthani R, et al. Magnetic resonance thermometry and laser interstitial thermal therapy for brain tumors. Neurosurg Clin N Am. 2017;28(4):525–533.
- Ashraf O, Patel NV, Hanft S, et al. Laser-induced thermal therapy in neuro-oncology: a review. World Neurosurg. 2018;112:166–177.
- Anzai Y, Lufkin RB, Hirschowitz S, et al. MR imaging-histopathologic correlation of thermal injuries induced with interstitial Nd:YAG laser irradiation in the chronic model. J Magn Reson Imaging. 1992;2(6):671–678.
- Schwarzmaier H-J, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208–215.
- Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr. 1994;18(4):519–532.
- Reimer P, Bremer C, Horch C, et al. MR-monitored LITT as a palliative concept in patients with high grade gliomas: preliminary clinical experience. J Magn Reson Imaging. 1998;8(1):240–244.
- Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 Suppl Operative):133–144.
- Ahrar K, Gowda A, Javadi S, et al. Preclinical assessment of a 980-nm diode laser ablation system in a large animal tumor model. J Vasc Interv Radiol. 2010;21(4):555–561.
- Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Oper Neurosurg. 2012;71(1 Suppl Operative):133–ons45.
- Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the neuroblate system first-in-humans phase I clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118(6):1202–1219.
- Diaz R, Ivan ME, Hanft S, et al. Laser interstitial thermal therapy: lighting the way to a new treatment option in neurosurgery. Neurosurgery. 2016;79(suppl_1):S3–s7.
- Norred S, Johnson J. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int. 2014;2014:761312.
- Rodriguez A, Tatter SB. Laser ablation of recurrent malignant gliomas: current status and future perspective. Neurosurgery. 2016;79(suppl_1):S35–s9.
- Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19(1):65.
- Stafford R, Fuentes D, Elliott A, et al. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010;38(1):79–100.
- Patel NV, Mian M, Stafford RJ, et al. Laser interstitial thermal therapy technology, physics of magnetic resonance imaging thermometry, and technical considerations for proper catheter placement during magnetic resonance imaging-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(suppl_1):S8–s16.
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008;27(2):376–390.
- Chang IA. Considerations for thermal injury analysis for RF ablation devices. Open Biomed Eng J. 2010;4:3–12.
- McNichols RJ, Gowda A, Kangasniemi M, et al. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med. 2004;34(1):48–55.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294.
- Bastos D, Rao G, Oliva ICG, et al. Predictors of local control of brain metastasis treated with laser interstitial thermal therapy. Neurosurgery. 2019 DOI:10.1093/neuros/nyz357.
- Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361–368.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21–8; discussion ONS8-9.
- Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56(1):101–113.
- Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57(2):325–334.
- Rao MS, Hargreaves EL, Khan AJ, et al. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74(6):658–667; discussion 67.
- Tatsui CE, Lee SH, Amini B, et al. Spinal laser interstitial thermal therapy: a novel alternative to surgery for metastatic epidural spinal cord compression. Neurosurgery. 2016;79(suppl_1):S73–S82.
- Thomas JG, Al-Holou WN, de Almeida Bastos DC, et al. A novel use of the intraoperative MRI for metastatic spine tumors: laser interstitial thermal therapy for percutaneous treatment of epidural metastatic spine disease. Neurosurg Clin N Am. 2017;28(4):513–524.
- Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74(6):569–584; discussion 84-5.
- Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–811.
- Beaumont TL, Mohammadi AM, Kim AH, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy for glioblastoma of the corpus callosum. Neurosurgery. 2018;83(3):556–565.
- Borghei-Razavi H, Koech H, Sharma M, et al. Laser interstitial thermal therapy for posterior fossa lesions: an initial experience. World Neurosurg. 2018;117:e146–e53.
- Chaunzwa TL, Deng D, Leuthardt EC, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63.
- Hale AT, Sen S, Haider AS, et al. Open resection versus laser interstitial thermal therapy for the treatment of pediatric insular epilepsy. Neurosurgery. 2019;85(4):E730–e6.
- Hernandez RN, Carminucci A, Patel P, et al. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019;85(1):84–90.
- Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843.
- Morris SA, Rollo M, Rollo P, et al. Prolonged blood-brain barrier disruption following laser interstitial ablation in epilepsy: a case series with a case report of postablation optic neuritis. World Neurosurg. 2017;104:467–475.
- Palma AE, Wicks RT, Popli G, et al. Corpus callosotomy via laser interstitial thermal therapy: a case series. J Neurosurg Pediatr. 2018;23(3):303–307.
- Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125(4):853–860.
- Rennert RC, Khan U, Tatter SB, et al. Patterns of clinical use of stereotactic laser ablation: analysis of a Multicenter Prospective Registry. World Neurosurg. 2018;116:e566–e70.
- Smith CJ, Myers CS, Chapple KM, et al. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(suppl_1):S59–s72.
- Sun XR, Patel NV, Danish SF. Tissue ablation dynamics during magnetic resonance-guided, laser-induced thermal therapy. Neurosurgery. 2015;77(1):51–58. discussion 8.
- Tovar-Spinoza Z, Choi H. Magnetic resonance-guided laser interstitial thermal therapy: report of a series of pediatric brain tumors. J Neurosurg Pediatr. 2016;17(6):723–733.
- Traylor JI, Patel R, Habib A, et al. Laser interstitial thermal therapy to the posterior fossa: challenges and nuances. World Neurosurg. 2019;132:e124–e32.
- Stupp R, Mason WP, van den Bent MJ, et al.; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
- Sampson JH, Gunn MD, Fecci PE, et al. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25.
- Stummer W, Pichlmeier U, Meinel T, et al.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401.
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316.
- Woroniecka KI, Rhodin KE, Chongsathidkiet P, et al. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24(16):3792–3802.
- Barnett GH, Voigt JD, Alhuwalia MS. A systematic review and meta-analysis of studies examining the use of brain laser interstitial thermal therapy versus craniotomy for the treatment of high-grade tumors in or near areas of eloquence: an examination of the extent of resection and major complication rates associated with each type of surgery. Stereotact Funct Neurosurg. 2016;94(3):164–173.
- Alattar AA, Bartek J, Jr., Chiang VL, et al. Stereotactic laser ablation as treatment of brain metastases recurring after stereotactic radiosurgery: a systematic literature review. World Neurosurg. 2019;128:134–142.
- Ivan ME, Mohammadi AM, De Deugd N, et al. Laser ablation of newly diagnosed malignant gliomas: a meta-analysis. Neurosurgery. 2016;79(suppl_1):S17–s23.
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38(3):E13.
- Schober R, Bettag M, Sabel M, et al. Fine structure of zonal changes in experimental Nd:YAG laser-induced interstitial hyperthermia. Lasers Surg Med. 1993;13(2):234–241.
- Tracz RA, Wyman DR, Little PB, et al. Comparison of magnetic resonance images and the histopathological findings of lesions induced by interstitial laser photocoagulation in the brain. Lasers Surg Med. 1993;13(1):45–54.
- Elder JB, Huntoon K, Otero J, et al. Histologic findings associated with laser interstitial thermotherapy for glioblastoma multiforme. Diagn Pathol. 2019;14(1):19.
- Patel PD, Patel NV, Davidson C, et al. The role of MRgLITT in overcoming the challenges in managing infield recurrence after radiation for brain metastasis. Neurosurgery. 2016;79(suppl_1):S40–S58.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349.
- Frey B, Weiss E-M, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542.
- Giuliano JS, Jr., Lahni PM, Wong HR, et al. Pediatric sepsis – part V: extracellular heat shock proteins: alarmins for the host immune system. Open Inflamm J. 2011;4:49–60.
- Guo D, Chen Y, Wang S, et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology. 2018;154(1):132–143.
- Hatzfeld-Charbonnier AS, Lasek A, Castera L, et al. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol. 2007;81(5):1179–1187.
- Ito A, Fujioka M, Tanaka K, et al. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J Biosci Bioeng. 2005;100(1):36–42.
- Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31(4):261–271.
- Mace TA, Zhong L, Kokolus KM, et al. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia. 2012;28(1):9–18.
- Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol. 2012;3:63.
- Nikfarjam M, Muralidharan V, Su K, et al. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperthermia. 2005;21(4):319–332.
- Ostberg JR, Dayanc BE, Yuan M, et al. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–1331.
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 2006;55(3):292–298.
- Suzue K, Zhou X, Eisen HN, et al. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94(24):13146–13151.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30(8):531–539.
- Toraya-Brown S, Sheen MR, Zhang P, et al. Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. Nanomedicine 2014;10:1273–1285.
- Tournier JN, Hellmann AQ, Lesca G, et al. Fever-like thermal conditions regulate the activation of maturing dendritic cells. J Leukoc Biol. 2003;73(4):493–501.
- Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91(8):3077–3081.
- van Bruggen I, Robertson TA, Papadimitriou JM. The effect of mild hyperthermia on the morphology and function of murine resident peritoneal macrophages. Exp Mol Pathol. 1991;55(2):119–134.
- Vogl TJ, Wissniowski TT, Naguib NN, et al. Activation of tumor-specific T lymphocytes after laser-induced thermotherapy in patients with colorectal liver metastases. Cancer Immunol Immunother. 2009;58(10):1557–1563.
- Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303.
- Zhang Y, Zheng L. Tumor immunotherapy based on tumor-derived heat shock proteins. Oncol Lett. 2013;6(6):1543–1549.
- Zheng H, Benjamin IJ, Basu S, et al. Heat shock factor 1-independent activation of dendritic cells by heat shock: implication for the uncoupling of heat-mediated immunoregulation from the heat shock response. Eur J Immunol. 2003;33(6):1754–1762.
- Zhong H, Yang Y, Ma S, et al. Induction of a tumour-specific CTL response by exosomes isolated from heat-treated malignant ascites of gastric cancer patients. Int J Hyperthermia. 2011;27(6):604–611.
- Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol. 2007;594:1–13.
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442.
- Multhoff G, Botzler C, Jennen L, et al. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158(9):4341–4350.
- Wallin RP, Lundqvist A, More SH, et al. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23(3):130–135.
- Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24(5):523–534.
- Javid B, MacAry PA, Lehner PJ. Structure and function: heat shock proteins and adaptive immunity. J Immunol. 2007;179(4):2035–2040.
- Tamura Y, Peng P, Liu K, et al. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278(5335):117–120.
- Chen T, Guo J, Yang M, et al. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186(4):2219–2228.
- Dai S, Wan T, Wang B, et al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11(20):7554–7563.
- Isbert C, Ritz JP, Roggan A, et al. Enhancement of the immune response to residual intrahepatic tumor tissue by laser-induced thermotherapy (LITT) compared to hepatic resection. Lasers Surg Med. 2004;35(4):284–292.
- Ivarsson K, Myllymaki L, Jansner K, et al. Resistance to tumour challenge after tumour laser thermotherapy is associated with a cellular immune response. Br J Cancer. 2005;93(4):435–440.
- Yanase M, Shinkai M, Honda H, et al. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Jpn J Cancer Res. 1998;89(7):775–782.
- Nah BS, Choi IB, Oh WY, et al. Vascular thermal adaptation in tumors and normal tissue in rats. Int J Radiat Oncol Biol Phys. 1996;35(1):95–101.
- Meyer RE, Braun RD, Rosner GL, et al. Local 42 degrees C hyperthermia improves vascular conductance of the R3230Ac rat mammary adenocarcinoma during sodium nitroprusside infusion. Radiat Res. 2000;154(2):196–201.
- Shakil A, Osborn JL, Song CW. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Radiat Oncol Biol Phys. 1999;43(4):859–865.
- Shivers RR, Wijsman JA. Blood-brain barrier permeability during hyperthermia. Prog Brain Res. 1998;115:413–424.
- Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613.
- McDannold N, Vykhodtseva N, Jolesz FA, et al. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51(5):913–923.
- Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161(3):926–939.
- Lampson LA. Monoclonal antibodies in neuro-oncology: Getting past the blood-brain barrier. MAbs. 2011;3(2):153–160.
- Husain B, Ellerman D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs. 2018;32(5):441–464.
- Romani M, Pistillo MP, Carosio R, et al. Immune checkpoints and innovative therapies in glioblastoma. Front Oncol. 2018;8:464.
- Galea I, Bernardes-Silva M, Forse PA, et al. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204(9):2023–2030.
- Miller RC, Roizin-Towle L, Komatsu K, et al. Interaction of heat with X-rays and cis-platinum; cell lethality and oncogenic transformation. Int J Hyperthermia. 1989;5(6):697–705.
- Baker D, Sager H, Constable W. The influence of levamisole and hyperthermia on the incidence of metastases from an X-irradiated tumor. Cancer Invest. 1986;4(4):287–292.
- Morita M, Kuwano H, Araki K, et al. Prognostic significance of lymphocyte infiltration following preoperative chemoradiotherapy and hyperthermia for esophageal cancer. Int J Radiat Oncol Biol Phys. 2001;49(5):1259–1266.
- Moy AJ, Tunnell JW. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv Drug Deliv Rev. 2017;114:175–183.
- Bear AS, Kennedy LC, Young JK, Perna SK, et al. Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive T cell transfer. PLoS One. 2013;8(7):e69073.
- Wang C, Xu L, Liang C, et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater Weinheim. 2014;26(48):8154–8162.
- Han X, Wang R, Xu J, et al. In situ thermal ablation of tumors in combination with nano-adjuvant and immune checkpoint blockade to inhibit cancer metastasis and recurrence. Biomaterials. 2019;224:119490.
- Luo L, Zhu C, Yin H, et al. Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS Nano. 2018;12(8):7647–7662.
- Cano-Mejia J, Burga RA, Sweeney EE, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomed Nanotechnol Biol Med. 2017;13(2):771–781.
- Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193.
- Liu Y, Chongsathidkiet P, Crawford BM, et al. Plasmonic gold nanostar-mediated photothermal immunotherapy for brain tumor ablation and immunologic memory. Immunotherapy. 2019;11(15):1293–1302.
- Paiva MB, Sercarz JA, Pantuck AJ, et al. Combined cytoreductive laser therapy and immunotherapy for palliation of metastatic renal cell carcinoma to the head and neck. Lasers Med Sci. 2007;22(1):60–63.
- Qiao G, Wang X, Zhou X, et al. Immune correlates of clinical benefit in a phase I study of hyperthermia with adoptive T cell immunotherapy in patients with solid tumors. Int J Hyperthermia. 2019;36(suppl 1):74–82.
- Avelumab with laser interstitial therapy for recurrent glioblastoma. Available from: https://ClinicalTrials.gov/show/NCT03341806.
- LITT and pembrolizumab in recurrent brain metastasis. Available from: https://ClinicalTrials.gov/show/NCT04187872.
- Laser interstitial thermotherapy (LITT) combined with checkpoint inhibitor for recurrent GBM (RGBM). Available from: https://ClinicalTrials.gov/show/NCT03277638.
